The Oral Administration of Lactobacillus delbrueckii subsp. lactis 557 (LDL557) Ameliorates the Progression of Monosodium Iodoacetate-Induced Osteoarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Preparation
2.3. Experimental Design
2.4. Preparing Frozen Sections of Rat Knee Joints
2.5. Hematoxylin-Eosin (H&E) Staining
2.6. Safranin O/Fast Green Staining
2.7. Measurement of Serum Biomarkers in Rats
2.8. Statistical Analysis
3. Results
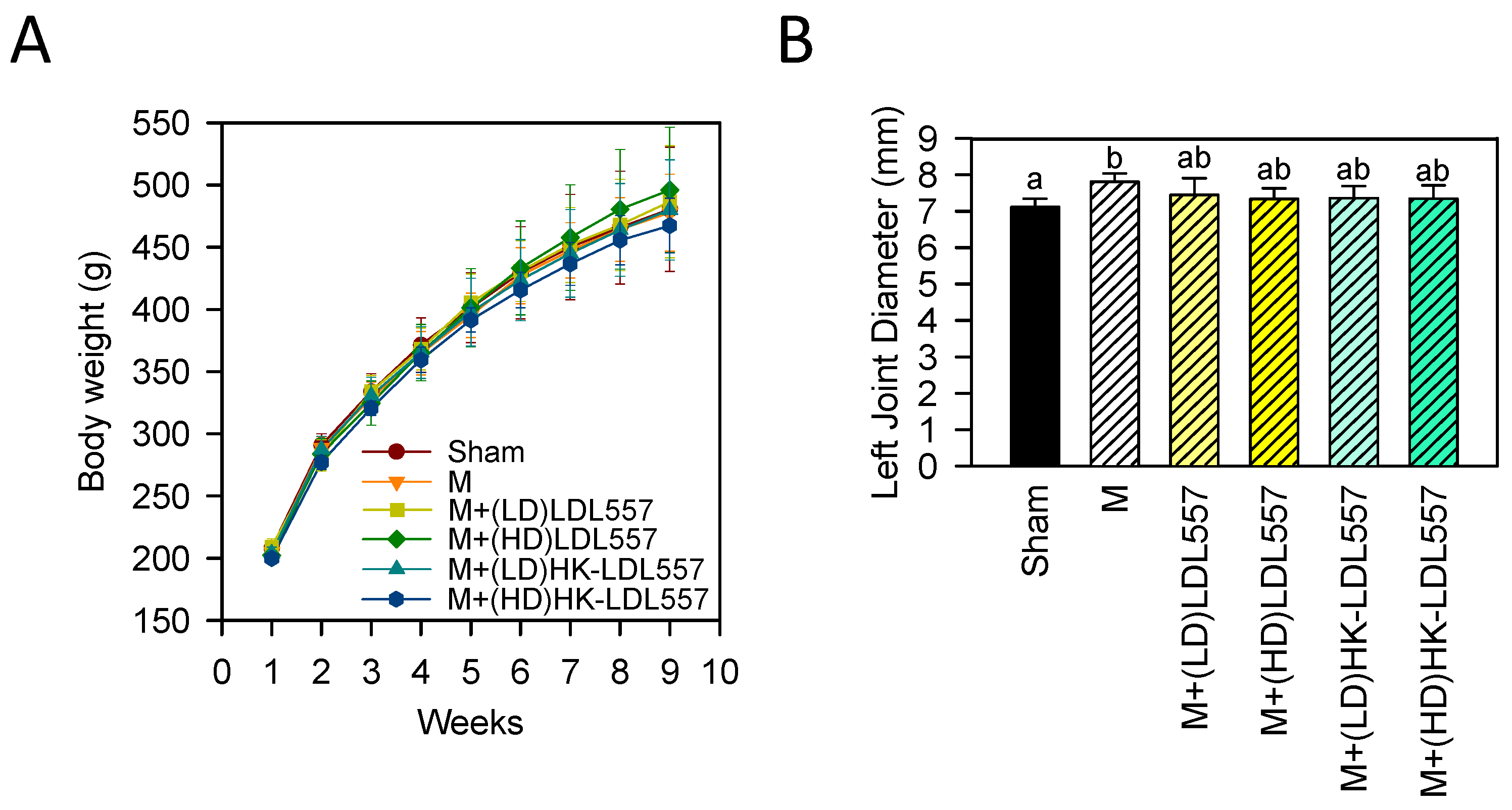
3.1. Effect of LDL557 on Body Weight and Joint Swelling
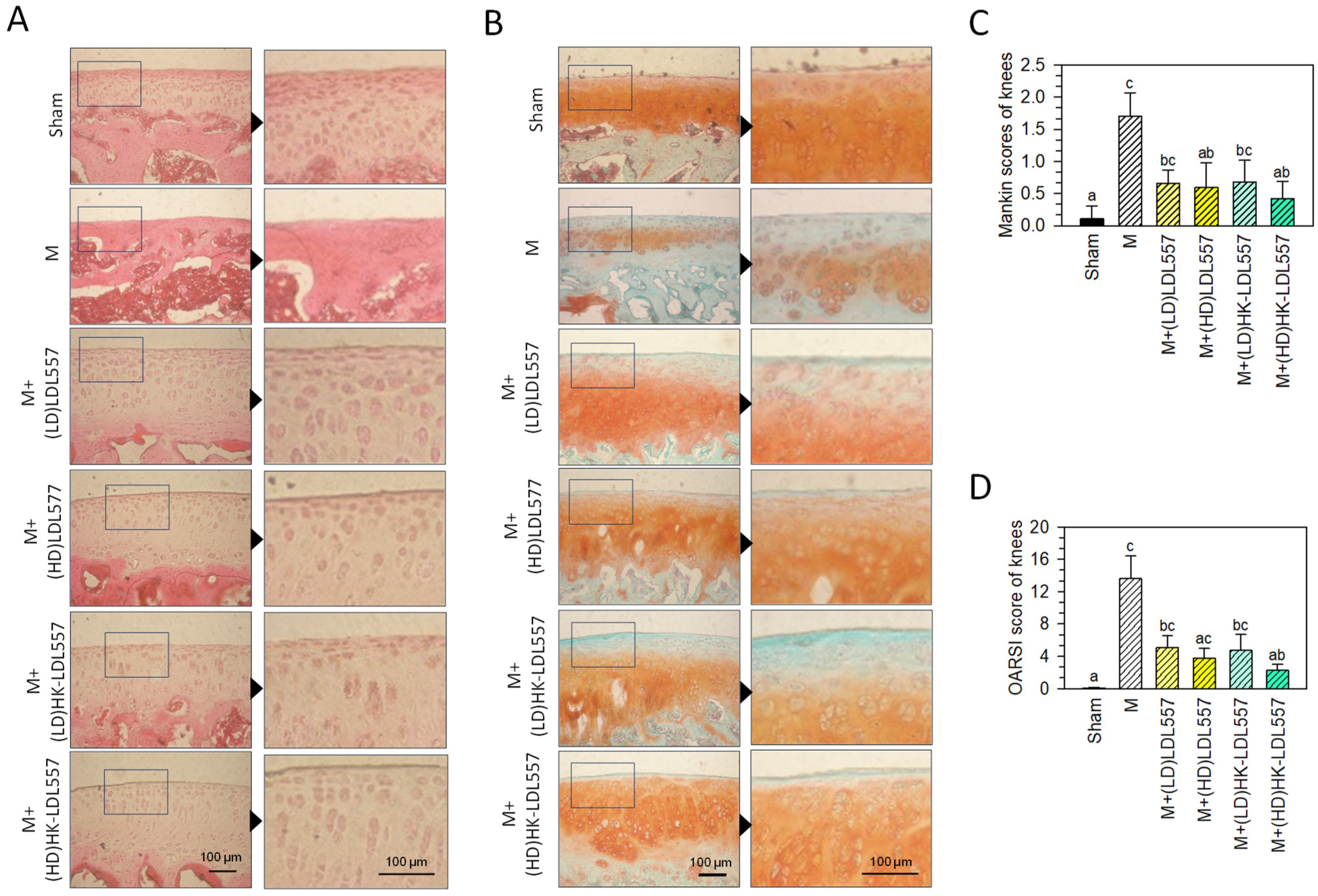
3.2. Effect of LDL557 on Knee-Joint Histopathologic Changes
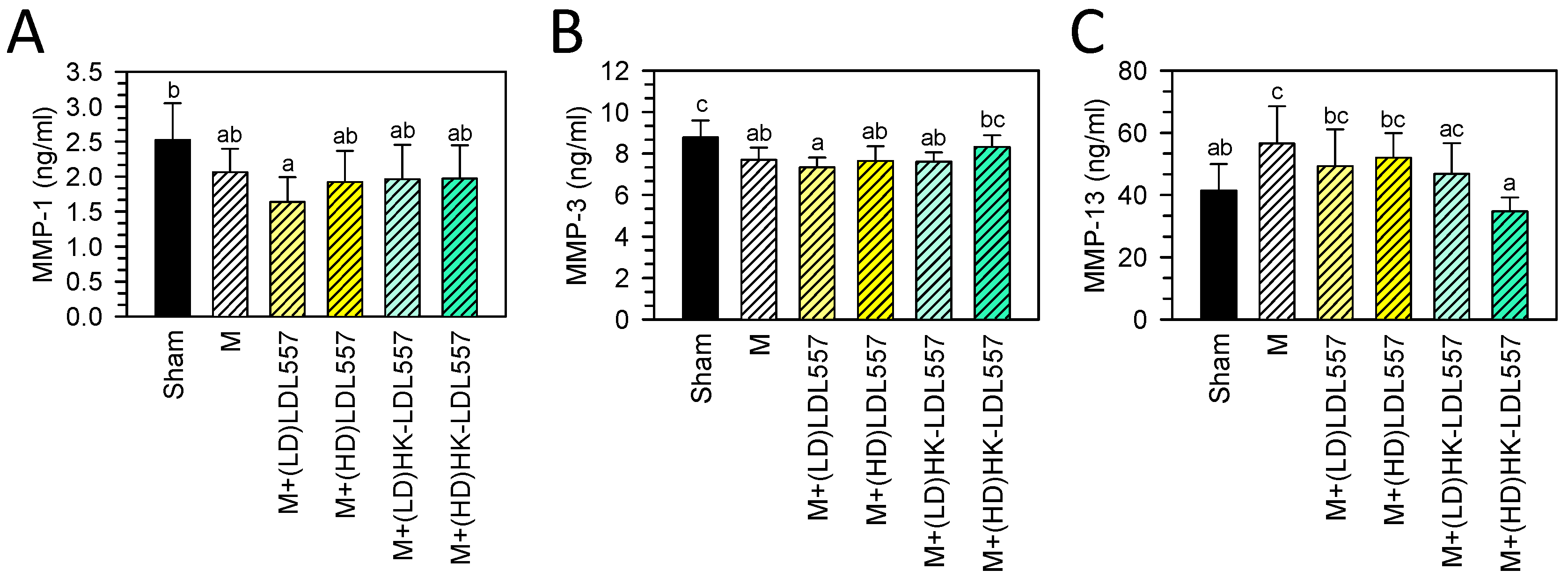
3.3. Effect of LDL557 on the Biomarkers of Matrix-Degrading Enzymes
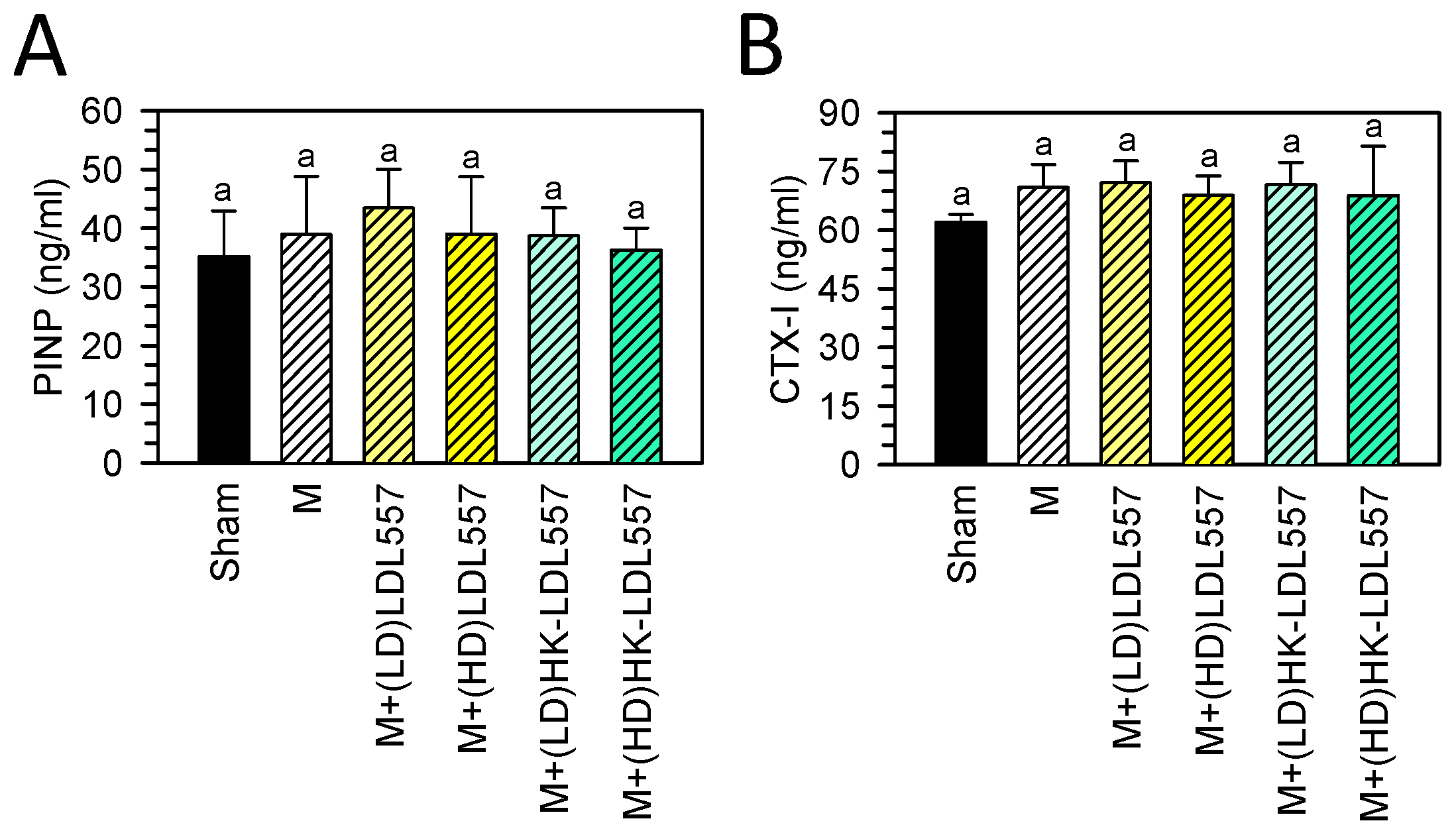
3.4. Effect of LDL557 on the Biomarkers of Bone Turnover
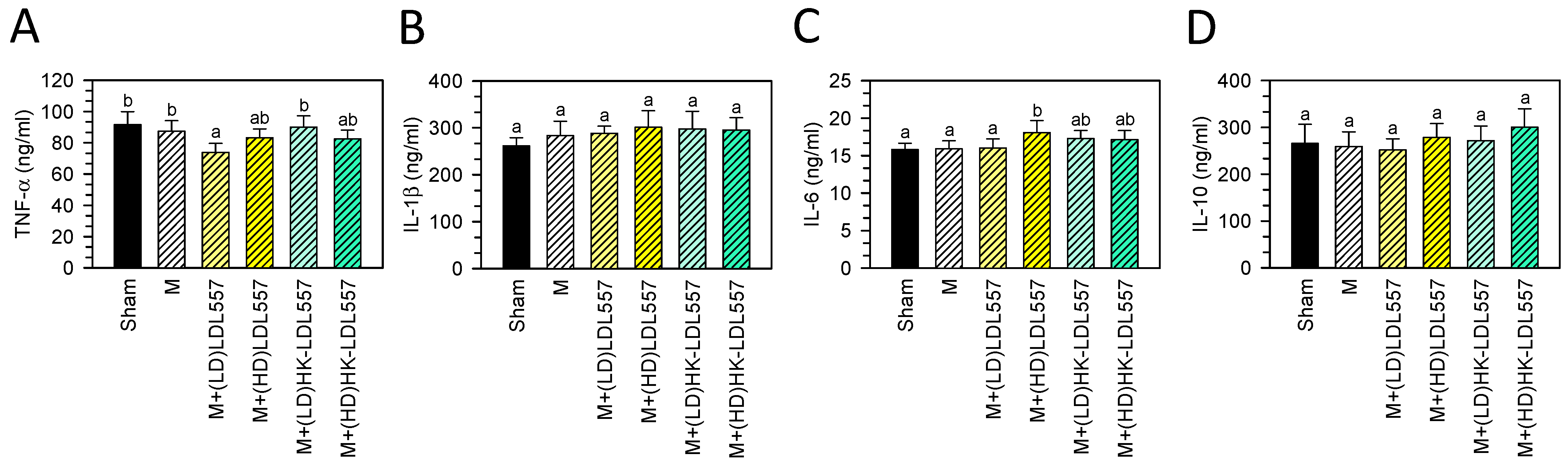
3.5. Effect of LDL557 on the Expressions of Inflammatory Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef]
- Neogi, T. The epidemiology and impact of pain in osteoarthritis. Osteoarthr. Cartil. 2013, 21, 1145–1153. [Google Scholar] [CrossRef]
- Agaliotis, M.; Fransen, M.; Bridgett, L.; Nairn, L.; Votrubec, M.; Jan, S.; Heard, R.; Mackey, M. Risk factors associated with reduced work productivity among people with chronic knee pain. Osteoarthr. Cartil. 2013, 21, 1160–1169. [Google Scholar] [CrossRef] [PubMed]
- Nazarinasab, M.; Motamedfar, A.; Moqadam, A.E. Investigating mental health in patients with osteoarthritis and its relationship with some clinical and demographic factors. Reumatologia 2017, 55, 183–188. [Google Scholar] [CrossRef]
- Nuesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Juni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Fahy, N.; Farrell, E.; Ritter, T.; Ryan, A.E.; Murphy, J.M. Immune modulation to improve tissue engineering outcomes for cartilage repair in the osteoarthritic joint. Tissue Eng. Part. B Rev. 2015, 21, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Rigoglou, S.; Papavassiliou, A.G. The NF-kappaB signalling pathway in osteoarthritis. Int. J. Biochem. Cell Biol. 2013, 45, 2580–2584. [Google Scholar] [CrossRef]
