Genomic Exploration of a Chitinolytic Streptomyces albogriseolus PMB5 Strain from European mantis (Mantis religiosa)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain Source and Sampling
2.2. Preparation of Colloidal Chitin
2.3. Screening of Chitinase-Producing Bacteria
2.4. Secondary Screening for Chitin-Degrading Actinobacteria Strains
2.5. DNA Extraction, Sequencing Library Generation, Sequencing, and Assembly
2.6. Genome-Based Strain Identification
2.7. Genome Annotation
2.8. Data Availability
3. Results and Discussion
3.1. Presumptive Identification of the Chitinase-Producing Bacteria
3.2. In Silico Genomic Landscape
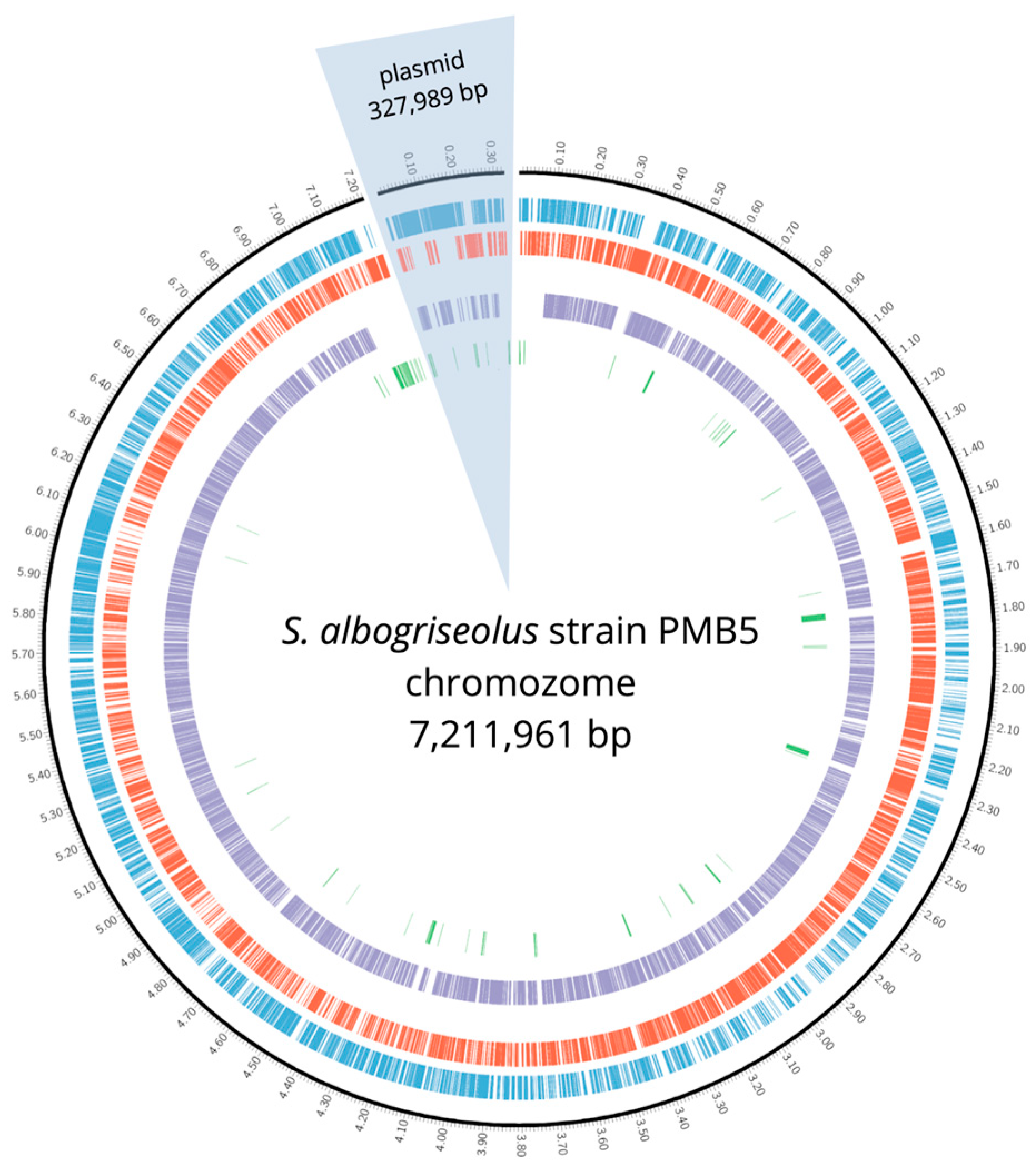
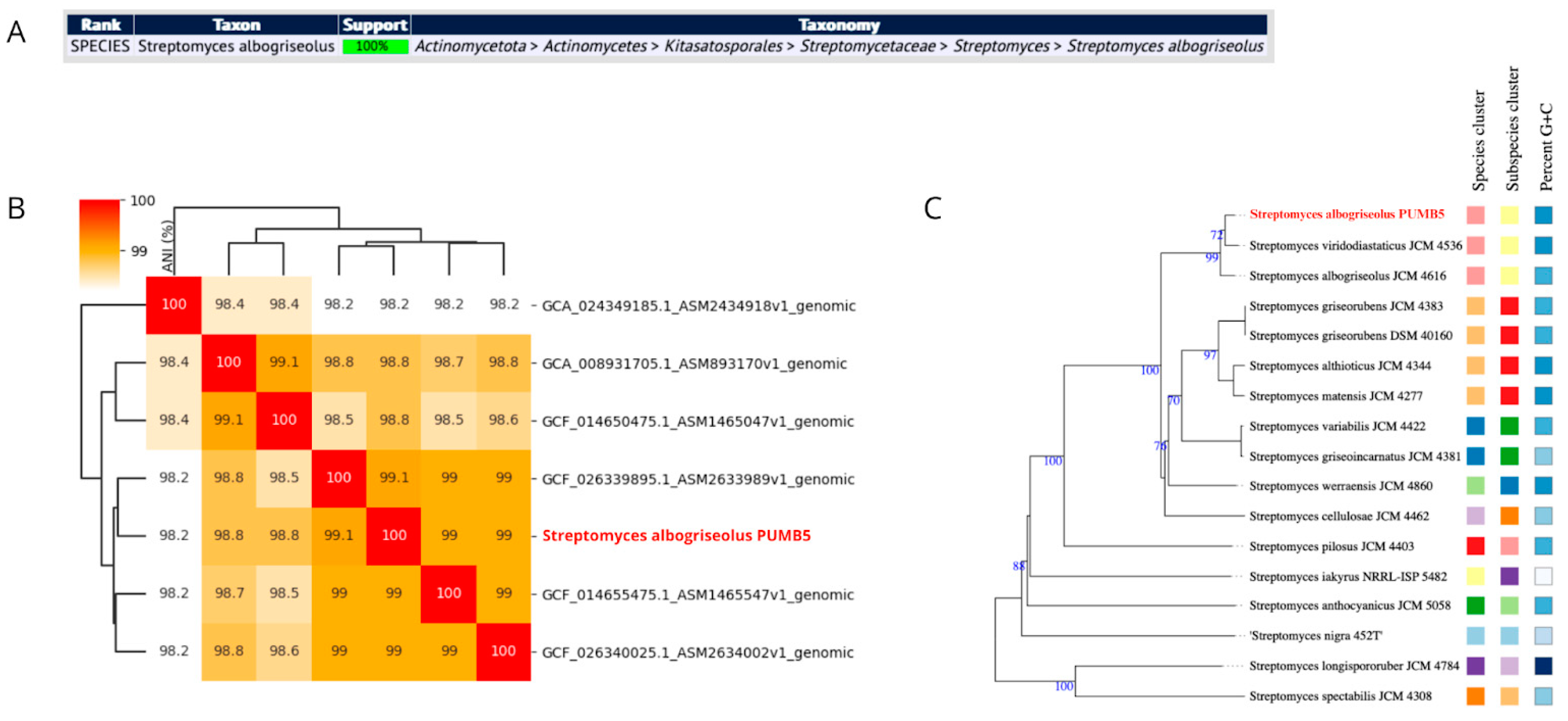
3.2.1. Genome Overview and Species Identification
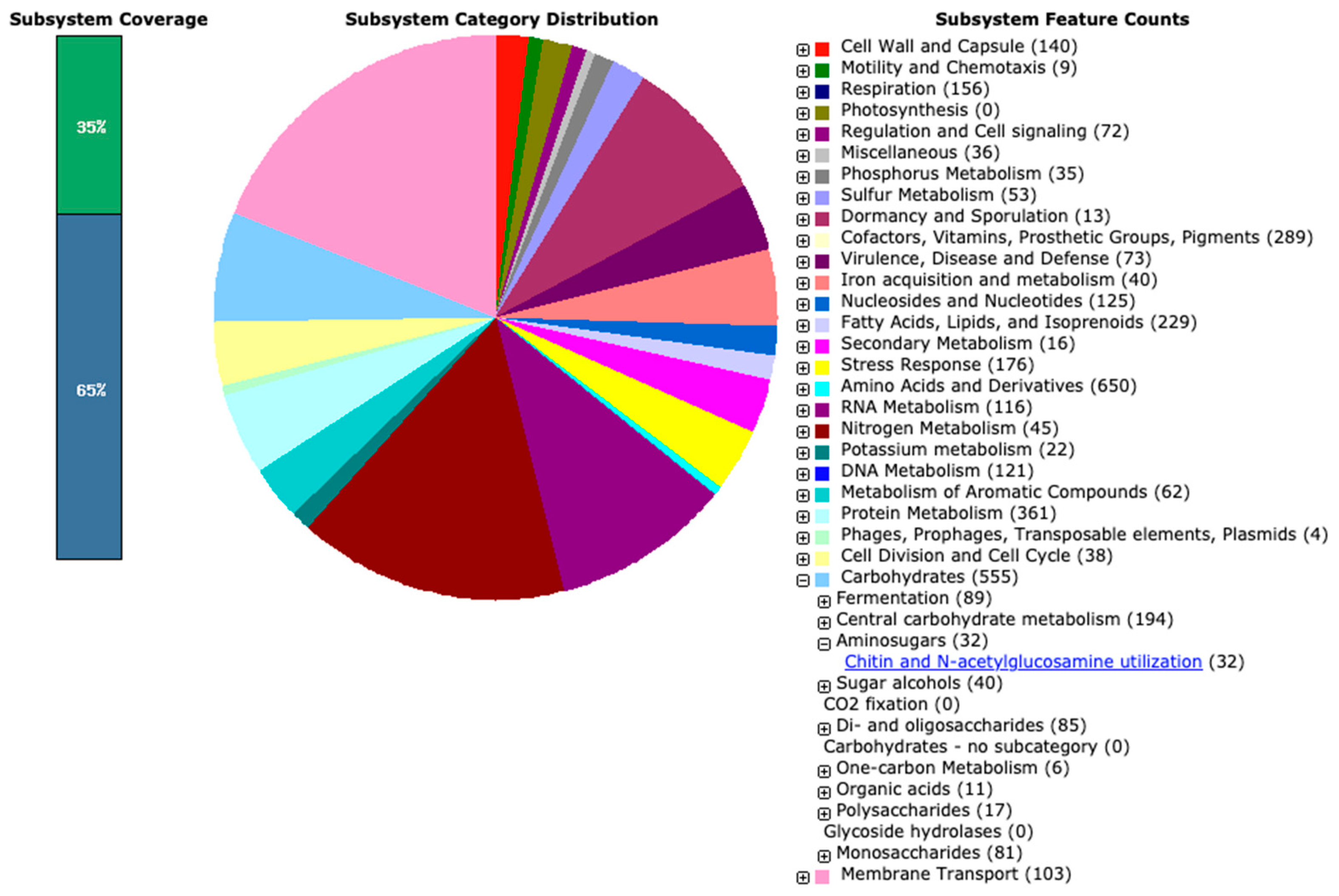
3.2.2. Genome Annotation
| Location | Type | From | To | Most Similar Known Cluster | Class | Similarity |
|---|---|---|---|---|---|---|
| chromosome | lassopeptide, T1PKS, NRPS | 468 | 70,358 | antimycin [60] | NRP + Polyketide | 100% |
| NRPS, RiPP-like | 323,156 | 401,724 | informatipeptin [64] | RiPP:Lanthipeptide | 57% | |
| terpene | 759,678 | 786,375 | hopene [65] | Terpene | 92% | |
| lassopeptide | 1,136,682 | 1,159,270 | citrulassin D | RiPP | 100% | |
| NI-siderophore | 1,206,990 | 1,238,161 | paulomycin [57,66] | Other | 13% | |
| terpene | 1,367,859 | 1,390,015 | geosmin [67] | Terpene | 100% | |
| RiPP-like | 1,413,136 | 1,424,449 | ||||
| LAP | 1,637,023 | 1,659,540 | streptamidine [68] | RiPP:Other | 100% | |
| terpene | 2,409,081 | 2,430,166 | albaflavenone [69] | Terpene | 100% | |
| RRE-containing | 2,554,459 | 2,575,655 | naphthomycin A [58] | Polyketide | 9% | |
| lassopeptide | 3,284,036 | 3,306,529 | aborycin [70] | RiPP | 100% | |
| NI-siderophore | 4,613,401 | 4,643,173 | desferrioxamin B/desferrioxamine E [71] | Other | 100% | |
| ectoine | 5,636,782 | 5,647,180 | ectoine | Other | 100% | |
| T2PKS | 6,450,985 | 6,523,494 | spore pigment [72] | Polyketide | 83% | |
| terpene | 6,710,643 | 6,734,757 | carotenoid [73] | Terpene | 54% | |
| T3PKS | 6,948,679 | 6,989,752 | alkylresorcinol [74] | Polyketide | 100% | |
| lanthipeptide-class-i | 7,026,827 | 7,051,355 | ||||
| lanthipeptide-class-i | 7,129,548 | 7,153,689 | ||||
| plasmid | transAT-PKS, NRPS, PKS-like, ectoine, T2PKS, hglE-KS, lanthipeptide-class-i, lanthipeptide-class-ii | 26,123 | 301,355 | kosinostatin [63] | NRP + Polyketide | 62% |
| lassopeptide | 304,861 | 327,519 | lagmysin | RiPP | 80% |
