The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases
Abstract
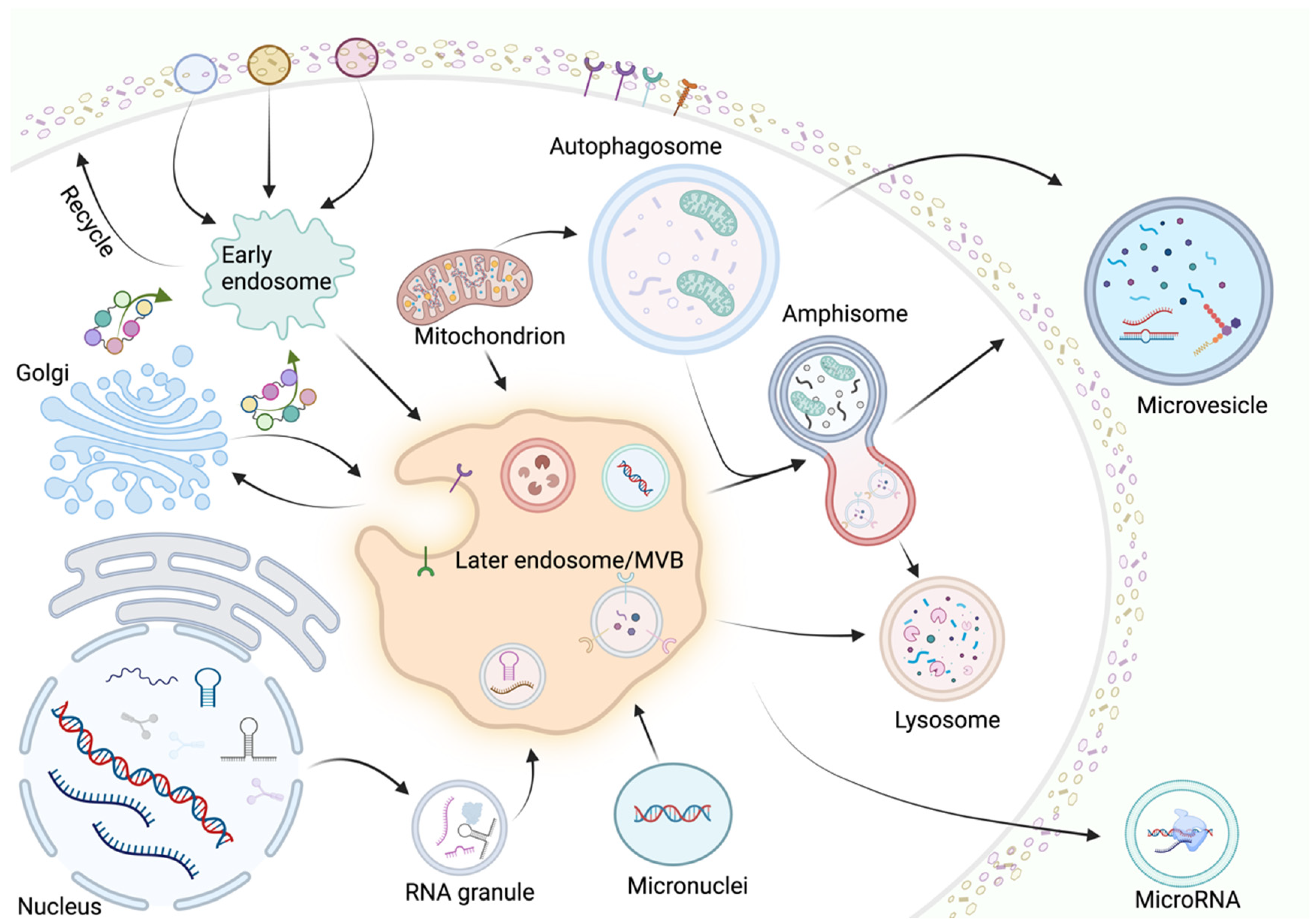
:1. Introduction
2. Biogenesis and Sorting of EVs
2.1. Exosomes
2.2. Microvesicles
2.3. Apoptotic Bodies
3. Regulation of Bone Regeneration by EVs
3.1. EV-Mediated Intercellular Interactions
3.1.1. Macrophages (Mφ)
3.1.2. Mesenchymal Stromal Cells
3.1.3. Osteoblasts
3.1.4. Osteoclasts
3.2. EVs Promote Angiogenesis
3.3. EVs Promote Cartilage Regeneration
4. The Role of EVs in Bone Diseases
4.1. Osteoporosis
4.2. Osteoarthritis
4.3. Fractures
5. Clinical Applications of Engineered EVs in Bone Regeneration-Related Diseases
5.1. Clinical Diagnostic Tools
5.2. Engineered EVs for Treatment
6. Conclusions
Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- He, Y.; Zhao, Y.; Fan, L.; Wang, X.; Duan, M.; Wang, H.; Zhu, X.; Liu, J. Injectable Affinity and Remote Magnetothermal Effects of Bi-Based Alloy for Long-Term Bone Defect Repair and Analgesia. Adv. Sci. 2021, 8, e2100719. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yu, W.; Yin, X.; Cui, L.; Tang, S.; Jiang, N.; Cui, L.; Zhao, N.; Lin, Q.; Chen, L.; et al. Prevalence of Osteoporosis and Fracture in China: The China Osteoporosis Prevalence Study. JAMA Netw. Open 2021, 4, e2121106. [Google Scholar] [CrossRef]
- Haugen, H.J.; Lyngstadaas, S.P.; Rossi, F.; Perale, G. Bone grafts: Which is the ideal biomaterial? J. Clin. Periodontol. 2019, 46 (Suppl. 21), 92–102. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Kuznetsova, A.V.; Popova, O.P.; Danilova, T.I.; Yanushevich, O.O. Modern Approaches to Acellular Therapy in Bone and Dental Regeneration. Int. J. Mol. Sci. 2021, 22, 13454. [Google Scholar] [CrossRef]
- van Niel, G.; D’Angelo, G.; Raposo, G. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 2018, 19, 213–228. [Google Scholar] [CrossRef]
- Marar, C.; Starich, B.; Wirtz, D. Extracellular vesicles in immunomodulation and tumor progression. Nat. Immunol. 2021, 22, 560–570. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular vesicles as drug delivery systems: Why and how? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Couch, Y.; Buzàs, E.I.; Di Vizio, D.; Gho, Y.S.; Harrison, P.; Hill, A.F.; Lötvall, J.; Raposo, G.; Stahl, P.D.; Théry, C.; et al. A brief history of nearly EV-erything-The rise and rise of extracellular vesicles. J. Extracell. Vesicles 2021, 10, e12144. [Google Scholar] [CrossRef]
- Song, X.; Xu, L.; Zhang, W. Biomimetic synthesis and optimization of extracellular vesicles for bone regeneration. J. Control Release 2023, 355, 18–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xing, J.; Liu, T.; Zhang, J.; Dai, Z.; Zhang, H.; Wang, D.; Tang, D. Small extracellular vesicles: From mediating cancer cell metastasis to therapeutic value in pancreatic cancer. Cell Commun. Signal 2022, 20, 1. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Shi, K.; Yang, S.; Liu, J.; Zhou, Q.; Wang, G.; Song, J.; Li, Z.; Zhang, Z.; Yuan, W. Effect of exosomal miRNA on cancer biology and clinical applications. Mol. Cancer 2018, 17, 147. [Google Scholar] [CrossRef] [PubMed]
- Mashouri, L.; Yousefi, H.; Aref, A.R.; Ahadi, A.M.; Molaei, F.; Alahari, S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol. Cancer 2019, 18, 75. [Google Scholar] [CrossRef]
- Colombo, M.; Raposo, G.; Théry, C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 2014, 30, 255–289. [Google Scholar] [CrossRef]
- Rädler, J.; Gupta, D.; Zickler, A.; Andaloussi, S.E. Exploiting the biogenesis of extracellular vesicles for bioengineering and therapeutic cargo loading. Mol. Ther. 2023, 31, 1231–1250. [Google Scholar] [CrossRef]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef]