- Zeng, G.Q.; Chen, A.B.; Li, W.; Song, J.H.; Gao, C.Y. High MMP-1, MMP-2, and MMP-9 protein levels in osteoarthritis. Genet. Mol. Res. 2015, 14, 14811–14822. [Google Scholar] [CrossRef]
- Yang, C.Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM metalloproteinases in osteoarthritis—Looking beyond the ‘usual suspects’. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Wang, W.; Niu, Y.; Jia, Q. Physical therapy as a promising treatment for osteoarthritis: A narrative review. Front. Physiol. 2022, 13, 1011407. [Google Scholar] [CrossRef] [PubMed]
- Terkawi, M.A.; Ebata, T.; Yokota, S.; Takahashi, D.; Endo, T.; Matsumae, G.; Shimizu, T.; Kadoya, K.; Iwasaki, N. Low-Grade Inflammation in the Pathogenesis of Osteoarthritis: Cellular and Molecular Mechanisms and Strategies for Future Therapeutic Intervention. Biomedicines 2022, 10, 1109. [Google Scholar] [CrossRef]
- Ramires, L.C.; Santos, G.S.; Ramires, R.P.; da Fonseca, L.F.; Jeyaraman, M.; Muthu, S.; Lana, A.V.; Azzini, G.; Smith, C.S.; Lana, J.F. The Association between Gut Microbiota and Osteoarthritis: Does the Disease Begin in the Gut? Int. J. Mol. Sci. 2022, 23, 1494. [Google Scholar] [CrossRef]
- Dunn, C.M.; Jeffries, M.A. The Microbiome in Osteoarthritis: A Narrative Review of Recent Human and Animal Model Literature. Curr. Rheumatol. Rep. 2022, 24, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Thoma, L.M.; Golightly, Y.M. Epidemiology of osteoarthritis. Osteoarthr. Cartil. 2022, 30, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Tap, J.; Mondot, S.; Levenez, F.; Pelletier, E.; Caron, C.; Furet, J.P.; Ugarte, E.; Munoz-Tamayo, R.; Paslier, D.L.; Nalin, R.; et al. Towards the human intestinal microbiota phylogenetic core. Environ. Microbiol. 2009, 11, 2574–2584. [Google Scholar] [CrossRef] [PubMed]
- Collins, K.H.; Paul, H.A.; Reimer, R.A.; Seerattan, R.A.; Hart, D.A.; Herzog, W. Relationship between inflammation, the gut microbiota, and metabolic osteoarthritis development: Studies in a rat model. Osteoarthr. Cartil. 2015, 23, 1989–1998. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Na, H.S.; Jhun, J.; Woo, J.S.; Lee, A.R.; Lee, S.Y.; Lee, J.S.; Um, I.G.; Kim, S.J.; Park, S.H.; et al. Lactobacillus (LA-1) and butyrate inhibit osteoarthritis by controlling autophagy and inflammatory cell death of chondrocytes. Front. Immunol. 2022, 13, 930511. [Google Scholar] [CrossRef] [PubMed]