3.2.3. The Repertoire of CAZymes and Genes for Chitin Utilization in the PMB5 Genome
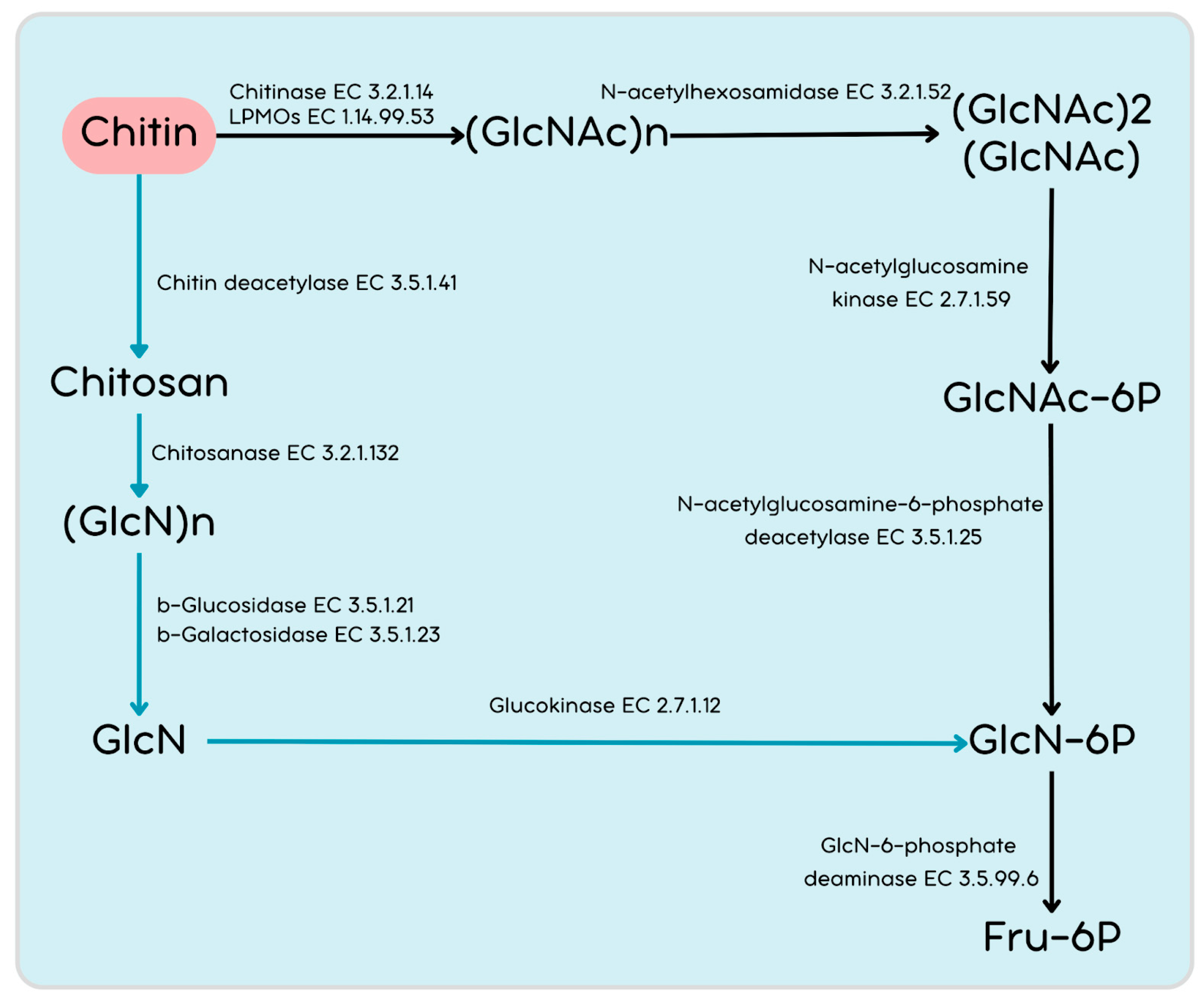
3.2.4. Genetic Repertoire of Chitin Breakdown and Utilization Genes in PMB5
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Fernando, L.D.; Dickwella Widanage, M.C.; Penfield, J.; Lipton, A.S.; Washton, N.; Latgé, J.P.; Wang, P.; Zhang, L.; Wang, T. Structural Polymorphism of Chitin and Chitosan in Fungal Cell Walls From Solid-State NMR and Principal Component Analysis. Front. Mol. Biosci. 2021, 8, 727053. [Google Scholar] [CrossRef]
- Le, B.; Yang, S.H. Microbial Chitinases: Properties, Current State and Biotechnological Applications. World J. Microbiol. Biotechnol. 2019, 35, 144. [Google Scholar] [CrossRef]
- He, X.; Yu, M.; Wu, Y.; Ran, L.; Liu, W.; Zhang, X.H. Two Highly Similar Chitinases from Marine Vibrio Species Have Different Enzymatic Properties. Mar. Drugs 2020, 18, 139. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Poria, V.; Rana, A.; Kumari, A.; Grewal, J.; Pranaw, K.; Singh, S. Current Perspectives on Chitinolytic Enzymes and Their Agro-Industrial Applications. Biology 2021, 10, 1319. [Google Scholar] [CrossRef]
- Mack, I.; Hector, A.; Ballbach, M.; Kohlhäufl, J.; Fuchs, K.J.; Weber, A.; Mall, M.A.; Hartl, D. The Role of Chitin, Chitinases, and Chitinase-like Proteins in Pediatric Lung Diseases. Mol. Cell. Pediatr. 2015, 2, 3. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Torres-Castillo, J.A.; Olazarán-Santibáñez, F.E. Insects as Source of Phenolic and Antioxidant Entomochemicals in the Food Industry. Front. Nutr. 2023, 10, 1133342. [Google Scholar]
- Nazari, B.; Saito, A.; Kobayashi, M.; Miyashita, K.; Wang, Y.; Fujii, T. High Expression Levels of Chitinase Genes in Streptomyces coelicolor A3(2) Grown in Soil. FEMS Microbiol. Ecol. 2011, 77, 623–635. [Google Scholar] [CrossRef] [PubMed]
- Lacombe-Harvey, M.È.; Brzezinski, R.; Beaulieu, C. Chitinolytic Functions in Actinobacteria: Ecology, Enzymes, and Evolution. Appl. Microbiol. Biotechnol. 2018, 102, 7219–7230. [Google Scholar] [PubMed]
- Mukherjee, G.; Sen, S.K. Purification, Characterization, and Antifungal Activity of Chitinase from Streptomyces venezuelae P10. Curr. Microbiol. 2006, 53, 265–269. [Google Scholar] [CrossRef]
- Joo, G.J. Purification and Characterization of an Extracellular Chitinase from the Antifungal Biocontrol Agent Streptomyces halstedii. Biotechnol. Lett. 2005, 27, 1483–1486. [Google Scholar] [CrossRef]