- Minciacchi, V.R.; Freeman, M.R.; Di Vizio, D. Extracellular vesicles in cancer: Exosomes, microvesicles and the emerging role of large oncosomes. Semin. Cell Dev. Biol. 2015, 40, 41–51. [Google Scholar] [CrossRef]
- Bian, X.; Xiao, Y.-T.; Wu, T.; Yao, M.; Du, L.; Ren, S.; Wang, J. Microvesicles and chemokines in tumor microenvironment: Mediators of intercellular communications in tumor progression. Mol. Cancer 2019, 18, 50. [Google Scholar] [CrossRef]
- Zou, X.; Lei, Q.; Luo, X.; Yin, J.; Chen, S.; Hao, C.; Shiyu, L.; Ma, D. Advances in biological functions and applications of apoptotic vesicles. Cell Commun. Signal 2023, 21, 260. [Google Scholar] [CrossRef]
- Atkin-Smith, G.K.; Tixeira, R.; Paone, S.; Mathivanan, S.; Collins, C.; Liem, M.; Goodall, K.J.; Ravichandran, K.S.; Hulett, M.D.; Poon, I.K.H. A novel mechanism of generating extracellular vesicles during apoptosis via a beads-on-a-string membrane structure. Nat. Commun. 2015, 6, 7439. [Google Scholar] [CrossRef] [PubMed]
- Moss, D.K.; Betin, V.M.; Malesinski, S.D.; Lane, J.D. A novel role for microtubules in apoptotic chromatin dynamics and cellular fragmentation. J. Cell Sci. 2006, 119 Pt 11, 2362–2374. [Google Scholar] [CrossRef] [PubMed]
- Tixeira, R.; Phan, T.K.; Caruso, S.; Shi, B.; Atkin-Smith, G.K.; Nedeva, C.; Chow, J.D.Y.; Puthalakath, H.; Hulett, M.D.; Herold, M.J.; et al. ROCK1 but not LIMK1 or PAK2 is a key regulator of apoptotic membrane blebbing and cell disassembly. Cell Death Differ. 2020, 27, 102–116. [Google Scholar] [CrossRef]
- Huang, X.; Lan, Y.; Shen, J.; Chen, Z.; Xie, Z. Extracellular Vesicles in Bone Homeostasis: Emerging Mediators of Osteoimmune Interactions and Promising Therapeutic Targets. Int. J. Biol. Sci. 2022, 18, 4088–4100. [Google Scholar] [CrossRef]
- Chu, C.; Deng, J.; Sun, X.; Qu, Y.; Man, Y. Collagen Membrane and Immune Response in Guided Bone Regeneration: Recent Progress and Perspectives. Tissue Eng. Part. B Rev. 2017, 23, 421–435. [Google Scholar] [CrossRef]
- Ekström, K.; Omar, O.; Granéli, C.; Wang, X.; Vazirisani, F.; Thomsen, P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS ONE 2013, 8, e75227. [Google Scholar] [CrossRef]
- Hao, Z.; Ren, L.; Zhang, Z.; Yang, Z.; Wu, S.; Liu, G.; Cheng, B.; Wu, J.; Xia, J. A multifunctional neuromodulation platform utilizing Schwann cell-derived exosomes orchestrates bone microenvironment via immunomodulation, angiogenesis and osteogenesis. Bioact. Mater. 2023, 23, 206–222. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Li, S.; Li, Y. Exosomes Derived From M2 Macrophages Facilitate Osteogenesis and Reduce Adipogenesis of BMSCs. Front. Endocrinol. 2021, 12, 680328. [Google Scholar] [CrossRef]
- Turturici, G.; Tinnirello, R.; Sconzo, G.; Geraci, F. Extracellular membrane vesicles as a mechanism of cell-to-cell communication: Advantages and disadvantages. Am. J. Physiol. Cell Physiol. 2014, 306, C621–C633. [Google Scholar] [CrossRef]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Weatherholt, A.M.; Fuchs, R.K.; Warden, S.J. Specialized connective tissue: Bone, the structural framework of the upper extremity. J. Hand Ther. 2012, 25, 123–131; quiz 132. [Google Scholar] [CrossRef] [PubMed]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Pankajakshan, D.; Agrawal, D.K. Mesenchymal Stem Cell Paracrine Factors in Vascular Repair and Regeneration. J. Biomed. Technol. Res. 2014, 1. [Google Scholar] [CrossRef]