- InSug, O.S.; Natarajan Anbazhagan, A.; Singh, G.; Ma, K.; Green, S.J.; Singhal, M.; Wang, J.; Kumar, A.; Dudeja, P.K.; Unterman, T.G.; et al. Lactobacillus acidophilus Mitigates Osteoarthritis-Associated Pain, Cartilage Disintegration and Gut Microbiota Dysbiosis in an Experimental Murine OA Model. Biomedicines 2022, 10, 1298. [Google Scholar] [CrossRef]
- Song, W.; Liu, Y.G.; Dong, X.H.; Song, C.; Bai, Y.Y.; Hu, P.P.; Li, L.; Wang, T.Y. M5 prevents osteoarthritis induced by a high-fat diet in mice. J. Funct. Foods 2020, 72, 104039. [Google Scholar] [CrossRef]
- Xu, T.; Yang, D.; Liu, K.; Gao, Q.; Liu, Z.; Li, G. Miya Improves Osteoarthritis Characteristics via the Gut-Muscle-Joint Axis According to Multi-Omics Analyses. Front. Pharmacol. 2022, 13, 816891. [Google Scholar] [CrossRef] [PubMed]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.J.; Bover-Cid, S.; Chemaly, M.; Davies, R.; Hilbert, F.; Lindqvist, R.; Nauta, M.; et al. Update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA 12: Suitability of taxonomic units notified to EFSA until March 2020. EFSA J. 2020, 18, e05965. [Google Scholar] [PubMed]
- Guzman, R.E.; Evans, M.G.; Bove, S.; Morenko, B.; Kilgore, K. Mono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: An animal model of osteoarthritis. Toxicol. Pathol. 2003, 31, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Morais, S.V.; Czeczko, N.G.; Malafaia, O.; Ribas, J.M.F.; Garcia, J.B.; Miguel, M.T.; Zini, C.; Massignan, A.G. Osteoarthritis model induced by intra-articular monosodium iodoacetate in rats knee. Acta Cir. Bras. 2016, 31, 765–773. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Udo, M.; Muneta, T.; Tsuji, K.; Ozeki, N.; Nakagawa, Y.; Ohara, T.; Saito, R.; Yanagisawa, K.; Koga, H.; Sekiya, I. Monoiodoacetic acid induces arthritis and synovitis in rats in a dose- and time-dependent manner: Proposed model-specific scoring systems. Osteoarthr. Cartil. 2016, 24, 1284–1291. [Google Scholar] [CrossRef] [PubMed]
- Savi, F.M.; Brierly, G.I.; Baldwin, J.; Theodoropoulos, C.; Woodruff, M.A. Comparison of Different Decalcification Methods Using Rat Mandibles as a Model. J. Histochem. Cytochem. 2017, 65, 705–722. [Google Scholar] [CrossRef]