- Gupta, R.; Saxena, R.K.; Chaturvedi, P.; Virdi, J.S. Chitinase Production by Streptomyces viridificans: Its Potential in Fungal Cell Wall Lysis. J. Appl. Bacteriol. 1995, 78, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Taechowisan, T.; Peberdy, J.F.; Lumyong, S. Chitinase Production by Endophytic Streptomyces aureofaciens CMUAc130 and Its Antagonism against Phytopathogenic Fungi. Ann. Microbiol. 2003, 53, 447–461. [Google Scholar]
- Narayana, K.J.P.; Vijayalakshmi, M. Chitinase Production by Streptomyces sp. ANU 6277. Braz. J. Microbiol. 2009, 40, 725–733. [Google Scholar] [CrossRef]
- Kim, K.J.; Yang, Y.J.; Kim, J.G. Purification and Characterization of Chitinase from Streptomyces sp. M-20. J. Biochem. Mol. Biol. 2003, 36, 185–189. [Google Scholar] [CrossRef]
- Han, Y.; Yang, B.; Zhang, F.; Miao, X.; Li, Z. Characterization of Antifungal Chitinase from Marine Streptomyces sp. DA11 Associated with South China Sea Sponge Craniella Australiensis. Mar. Biotechnol. 2009, 11, 132–140. [Google Scholar] [CrossRef]
- Ueno, H.; Oba, Y.; Miyashita, K.; Sawada, Y. Purification and Some Properties of Extracellular Chitinases from Streptomyces sp. S-84. J. Gen. Appl. Microbiol. 1990, 36, 377–392. [Google Scholar] [CrossRef]
- Xu, T.; Qi, M.; Liu, H.; Cao, D.; Xu, C.; Wang, L.; Qi, B. Chitin Degradation Potential and Whole-Genome Sequence of Streptomyces diastaticus Strain CS1801. AMB Express 2020, 10, 29. [Google Scholar] [CrossRef]
- Ngoc Tung, Q.; Thu An, N.T.; Hanh Nguyen, V.T.; Quyet Tien, P. Genome Mining Reveals Chitin Degradation Potential of Streptomyces parvulus VCCM 22513. Acad. J. Biol. 2023, 45, 27–36. [Google Scholar] [CrossRef]
- Hooper, L.V.; Gordon, J.I. Commensal Host-Bacterial Relationships in the Gut. Science 2001, 292, 1115–1118. [Google Scholar]
- Marchiori, C.H. Praying Mantis (Insecta: Mantodea). In Definitions; Qeios: London, UK, 2024. [Google Scholar] [CrossRef]
- Chevrette, M.G.; Carlson, C.M.; Ortega, H.E.; Thomas, C.; Ananiev, G.E.; Barns, K.J.; Book, A.J.; Cagnazzo, J.; Carlos, C.; Flanigan, W.; et al. The Antimicrobial Potential of Streptomyces from Insect Microbiomes. Nat. Commun. 2019, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.C.; Lockwood, J.L. Powdered Chitin Agar as a Selective Medium for Enumeration of Actinomycetes in Water and Soil. Appl. Microbiol. 1975, 29, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.S.; Park, I.H.; Yoo, J.S.; Chung, S.Y.; Lee, Y.C.; Cho, Y.S.; Ahn, S.C.; Kim, C.M.; Choi, Y.L. Cloning, Purification, and Characterization of Chitinase from Bacillus sp. DAU101. Bioresour. Technol. 2007, 98, 2734–2741. [Google Scholar] [CrossRef]