- Pan, G.; Yang, Y.; Zhang, J.; Liu, W.; Wang, G.; Zhang, Y.; Yang, Q.; Zhai, F.; Tai, Y.; Liu, J.; et al. Bone marrow mesenchymal stem cells ameliorate hepatic ischemia/reperfusion injuries via inactivation of the MEK/ERK signaling pathway in rats. J. Surg. Res. 2012, 178, 935–948. [Google Scholar] [CrossRef] [PubMed]
- Di Nicola, M.; Carlo-Stella, C.; Magni, M.; Milanesi, M.; Longoni, P.D.; Matteucci, P.; Grisanti, S.; Gianni, A.M. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 2002, 99, 3838–3843. [Google Scholar] [CrossRef] [PubMed]
- Chiossone, L.; Conte, R.; Spaggiari, G.M.; Serra, M.; Romei, C.; Bellora, F.; Becchetti, F.; Andaloro, A.; Moretta, L.; Bottino, C. Mesenchymal Stromal Cells Induce Peculiar Alternatively Activated Macrophages Capable of Dampening Both Innate and Adaptive Immune Responses. Stem Cells 2016, 34, 1909–1921. [Google Scholar] [CrossRef] [PubMed]
- Dyer, D.P.; Thomson, J.M.; Hermant, A.; Jowitt, T.A.; Handel, T.M.; Proudfoot, A.E.I.; Day, A.J.; Milner, C.M. TSG-6 inhibits neutrophil migration via direct interaction with the chemokine CXCL8. J. Immunol. 2014, 192, 2177–2185. [Google Scholar] [CrossRef]
- Phinney, D.G.; Pittenger, M.F. Concise Review: MSC-Derived Exosomes for Cell-Free Therapy. Stem Cells 2017, 35, 851–858. [Google Scholar] [CrossRef]
- Nakao, Y.; Fukuda, T.; Zhang, Q.; Sanui, T.; Shinjo, T.; Kou, X.; Chen, C.; Liu, D.; Watanabe, Y.; Hayashi, C.; et al. Exosomes from TNF-α-treated human gingiva-derived MSCs enhance M2 macrophage polarization and inhibit periodontal bone loss. Acta Biomater. 2021, 122, 306–324. [Google Scholar] [CrossRef]
- Wang, D.; Cao, H.; Hua, W.; Gao, L.; Yuan, Y.; Zhou, X.; Zeng, Z. Mesenchymal Stem Cell-Derived Extracellular Vesicles for Bone Defect Repair. Membranes 2022, 12, 716. [Google Scholar] [CrossRef]
- Qin, Y.; Wang, L.; Gao, Z.; Chen, G.; Zhang, C. Bone marrow stromal/stem cell-derived extracellular vesicles regulate osteoblast activity and differentiation in vitro and promote bone regeneration in vivo. Sci. Rep. 2016, 6, 21961. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Omar, O.; Vazirisani, F.; Thomsen, P.; Ekström, K. Mesenchymal stem cell-derived exosomes have altered microRNA profiles and induce osteogenic differentiation depending on the stage of differentiation. PLoS ONE 2018, 13, e0193059. [Google Scholar] [CrossRef]
- Salhotra, A.; Shah, H.N.; Levi, B.; Longaker, M.T. Mechanisms of bone development and repair. Nat. Rev. Mol. Cell Biol. 2020, 21, 696–711. [Google Scholar] [CrossRef]
- Lee, W.-C.; Guntur, A.R.; Long, F.; Rosen, C.J. Energy Metabolism of the Osteoblast: Implications for Osteoporosis. Endocr. Rev. 2017, 38, 255–266. [Google Scholar] [CrossRef]
- Kito, H.; Ohya, S. Role of K+ and Ca2+-Permeable Channels in Osteoblast Functions. Int. J. Mol. Sci. 2021, 22, 10459. [Google Scholar] [CrossRef]
- Makino, T.; Tsukazaki, H.; Ukon, Y.; Tateiwa, D.; Yoshikawa, H.; Kaito, T. The Biological Enhancement of Spinal Fusion for Spinal Degenerative Disease. Int. J. Mol. Sci. 2018, 19, 2430. [Google Scholar] [CrossRef] [PubMed]
- Henry, J.P.; Bordoni, B. Histology, Osteoblasts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Cappariello, A.; Loftus, A.; Muraca, M.; Maurizi, A.; Rucci, N.; Teti, A. Osteoblast-Derived Extracellular Vesicles Are Biological Tools for the Delivery of Active Molecules to Bone. J. Bone Miner. Res. 2018, 33, 517–533. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Luan, J.; Li, H.; Zhou, X.; Han, J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016, 590, 185–192. [Google Scholar] [CrossRef]