- Sahin, K.; Kucuk, O.; Orhan, C.; Tuzcu, M.; Durmus, A.S.; Ozercan, I.H.; Sahin, N.; Juturu, V. Niacinamide and undenatured type II collagen modulates the inflammatory response in rats with monoiodoacetate-induced osteoarthritis. Sci. Rep. 2021, 11, 14724. [Google Scholar] [CrossRef]
- Pritzker, K.P.; Gay, S.; Jimenez, S.A.; Ostergaard, K.; Pelletier, J.P.; Revell, P.A.; Salter, D.; van den Berg, W.B. Osteoarthritis cartilage histopathology: Grading and staging. Osteoarthr. Cartil. 2006, 14, 13–29. [Google Scholar] [CrossRef]
- Pengas, I.; Eldridge, S.; Assiotis, A.; McNicholas, M.; Mendes, J.E.; Laver, L. MMP-3 in the peripheral serum as a biomarker of knee osteoarthritis, 40 years after open total knee meniscectomy. J. Exp. Orthop. 2018, 5, 21. [Google Scholar] [CrossRef]
- Jarecki, J.; Malecka-Masalska, T.; Kosior-Jarecka, E.; Widuchowski, W.; Krasowski, P.; Gutbier, M.; Dobrzynski, M.; Blicharski, T. Concentration of Selected Metalloproteinases and Osteocalcin in the Serum and Synovial Fluid of Obese Women with Advanced Knee Osteoarthritis. Int. J. Environ. Res. Public. Health 2022, 19, 3530. [Google Scholar] [CrossRef]
- Singh, S.; Jindal, D.; Khanna, R. Can serum MMP-3 diagnose early knee osteoarthritis? J. Orthop. 2023, 38, 42–46. [Google Scholar] [CrossRef]
- Kumm, J.; Tamm, A.; Lintrop, M.; Tamm, A. Diagnostic and prognostic value of bone biomarkers in progressive knee osteoarthritis: A 6-year follow-up study in middle-aged subjects. Osteoarthr. Cartil. 2013, 21, 815–822. [Google Scholar] [CrossRef]
- Gillett, M.J.; Vasikaran, S.D.; Inderjeeth, C.A. The Role of PINP in Diagnosis and Management of Metabolic Bone Disease. Clin. Biochem. Rev. 2021, 42, 3–10. [Google Scholar] [CrossRef]
- Eastell, R.; Pigott, T.; Gossiel, F.; Naylor, K.E.; Walsh, J.S.; Peel, N.F.A. DIAGNOSIS OF ENDOCRINE DISEASE: Bone turnover markers: Are they clinically useful? Eur. J. Endocrinol. 2018, 178, R19–R31. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, S.; Su, H.; Cheng, J. Isoliquiritigenin Inhibits IL-1beta-Induced Production of Matrix Metalloproteinase in Articular Chondrocytes. Mol. Ther. Methods Clin. Dev. 2018, 9, 153–159. [Google Scholar] [CrossRef]