- Saima; Kuddus, M.; Roohi; Ahmad, I.Z. Isolation of Novel Chitinolytic Bacteria and Production Optimization of Extracellular chitinase. J. Genet. Eng. Biotechnol. 2013, 11, 39–46. [Google Scholar] [CrossRef]
- Aladame, N. Bergey’s Manual of Systematic Bacteriology. Ann. Inst. Pasteur. Microbiol. 1987, 138, 146. [Google Scholar] [CrossRef]
- Antido, J.W.A.; Climacosa, F.M.M. Enhanced Isolation of Streptomyces from Different Soil Habitats in Calamba City, Laguna, Philippines Using a Modified Integrated Approach. Int. J. Microbiol. 2022, 2022, 2598963. [Google Scholar] [CrossRef]
- Parks, D.H.; Imelfort, M.; Skennerton, C.T.; Hugenholtz, P.; Tyson, G.W. CheckM: Assessing the Quality of Microbial Genomes Recovered from Isolates, Single Cells, and Metagenomes. Genome Res. 2015, 25, 1043–1055. [Google Scholar] [CrossRef]
- Grant, J.R.; Enns, E.; Marinier, E.; Mandal, A.; Herman, E.K.; Chen, C.Y.; Graham, M.; Van Domselaar, G.; Stothard, P. Proksee: In-Depth Characterization and Visualization of Bacterial Genomes. Nucleic Acids Res. 2023, 51, W484–W492. [Google Scholar] [CrossRef]
- Krzywinski, M.; Schein, J.; Birol, I.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef]
- Jolley, K.A.; Bray, J.E.; Maiden, M.C.J. Open-Access Bacterial Population Genomics: BIGSdb Software, the PubMLST.Org Website and Their Applications. Wellcome Open Res. 2018, 3, 124. [Google Scholar] [CrossRef]
- Jain, C.; Rodriguez-R, L.M.; Phillippy, A.M.; Konstantinidis, K.T.; Aluru, S. High Throughput ANI Analysis of 90K Prokaryotic Genomes Reveals Clear Species Boundaries. Nat. Commun. 2018, 9, 5114. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Göker, M. TYGS Is an Automated High-Throughput Platform for State-of-the-Art Genome-Based Taxonomy. Nat. Commun. 2019, 10, 2182. [Google Scholar] [CrossRef] [PubMed]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A Modular and Extensible Implementation of the RAST Algorithm for Building Custom Annotation Pipelines and Annotating Batches of Genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Morishima, K. BlastKOALA and GhostKOALA: KEGG Tools for Functional Characterization of Genome and Metagenome Sequences. J. Mol. Biol. 2016, 428, 726–731. [Google Scholar] [CrossRef]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.-L.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic Resistome Surveillance with the Comprehensive Antibiotic Resistance Database. Nucleic Acids Res. 2019, 48, D517–D525. [Google Scholar] [CrossRef]