- Ikebuchi, Y.; Aoki, S.; Honma, M.; Hayashi, M.; Sugamori, Y.; Khan, M.; Kariya, Y.; Kato, G.; Tabata, Y.; Penninger, J.M.; et al. Coupling of bone resorption and formation by RANKL reverse signalling. Nature 2018, 561, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Lopatina, T.; Bruno, S.; Tetta, C.; Kalinina, N.; Porta, M.; Camussi, G. Platelet-derived growth factor regulates the secretion of extracellular vesicles by adipose mesenchymal stem cells and enhances their angiogenic potential. Cell Commun. Signal 2014, 12, 26. [Google Scholar] [CrossRef]
- Bian, S.; Zhang, L.; Duan, L.; Wang, X.; Min, Y.; Yu, H. Extracellular vesicles derived from human bone marrow mesenchymal stem cells promote angiogenesis in a rat myocardial infarction model. J. Mol. Med. 2014, 92, 387–397. [Google Scholar] [CrossRef]
- Zhang, H.-C.; Liu, X.-B.; Huang, S.; Bi, X.-Y.; Wang, H.-X.; Xie, L.-X.; Wang, Y.-Q.; Cao, X.-F.; Lv, J.; Xiao, F.-J.; et al. Microvesicles derived from human umbilical cord mesenchymal stem cells stimulated by hypoxia promote angiogenesis both in vitro and in vivo. Stem Cells Dev. 2012, 21, 3289–3297. [Google Scholar] [CrossRef]
- Zhang, Y.; Hao, Z.; Wang, P.; Xia, Y.; Wu, J.; Xia, D.; Fang, S.; Xu, S. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF-1α-mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Prolif. 2019, 52, e12570. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, X.; Chen, X.; Wei, Y.; Du, W.; Wang, Y.; Liu, L.; Zhao, W.; Han, Z.; Kong, D.; et al. Enhanced Therapeutic Effects of Mesenchymal Stem Cell-Derived Exosomes with an Injectable Hydrogel for Hindlimb Ischemia Treatment. ACS Appl. Mater. Interfaces 2018, 10, 30081–30091. [Google Scholar] [CrossRef]
- Gonzalez-King, H.; García, N.A.; Ontoria-Oviedo, I.; Ciria, M.; Montero, J.A.; Sepúlveda, P. Hypoxia Inducible Factor-1α Potentiates Jagged 1-Mediated Angiogenesis by Mesenchymal Stem Cell-Derived Exosomes. Stem Cells 2017, 35, 1747–1759. [Google Scholar] [CrossRef]
- Xie, H.; Wang, Z.; Zhang, L.; Lei, Q.; Zhao, A.; Wang, H.; Li, Q.; Cao, Y.; Jie Zhang, W.; Chen, Z. Extracellular Vesicle-functionalized Decalcified Bone Matrix Scaffolds with Enhanced Pro-angiogenic and Pro-bone Regeneration Activities. Sci. Rep. 2017, 7, 45622. [Google Scholar] [CrossRef]
- Nakajima, A.; Seroogy, C.M.; Sandora, M.R.; Tarner, I.H.; Costa, G.L.; Taylor-Edwards, C.; Bachmann, M.H.; Contag, C.H.; Fathman, C.G. Antigen-specific T cell-mediated gene therapy in collagen-induced arthritis. J. Clin. Investig. 2001, 107, 1293–1301. [Google Scholar] [CrossRef]
- Patel, Z.S.; Young, S.; Tabata, Y.; Jansen, J.A.; Wong, M.E.K.; Mikos, A.G. Dual delivery of an angiogenic and an osteogenic growth factor for bone regeneration in a critical size defect model. Bone 2008, 43, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Park, H.-K.; Auh, Q.-S.; Nah, H.; Lee, J.S.; Moon, H.-J.; Heo, D.N.; Kim, I.S.; Kwon, I.K. Emerging Potential of Exosomes in Regenerative Medicine for Temporomandibular Joint Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 1541. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xue, K.; Zhang, X.; Zheng, Z.; Liu, K. Exosomes derived from mature chondrocytes facilitate subcutaneous stable ectopic chondrogenesis of cartilage progenitor cells. Stem Cell Res. Ther. 2018, 9, 318. [Google Scholar] [CrossRef]
- Ferreira, E.; Porter, R.M. Harnessing extracellular vesicles to direct endochondral repair of large bone defects. Bone Joint Res. 2018, 7, 263–273. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, Y.; Hyman, M.E.; Li, J.; Chen, Y. Osteoporosis in china. Osteoporos. Int. 2009, 20, 1651–1662. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy, March 7-29, 2000: Highlights of the conference. South. Med. J. 2001, 94, 569–573.