- Kunisch, E.; Kinne, R.W.; Alsalameh, R.J.; Alsalameh, S. Pro-inflammatory IL-1beta and/or TNF-alpha up-regulate matrix metalloproteases-1 and -3 mRNA in chondrocyte subpopulations potentially pathogenic in osteoarthritis: In situ hybridization studies on a single cell level. Int. J. Rheum. Dis. 2016, 19, 557–566. [Google Scholar] [CrossRef]
- Chan, C.M.; Macdonald, C.D.; Litherland, G.J.; Wilkinson, D.J.; Skelton, A.; Europe-Finner, G.N.; Rowan, A.D. Cytokine-induced MMP13 Expression in Human Chondrocytes Is Dependent on Activating Transcription Factor 3 (ATF3) Regulation. J. Biol. Chem. 2017, 292, 1625–1636. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, Y.; Dong, H.; Cui, Y.; Du, Q.; Wang, X.; Li, L.; Zhang, H. Telmisartan Mitigates TNF-alpha-Induced Type II Collagen Reduction by Upregulating SOX-9. ACS Omega 2021, 6, 11756–11761. [Google Scholar] [CrossRef]
- Chadjichristos, C.; Ghayor, C.; Kypriotou, M.; Martin, G.; Renard, E.; Ala-Kokko, L.; Suske, G.; de Crombrugghe, B.; Pujol, J.P.; Galera, P. Sp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytes. J. Biol. Chem. 2003, 278, 39762–39772. [Google Scholar] [CrossRef] [PubMed]
- Neidel, J.; Zeidler, U. Independent effects of interleukin 1 on proteoglycan synthesis and proteoglycan breakdown of bovine articular cartilage in vitro. Agents Actions 1993, 39, 82–90. [Google Scholar] [CrossRef]
- Jiang, J.; Cai, M. Cardamonin Inhibited IL-1beta Induced Injury by Inhibition of NLRP3 Inflammasome via Activating Nrf2/NQO-1 Signaling Pathway in Chondrocyte. J. Microbiol. Biotechnol. 2021, 31, 794–802. [Google Scholar] [CrossRef]
- Koyama, T.; Uchida, K.; Fukushima, K.; Ohashi, Y.; Uchiyama, K.; Inoue, G.; Takahira, N.; Takaso, M. Elevated levels of TNF-alpha, IL-1beta and IL-6 in the synovial tissue of patients with labral tear: A comparative study with hip osteoarthritis. BMC Musculoskelet. Disord. 2021, 22, 33. [Google Scholar] [CrossRef]
- Sahu, N.; Viljoen, H.J.; Subramanian, A. Continuous low-intensity ultrasound attenuates IL-6 and TNFalpha-induced catabolic effects and repairs chondral fissures in bovine osteochondral explants. BMC Musculoskelet. Disord. 2019, 20, 193. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yang, S.; Shin, Y.; Rhee, J.; Chun, C.H.; Chun, J.S. Interleukin-6 plays an essential role in hypoxia-inducible factor 2alpha-induced experimental osteoarthritic cartilage destruction in mice. Arthritis Rheum. 2011, 63, 2732–2743. [Google Scholar] [CrossRef]