- Doster, E.; Lakin, S.M.; Dean, C.J.; Wolfe, C.; Young, J.G.; Boucher, C.; Belk, K.E.; Noyes, N.R.; Morley, P.S. MEGARes 2.0: A Database for Classification of Antimicrobial Drug, Biocide and Metal Resistance Determinants in Metagenomic Sequence Data. Nucleic Acids Res. 2020, 48, D561–D569. [Google Scholar] [CrossRef] [PubMed]
- Bonin, N.; Doster, E.; Worley, H.; Pinnell, L.J.; Bravo, J.E.; Ferm, P.; Marini, S.; Prosperi, M.; Noyes, N.; Morley, P.S.; et al. MEGARes and AMR++, v3.0: An Updated Comprehensive Database of Antimicrobial Resistance Determinants and an Improved Software Pipeline for Classification Using High-Throughput Sequencing. Nucleic Acids Res. 2023, 51, D744–D752. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, B.; Zhang, X.; Ge, Q.; Yan, Y.; Akresi, J.; Piyush, V.; Huang, L.; Yin, Y. DbCAN-Seq Update: CAZyme Gene Clusters and Substrates in Microbiomes. Nucleic Acids Res. 2023, 51, D557–D563. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, H.; Wu, P.; Entwistle, S.; Li, X.; Yohe, T.; Yi, H.; Yang, Z.; Yin, Y. DbCAN-Seq: A Database of Carbohydrate-Active Enzyme (CAZyme) Sequence and Annotation. Nucleic Acids Res. 2018, 46, D516–D521. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Augustijn, H.E.; Reitz, Z.L.; Biermann, F.; Alanjary, M.; Fetter, A.; Terlouw, B.R.; Metcalf, W.W.; Helfrich, E.J.N.; et al. AntiSMASH 7.0: New and Improved Predictions for Detection, Regulation, Chemical Structures and Visualisation. Nucleic Acids Res. 2023, 51, W46–W50. [Google Scholar] [CrossRef]
- Prete, R.; Long, S.L.; Joyce, S.A.; Corsetti, A. Genotypic and Phenotypic Characterization of Food-Associated Lactobacillus plantarum Isolates for Potential Probiotic Activities. FEMS Microbiol. Lett. 2020, 367, fnaa076. [Google Scholar] [CrossRef]
- Xu, H.; Liu, W.; Zhang, W.; Yu, J.; Song, Y.; Menhe, B.; Zhang, H.; Sun, Z. Use of Multilocus Sequence Typing to Infer Genetic Diversity and Population Structure of Lactobacillus plantarum Isolates from Different Sources. BMC Microbiol. 2015, 15, 241. [Google Scholar] [CrossRef]
- Shao, H.; Chen, M.; Fei, X.; Zhang, R.; Zhong, Y.; Ni, W.; Tao, X.; He, X.; Zhang, E.; Yong, B.; et al. Complete Genome Sequence and Characterization of a Polyethylene Biodegradation Strain, Streptomyces albogriseolus LBX-2. Microorganisms 2019, 7, 379. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Z.; Yang, T.; Chen, M.; Li, J.; Chen, F.; Yang, J.; Li, W.; Zhang, B.; Zhang, Z.; et al. Comparative Genomics Analysis of Streptomyces Species Reveals Their Adaptation to the Marine Environment and Their Diversity at the Genomic Level. Front. Microbiol. 2016, 7, 998. [Google Scholar] [CrossRef]