- Kassel, K.M.; Owens, A.P.; Rockwell, C.E.; Sullivan, B.P.; Wang, R.; Tawfik, O.; Li, G.; Guo, G.L.; Mackman, N.; Luyendyk, J.P. Protease-activated receptor 1 and hematopoietic cell tissue factor are required for hepatic steatosis in mice fed a Western diet. Am. J. Pathol. 2011, 179, 2278–2289. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Clinical Practice. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- He, X.-Y.; Yu, H.-M.; Lin, S.; Li, Y.-Z. Advances in the application of mesenchymal stem cells, exosomes, biomimetic materials, and 3D printing in osteoporosis treatment. Cell Mol. Biol. Lett. 2021, 26, 47. [Google Scholar] [CrossRef]
- Yang, X.; Yang, J.; Lei, P.; Wen, T. LncRNA MALAT1 shuttled by bone marrow-derived mesenchymal stem cells-secreted exosomes alleviates osteoporosis through mediating microRNA-34c/SATB2 axis. Aging 2019, 11, 8777–8791. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Q.; Su, H.; Cheng, J. Exosomes from adipose derived mesenchymal stem cells alleviate diabetic osteoporosis in rats through suppressing NLRP3 inflammasome activation in osteoclasts. J. Biosci. Bioeng. 2021, 131, 671–678. [Google Scholar] [CrossRef]
- Yu, L.; Hu, M.; Cui, X.; Bao, D.; Luo, Z.; Li, D.; Li, L.; Liu, N.; Wu, Y.; Luo, X.; et al. M1 macrophage-derived exosomes aggravate bone loss in postmenopausal osteoporosis via a microRNA-98/DUSP1/JNK axis. Cell Biol. Int. 2021, 45, 2452–2463. [Google Scholar] [CrossRef]
- Yang, R.-Z.; Xu, W.-N.; Zheng, H.-L.; Zheng, X.-F.; Li, B.; Jiang, L.-S.; Jiang, S.-D. Exosomes derived from vascular endothelial cells antagonize glucocorticoid-induced osteoporosis by inhibiting ferritinophagy with resultant limited ferroptosis of osteoblasts. J. Cell Physiol. 2021, 236, 6691–6705. [Google Scholar] [CrossRef]
- Fang, F.; Yang, J.; Wang, J.; Li, T.; Wang, E.; Zhang, D.; Liu, X.; Zhou, C. The role and applications of extracellular vesicles in osteoporosis. Bone Res. 2024, 12, 4. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Risk Factor Collaborators Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.-P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Lafeber, F.P.J.G.; van Laar, J.M. Strontium ranelate: Ready for clinical use as disease-modifying osteoarthritis drug? Ann. Rheum. Dis. 2013, 72, 157–161. [Google Scholar] [CrossRef]
- Bultink, I.E.M.; Lems, W.F. Osteoarthritis and osteoporosis: What is the overlap? Curr. Rheumatol. Rep. 2013, 15, 328. [Google Scholar] [CrossRef]
- Cosenza, S.; Ruiz, M.; Toupet, K.; Jorgensen, C.; Noël, D. Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci. Rep. 2017, 7, 16214. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, Y.; Qiu, P.; Xia, C.; Fang, Y.; Mei, S.; Fang, C.; Shi, Y.; Wu, K.; Chen, Z.; et al. Primary chondrocyte exosomes mediate osteoarthritis progression by regulating mitochondrion and immune reactivity. Nanomedicine 2019, 14, 3193–3212. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Wu, Q.; Zhang, Y.; You, Y.; Jiang, W.; Dai, K. Chondrogenic primed extracellular vesicles activate miR-455/SOX11/FOXO axis for cartilage regeneration and osteoarthritis treatment. NPJ Regen. Med. 2022, 7, 53. [Google Scholar] [CrossRef]
- Zhang, S.; Teo, K.Y.W.; Chuah, S.J.; Lai, R.C.; Lim, S.K.; Toh, W.S. MSC exosomes alleviate temporomandibular joint osteoarthritis by attenuating inflammation and restoring matrix homeostasis. Biomaterials 2019, 200, 35–47. [Google Scholar] [CrossRef]