- Grunke, M.; Schulze-Koops, H. Successful treatment of inflammatory knee osteoarthritis with tumour necrosis factor blockade. Ann. Rheum. Dis. 2006, 65, 555–556. [Google Scholar] [CrossRef] [PubMed]
- Latourte, A.; Cherifi, C.; Maillet, J.; Ea, H.K.; Bouaziz, W.; Funck-Brentano, T.; Cohen-Solal, M.; Hay, E.; Richette, P. Systemic inhibition of IL-6/Stat3 signalling protects against experimental osteoarthritis. Ann. Rheum. Dis. 2017, 76, 748–755. [Google Scholar] [CrossRef]
- Yu, L.; Luo, R.; Qin, G.; Zhang, Q.; Liang, W. Efficacy and safety of anti-interleukin-1 therapeutics in the treatment of knee osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2023, 18, 100. [Google Scholar] [CrossRef]
- Gallelli, L.; Galasso, O.; Falcone, D.; Southworth, S.; Greco, M.; Ventura, V.; Romualdi, P.; Corigliano, A.; Terracciano, R.; Savino, R.; et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthr. Cartil. 2013, 21, 1400–1408. [Google Scholar] [CrossRef]
- Hunter, L.J.; Wood, D.M.; Dargan, P.I. The patterns of toxicity and management of acute nonsteroidal anti-inflammatory drug (NSAID) overdose. Open Access Emerg. Med. 2011, 3, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fuochi, V.; Furneri, P.M. Applications of Probiotics and Their Potential Health Benefits. Int. J. Mol. Sci. 2023, 24, 15915. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, W.; Ran, C.; Hu, J.; Zhou, Z. Abrupt suspension of probiotics administration may increase host pathogen susceptibility by inducing gut dysbiosis. Sci. Rep. 2016, 6, 23214. [Google Scholar] [CrossRef]
- Liong, M.T. Safety of probiotics: Translocation and infection. Nutr. Rev. 2008, 66, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, D.; GianVincenzo, Z.; Carlo Luca, R.; Karan, G.; Jorge, V.; Roberto, M.; Javad, P. Oral-Gut Microbiota and Arthritis: Is There an Evidence-Based Axis? J. Clin. Med. 2019, 8, 1753. [Google Scholar] [CrossRef] [PubMed]