- Lerminiaux, N.A.; Cameron, A.D.S. Horizontal Transfer of Antibiotic Resistance Genes in Clinical Environments. Can. J. Microbiol. 2019, 65, 34–44. [Google Scholar] [CrossRef]
- McDonald, B.R.; Curriea, C.R. Lateral Gene Transfer Dynamics in the Ancient Bacterial Genus Streptomyces. mBio 2017, 8, e00644-17. [Google Scholar] [CrossRef] [PubMed]
- Ogawara, H. Self-Resistance in Streptomyces, with Special Reference to β-Lactam Antibiotics. Molecules 2016, 21, 605. [Google Scholar] [CrossRef] [PubMed]
- Kotrbová, L.; Lara, A.C.; Corretto, E.; Scharfen, J.; Ulmann, V.; Petříčková, K.; Chroňáková, A. Evaluation and Comparison of Antibiotic Susceptibility Profiles of Streptomyces Spp. from Clinical Specimens Revealed Common and Region-Dependent Resistance Patterns. Sci. Rep. 2022, 12, 9353. [Google Scholar] [CrossRef]
- Schlatter, D.C.; Kinkel, L.L. Global Biogeography of Streptomyces Antibiotic Inhibition, Resistance, and Resource Use. FEMS Microbiol. Ecol. 2014, 88, 386–397. [Google Scholar] [CrossRef]
- Kinashi, H. Giant Linear Plasmids in Streptomyces: A Treasure Trove of Antibiotic Biosynthetic Clusters. J. Antibiot. 2011, 64, 19–25. [Google Scholar]
- Liu, G.; Chater, K.F.; Chandra, G.; Niu, G.; Tan, H. Molecular Regulation of Antibiotic Biosynthesis in Streptomyces. Microbiol. Mol. Biol. Rev. 2013, 77, 112–143. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhao, Y.; Huang, C.; Luo, Y. Recent Advances in Silent Gene Cluster Activation in Streptomyces. Front. Bioeng. Biotechnol. 2021, 9, 632230. [Google Scholar]
- Iftime, D.; Kulik, A.; Härtner, T.; Rohrer, S.; Niedermeyer, T.H.J.; Stegmann, E.; Weber, T.; Wohlleben, W. Identification and Activation of Novel Biosynthetic Gene Clusters by Genome Mining in the Kirromycin Producer Streptomyces collinus Tü 365. J. Ind. Microbiol. Biotechnol. 2016, 43, 277–291. [Google Scholar] [CrossRef]
- Tietz, J.; Mitchell, A. Using Genomics for Natural Product Structure Elucidation. Curr. Top. Med. Chem. 2015, 16, 1645–1694. [Google Scholar] [CrossRef]
- Majer, J.; Chater, K.F. Streptomyces Albus G Produces an Antibiotic Complex Identical to Paulomycins A and B. J. Gen. Microbiol. 1987, 133, 2503–2507. [Google Scholar] [CrossRef]