- Liu, Z.; Zhuang, Y.; Fang, L.; Yuan, C.; Wang, X.; Lin, K. Breakthrough of extracellular vesicles in pathogenesis, diagnosis and treatment of osteoarthritis. Bioact. Mater. 2023, 22, 423–452. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Robbins, P.D. Immunosuppressive exosomes: A new approach for treating arthritis. Int. J. Rheumatol. 2012, 2012, 573528. [Google Scholar] [CrossRef]
- Hu, H.; Wang, D.; Li, L.; Yin, H.; He, G.; Zhang, Y. Role of microRNA-335 carried by bone marrow mesenchymal stem cells-derived extracellular vesicles in bone fracture recovery. Cell Death Dis. 2021, 12, 156. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Cui, R.; Chen, X.; Hu, H.; Qiu, Y. Bone marrow mesenchymal stem cells derived exosomal Lnc TUG1 promotes bone fracture recovery via miR-22-5p/Anxa8 axis. Hum. Cell 2023, 36, 1041–1053. [Google Scholar] [CrossRef]
- Zhang, L.; Jiao, G.; Ren, S.; Zhang, X.; Li, C.; Wu, W.; Wang, H.; Liu, H.; Zhou, H.; Chen, Y. Exosomes from bone marrow mesenchymal stem cells enhance fracture healing through the promotion of osteogenesis and angiogenesis in a rat model of nonunion. Stem Cell Res. Ther. 2020, 11, 38. [Google Scholar] [CrossRef]
- Liu, W.; Li, L.; Rong, Y.; Qian, D.; Chen, J.; Zhou, Z.; Luo, Y.; Jiang, D.; Cheng, L.; Zhao, S.; et al. Hypoxic mesenchymal stem cell-derived exosomes promote bone fracture healing by the transfer of miR-126. Acta Biomater. 2020, 103, 196–212. [Google Scholar] [CrossRef]
- Huber, J.; Longaker, M.T.; Quarto, N. Circulating and extracellular vesicle-derived microRNAs as biomarkers in bone-related diseases. Front. Endocrinol. 2023, 14, 1168898. [Google Scholar] [CrossRef]
- Sun, W.; Zhao, C.; Li, Y.; Wang, L.; Nie, G.; Peng, J.; Wang, A.; Zhang, P.; Tian, W.; Li, Q.; et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016, 2, 16015. [Google Scholar] [CrossRef]
- Xiong, Y.; Cao, F.; Chen, L.; Yan, C.; Zhou, W.; Chen, Y.; Endo, Y.; Leng, X.; Mi, B.; Liu, G. Identification of key microRNAs and target genes for the diagnosis of bone nonunion. Mol. Med. Rep. 2020, 21, 1921–1933. [Google Scholar] [CrossRef]
- Zhao, Y.; Xu, J. Synovial fluid-derived exosomal lncRNA PCGEM1 as biomarker for the different stages of osteoarthritis. Int. Orthop. 2018, 42, 2865–2872. [Google Scholar] [CrossRef]
- Ali, S.A.; Espin-Garcia, O.; Wong, A.K.; Potla, P.; Pastrello, C.; McIntyre, M.; Lively, S.; Jurisica, I.; Gandhi, R.; Kapoor, M. Circulating microRNAs differentiate fast-progressing from slow-progressing and non-progressing knee osteoarthritis in the Osteoarthritis Initiative cohort. Ther. Adv. Musculoskelet. Dis. 2022, 14, 1759720X221082917. [Google Scholar] [CrossRef]
- Urdinez, J.; Boro, A.; Mazumdar, A.; Arlt, M.J.; Muff, R.; Botter, S.M.; Bode-Lesniewska, B.; Fuchs, B.; Snedeker, J.G.; Gvozdenovic, A. The miR-143/145 Cluster, a Novel Diagnostic Biomarker in Chondrosarcoma, Acts as a Tumor Suppressor and Directly Inhibits Fascin-1. J. Bone Miner. Res. 2020, 35, 1077–1091. [Google Scholar] [CrossRef]
- Raimondo, S.; Urzì, O.; Conigliaro, A.; Bosco, G.L.; Parisi, S.; Carlisi, M.; Siragusa, S.; Raimondi, L.; Luca, A.D.; Giavaresi, G.; et al. Extracellular Vesicle microRNAs Contribute to the Osteogenic Inhibition of Mesenchymal Stem Cells in Multiple Myeloma. Cancers 2020, 12, 449. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Wang, H.; Guo, W.; He, E.; Huang, K.; Zhao, Q. Osteosarcoma-derived exosomal miR-501-3p promotes osteoclastogenesis and aggravates bone loss. Cell Signal 2021, 82, 109935. [Google Scholar] [CrossRef]