- Kalinkovich, A.; Livshits, G. A cross talk between dysbiosis and gut-associated immune system governs the development of inflammatory arthropathies. Semin. Arthritis Rheum. 2019, 49, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Kraus, V.B. Does lipopolysaccharide-mediated inflammation have a role in OA? Nat. Rev. Rheumatol. 2016, 12, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Whitley, C.S.; Haribabu, B.; Jala, V.R. Regulation of Intestinal Barrier Function by Microbial Metabolites. Cell Mol. Gastroenterol. Hepatol. 2021, 11, 1463–1482. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Shang, X.; Liu, J.; Chi, R.; Zhang, J.; Xu, T. The gut microbiota in osteoarthritis: Where do we stand and what can we do? Arthritis Res. Ther. 2021, 23, 42. [Google Scholar] [CrossRef]
- Marchese, L.; Contartese, D.; Giavaresi, G.; Di Sarno, L.; Salamanna, F. The Complex Interplay between the Gut Microbiome and Osteoarthritis: A Systematic Review on Potential Correlations and Therapeutic Approaches. Int. J. Mol. Sci. 2024, 25, 143. [Google Scholar] [CrossRef]
- Jhun, J.; Cho, K.H.; Lee, D.H.; Kwon, J.Y.; Woo, J.S.; Kim, J.; Na, H.S.; Park, S.H.; Kim, S.J.; Cho, M.L. Oral Administration of Lactobacillus rhamnosus Ameliorates the Progression of Osteoarthritis by Inhibiting Joint Pain and Inflammation. Cells 2021, 10, 1057. [Google Scholar] [CrossRef]
- Lin, Y.Y.; Chang, S.L.; Liu, S.C.; Achudhan, D.; Tsai, Y.S.; Lin, S.W.; Chen, Y.L.; Chen, C.C.; Chang, J.W.; Fong, Y.C.; et al. Therapeutic Effects of Live Lactobacillus plantarum GKD7 in a Rat Model of Knee Osteoarthritis. Nutrients 2022, 14, 3170. [Google Scholar] [CrossRef] [PubMed]
- So, J.S.; Song, M.K.; Kwon, H.K.; Lee, C.G.; Chae, C.S.; Sahoo, A.; Jash, A.; Lee, S.H.; Park, Z.Y.; Im, S.H. Lactobacillus casei enhances type II collagen/glucosamine-mediated suppression of inflammatory responses in experimental osteoarthritis. Life Sci. 2011, 88, 358–366. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.K.; Na, H.S.; Jhun, J.Y.; Lee, J.S.; Um, I.G.; Lee, S.Y.; Park, M.S.; Cho, M.L.; Park, S.H. Bifidobacterium longum BORI inhibits pain behavior and chondrocyte death, and attenuates osteoarthritis progression. PLoS ONE 2023, 18, e0286456. [Google Scholar] [CrossRef]