- Kaewkla, O.; Perkins, M.; Thamchaipenet, A.; Saijuntha, W.; Sukpanoa, S.; Suriyachadkun, C.; Chamroensaksri, N.; Chumroenphat, T.; Franco, C.M.M. Description of Streptomyces Naphthomycinicus sp. Nov., an Endophytic Actinobacterium Producing Naphthomycin A and Its Genome Insight for Discovering Bioactive Compounds. Front. Microbiol. 2024, 15, 1353511. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, D.E.; Hong, S.C.; Kim, S.Y.; Kwon, H.C.; Hyun, C.G.; Choi, J. Genome Analysis of Streptomyces nojiriensis Jcm 3382 and Distribution of Gene Clusters for Three Antibiotics and an Azasugar across the Genus Streptomyces. Microorganisms 2021, 9, 1802. [Google Scholar] [CrossRef]
- Joynt, R.; Seipke, R.F. A Phylogenetic and Evolutionary Analysis of Antimycin Biosynthesis. Microbiology 2018, 164, 28–39. [Google Scholar] [CrossRef]
- Lioy, V.S.; Lorenzi, J.N.; Najah, S.; Poinsignon, T.; Leh, H.; Saulnier, C.; Aigle, B.; Lautru, S.; Thibessard, A.; Lespinet, O.; et al. Dynamics of the Compartmentalized Streptomyces Chromosome during Metabolic Differentiation. Nat. Commun. 2021, 12, 5221. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.M.; Thibessard, A.; Lorenzi, J.N.; Benhadj, M.; Hôtel, L.; Gacemi-Kirane, D.; Lespinet, O.; Leblond, P.; Aigle, B. Comparative Genomics among Closely Related Streptomyces Strains Revealed Specialized Metabolite Biosynthetic Gene Cluster Diversity. Antibiotics 2018, 7, 86. [Google Scholar] [CrossRef] [PubMed]
- Rambabu, V.; Suba, S.; Vijayakumar, S. Antimicrobial and Antiproliferative Prospective of Kosinostatin—A Secondary Metabolite Isolated from Streptomyces sp. J. Pharm. Anal. 2015, 5, 378–382. [Google Scholar] [CrossRef]
- Mohimani, H.; Kersten, R.D.; Liu, W.T.; Wang, M.; Purvine, S.O.; Wu, S.; Brewer, H.M.; Pasa-Tolic, L.; Bandeira, N.; Moore, B.S.; et al. Automated Genome Mining of Ribosomal Peptide Natural Products. ACS Chem. Biol. 2014, 9, 1545–1551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Sun, J.; Huang, W.C.; Xue, C.; Mao, X. A Novel Soluble Squalene-Hopene Cyclase and Its Application in Efficient Synthesis of Hopene. Front. Bioeng. Biotechnol. 2020, 8, 426. [Google Scholar] [CrossRef]
- González, A.; Rodríguez, M.; Braña, A.F.; Méndez, C.; Salas, J.A.; Olano, C. New Insights into Paulomycin Biosynthesis Pathway in Streptomyces albus J1074 and Generation of Novel Derivatives by Combinatorial Biosynthesis. Microb. Cell Fact. 2016, 15, 56. [Google Scholar] [CrossRef] [PubMed]