- Man, K.; Brunet, M.Y.; Jones, M.-C.; Cox, S.C. Engineered Extracellular Vesicles: Tailored-Made Nanomaterials for Medical Applications. Nanomaterials 2020, 10, 1838. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Huang, Y. Bioinspired exosome-like therapeutics and delivery nanoplatforms. Biomaterials 2020, 242, 119925. [Google Scholar] [CrossRef]
- Pizzicannella, J.; Gugliandolo, A.; Orsini, T.; Fontana, A.; Ventrella, A.; Mazzon, E.; Bramanti, P.; Diomede, F.; Trubiani, O. Engineered Extracellular Vesicles From Human Periodontal-Ligament Stem Cells Increase VEGF/VEGFR2 Expression During Bone Regeneration. Front. Physiol. 2019, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Pizzicannella, J.; Diomede, F.; Gugliandolo, A.; Chiricosta, L.; Bramanti, P.; Merciaro, I.; Orsini, T.; Mazzon, E.; Trubiani, O. 3D Printing PLA/Gingival Stem Cells/ EVs Upregulate miR-2861 and -210 during Osteoangiogenesis Commitment. Int. J. Mol. Sci. 2019, 20, 3256. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Guo, S.; Shi, W.; Liu, Q.; Huo, F.; Wu, Y.; Tian, W. Bone Marrow Mesenchymal Stem Cell-Derived Small Extracellular Vesicles Promote Periodontal Regeneration. Tissue Eng. Part. A 2021, 27, 962–976. [Google Scholar] [CrossRef]
- Song, H.; Li, X.; Zhao, Z.; Qian, J.; Wang, Y.; Cui, J.; Weng, W.; Cao, L.; Chen, X.; Hu, Y.; et al. Reversal of Osteoporotic Activity by Endothelial Cell-Secreted Bone Targeting and Biocompatible Exosomes. Nano Lett. 2019, 19, 3040–3048. [Google Scholar] [CrossRef]
- Hao, H.; Liu, Q.; Zheng, T.; Li, J.; Zhang, T.; Yao, Y.; Liu, Y.; Lin, K.; Liu, T.; Gong, P.; et al. Oral Milk-Derived Extracellular Vesicles Inhibit Osteoclastogenesis and Ameliorate Bone Loss in Ovariectomized Mice by Improving Gut Microbiota. J. Agric. Food Chem. 2024, 72, 4726–4736. [Google Scholar] [CrossRef]
- Jin, T.; Lu, Y.; He, Q.X.; Wang, H.; Li, B.F.; Zhu, L.Y.; Xu, Q.Y. The Role of MicroRNA, miR-24, and Its Target CHI3L1 in Osteomyelitis Caused by Staphylococcus aureus. J. Cell Biochem. 2015, 116, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef] [PubMed]
- Smyth, T.; Kullberg, M.; Malik, N.; Smith-Jones, P.; Graner, M.W.; Anchordoquy, T.J. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control Release 2015, 199, 145–155. [Google Scholar] [CrossRef]
- Lu, Y.; Mai, Z.; Cui, L.; Zhao, X. Engineering exosomes and biomaterial-assisted exosomes as therapeutic carriers for bone regeneration. Stem Cell Res. Ther. 2023, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Zhang, J.; Yuan, H.; Xu, Z.; Li, Q.; Niu, X.; Hu, B.; Wang, Y.; Li, X. Exosomes Secreted by Human-Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Repair Critical-Sized Bone Defects through Enhanced Angiogenesis and Osteogenesis in Osteoporotic Rats. Int. J. Biol. Sci. 2016, 12, 836–849. [Google Scholar] [CrossRef]
- Wei, F.; Li, M.; Crawford, R.; Zhou, Y.; Xiao, Y. Exosome-integrated titanium oxide nanotubes for targeted bone regeneration. Acta Biomater. 2019, 86, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Gu, R.; Li, W.; Zeng, L.; Zhu, Y.; Heng, B.C.; Liu, Y.; Zhou, Y. Engineering 3D-Printed Strontium-Titanium Scaffold-Integrated Highly Bioactive Serum Exosomes for Critical Bone Defects by Osteogenesis and Angiogenesis. ACS Appl. Mater. Interfaces 2023, 15, 27486–27501. [Google Scholar] [CrossRef]
- Feng, K.; Xie, X.; Yuan, J.; Gong, L.; Zhu, Z.; Zhang, J.; Li, H.; Yang, Y.; Wang, Y. Reversing the surface charge of MSC-derived small extracellular vesicles by εPL-PEG-DSPE for enhanced osteoarthritis treatment. J. Extracell. Vesicles 2021, 10, e12160. [Google Scholar] [CrossRef]