- Henrotin, Y.; Patrier, S.; Pralus, A.; Roche, M.; Nivoliez, A. Protective Actions of Oral Administration of Bifidobacterium longum CBi0703 in Spontaneous Osteoarthritis in Dunkin Hartley Guinea Pig Model. Cartilage 2021, 13, 1204S–1213S. [Google Scholar] [CrossRef]
- Boer, C.G.; Radjabzadeh, D.; Medina-Gomez, C.; Garmaeva, S.; Schiphof, D.; Arp, P.; Koet, T.; Kurilshikov, A.; Fu, J.; Ikram, M.A.; et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat. Commun. 2019, 10, 4881. [Google Scholar] [CrossRef]
- Longo, U.G.; Papalia, R.; De Salvatore, S.; Picozzi, R.; Sarubbi, A.; Denaro, V. Induced Models of Osteoarthritis in Animal Models: A Systematic Review. Biology 2023, 12, 283. [Google Scholar] [CrossRef]
- Rai, M.F.; Duan, X.; Quirk, J.D.; Holguin, N.; Schmidt, E.J.; Chinzei, N.; Silva, M.J.; Sandell, L.J. Post-Traumatic Osteoarthritis in Mice Following Mechanical Injury to the Synovial Joint. Sci. Rep. 2017, 7, 45223. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Nakamura, J.; Ohtori, S.; Orita, S.; Omae, T.; Nakajima, T.; Suzuki, T.; Takahashi, K. Intra-articular injection of mono-iodoacetate induces osteoarthritis of the hip in rats. BMC Musculoskelet. Disord. 2016, 17, 132. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, L.-W.; Huang, T.-C.; Hu, Y.-C.; Hsieh, B.-S.; Lin, J.-S.; Hsu, H.-Y.; Lee, C.-C.; Chang, K.-L. The Oral Administration of Lactobacillus delbrueckii subsp. lactis 557 (LDL557) Ameliorates the Progression of Monosodium Iodoacetate-Induced Osteoarthritis. Curr. Issues Mol. Biol. 2024, 46, 8969-8980. https://doi.org/10.3390/cimb46080530
Huang L-W, Huang T-C, Hu Y-C, Hsieh B-S, Lin J-S, Hsu H-Y, Lee C-C, Chang K-L. The Oral Administration of Lactobacillus delbrueckii subsp. lactis 557 (LDL557) Ameliorates the Progression of Monosodium Iodoacetate-Induced Osteoarthritis. Current Issues in Molecular Biology. 2024; 46(8):8969-8980. https://doi.org/10.3390/cimb46080530
Chicago/Turabian StyleHuang, Li-Wen, Tzu-Ching Huang, Yu-Chen Hu, Bau-Shan Hsieh, Jin-Seng Lin, Han-Yin Hsu, Chia-Chia Lee, and Kee-Lung Chang. 2024. "The Oral Administration of Lactobacillus delbrueckii subsp. lactis 557 (LDL557) Ameliorates the Progression of Monosodium Iodoacetate-Induced Osteoarthritis" Current Issues in Molecular Biology 46, no. 8: 8969-8980. https://doi.org/10.3390/cimb46080530