- Hoshino, A.; Costa-Silva, B.; Shen, T.-L.; Rodrigues, G.; Hashimoto, A.; Tesic Mark, M.; Molina, H.; Kohsaka, S.; Di Giannatale, A.; Ceder, S.; et al. Tumour exosome integrins determine organotropic metastasis. Nature 2015, 527, 329–335. [Google Scholar] [CrossRef]

| Bone Disease | Role and Specific Effects of EVs | Mechanisms and Pathways | Ref. |
|---|---|---|---|
| Osteoporosis | EVs from MSCs enhance osteoblast activity in osteoporotic mice. | miR-34c/SATB2 axis in BMSC-derived exosomes | [65] |
| Adipose MSC-derived exosomes attenuate bone loss by inhibiting osteoclast activity. | Inhibition of NLRP3 inflammasome and reduction of IL-1β and IL-18 secretion | [66] | |
| M1 macrophage-derived EVs exacerbate bone loss by altering signaling pathways. | Downregulation of DUSP1 and activation of JNK signaling in osteoclasts | [67] | |
| Vascular endothelial cell-derived exosomes reverse glucocorticoid-induced osteoporosis. | Inhibition of ferritin autophagy-dependent iron concentration | [68] | |
| Osteoarthritis | BMSC-derived exosomes prevent apoptosis and enhance survival of chondrocytes in osteoarthritic environments. | Direct effect on chondrocyte viability, | [74] |
| Exosomes from normal primary chondrocytes restore mitochondrial function and influence macrophage behavior. | Polarization of macrophages to M2 phenotype, enhancing repair processes, | [75] | |
| TGFβ3-pretreated MSC-derived EVs promote remission of OA and regenerate cartilage by modulating cellular pathways. | Activation of SOX11/FOXO signaling pathway, | [76] | |
| Stem cell-derived EVs modulate immune responses, reducing inflammatory burden in osteoarthritic joints. | Immunomodulatory effects, suppression of pro-inflammatory cytokines | [77,78] |
| NCT Number | Title | Status | Conditions | Interventions | Characteristics |
|---|---|---|---|---|---|
| NCT06368154 | Exosome microRNAs as Potential Biomarkers of Metabolic Bone Disease of Prematurity | Recruiting |
| Not applicable | Phase: not applicable |
| NCT05520125 | Treatment of Patients with Bone Tissue Defects Using Mesenchymal Stem Cells Enriched by Extracellular Vesicles | Not yet recruiting |
|
| Phase:
|
| NCT04998058 | Autogenous Mesenchymal Stem Cell Culture-Derived Signalling Molecules as Enhancers of Bone Formation in Bone Grafting | Not yet recruiting |
|
| Phase:
|
| NCT04849429 | Intra-discal Injection of Platelet-rich Plasma (PRP) Enriched with Exosomes in Chronic Low Back Pain | Completed |
|
| Phase: Phase 1 |
| NCT04281901 | Efficacy of Platelet- and Extracellular Vesicle-rich Plasma in Chronic Postsurgical Temporal Bone Inflammations | Completed |
|
| Phase: not applicable |
| NCT03895216 | Identification and Characterization of Predictive Factors of Onset of Bone Metastases in Cancer Patients | Completed |
| Not applicable | Phase: not applicable |
| NCT03108677 | Circulating Exosome RNA in Lung Metastases of Primary High-Grade Osteosarcoma | Active, not recruiting |
|
| Phase: not applicable |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wan, X.; Zhang, W.; Dai, L.; Chen, L. The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases. Curr. Issues Mol. Biol. 2024, 46, 9269-9285. https://doi.org/10.3390/cimb46090548
Wan X, Zhang W, Dai L, Chen L. The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases. Current Issues in Molecular Biology. 2024; 46(9):9269-9285. https://doi.org/10.3390/cimb46090548
Chicago/Turabian StyleWan, Xinyue, Wenjie Zhang, Lingyan Dai, and Liang Chen. 2024. "The Role of Extracellular Vesicles in Bone Regeneration and Associated Bone Diseases" Current Issues in Molecular Biology 46, no. 9: 9269-9285. https://doi.org/10.3390/cimb46090548