- Cane, D.E.; He, X.; Kobayashi, S.; Omura, S.; Ikeda, H. Geosmin Biosynthesis in Streptomyces avermitilis. Molecular Cloning, Expression, and Mechanistic Study of the Germacradienol/Geosmin Synthase. J. Antibiot. 2006, 59, 471–479. [Google Scholar] [CrossRef]
- Russell, A.H.; Vior, N.M.; Hems, E.S.; Lacret, R.; Truman, A.W. Discovery and Characterisation of an Amidine-Containing Ribosomally-Synthesised Peptide That Is Widely Distributed in Nature. Chem. Sci. 2021, 12, 11769–11778. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Lin, X.; Lei, L.; Lamb, D.C.; Kelly, S.L.; Waterman, M.R.; Cane, D.E. Biosynthesis of the Sesquiterpene Antibiotic Albaflavenone in Streptomyces coelicolor A3(2). J. Biol. Chem. 2008, 283, 8183–8189. [Google Scholar] [CrossRef]
- Potterat, O.; Stephan, H.; Metzger, J.W.; Gnau, V.; Zähner, H.; Jung, G. Aborycin—A Tricyclic 21-Peptide Antibiotic Isolated from Streptomyces griseoflavus. Liebigs Ann. Chem. 1994, 1994, 741–743. [Google Scholar]
- Chávez-Avila, S.; Valencia-Marin, M.F.; Guzmán-Guzmán, P.; Kumar, A.; Babalola, O.O.; del Carmen Orozco-Mosqueda, M.; de los Santos-Villalobos, S.; Santoyo, G. Deciphering the Antifungal and Plant Growth-Stimulating Traits of the Stress-Tolerant Streptomyces achromogenes subsp. Achromogenes Strain UMAF16, a Bacterium Isolated from Soils Affected by Underground Fires. Biocatal. Agric. Biotechnol. 2023, 53, 102859. [Google Scholar] [CrossRef]
- Kelemen, G.H.; Brian, P.; Flärdh, K.; Chamberlin, L.; Chater, K.F.; Buttner, M.J. Developmental Regulation of Transcription of WhiE, a Locus Specifying the Polyketide Spore Pigment in Streptomyces coelicolor A3(2). J. Bacteriol. 1998, 180, 2515–2521. [Google Scholar] [CrossRef]
- Baskar, V.; Kanimozhi, P.; Panneerselvam, K. Characterization of Carotenoids from Selected Strains of Streptomyces sp. Sch. Res. Libr. Ann. Biol. Res. 2010, 1, 194–200. [Google Scholar]
- Funabashi, M.; Funa, N.; Horinouchi, S. Phenolic Lipids Synthesized by Type III Polyketide Synthase Confer Penicillin Resistance on Streptomyces griseus. J. Biol. Chem. 2008, 283, 13983–13991. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, M.; Hesketh, A.; Frangou, N.; Mossialos, D.; Van de Peer, Y.; Oliver, S.G.; Amoutzias, G.D. A Panoramic View of the Genomic Landscape of the Genus Streptomyces. Microb. Genom. 2023, 9, 001028. [Google Scholar] [CrossRef]
- Porst, H. The Novel Streptomyces Olivaceoviridis ABC Transporter Ngc Mediates Uptake of N-Acetylglucosamine and N,N′-Diacetylchitobiose. Mol. Genet. Genom. 2002, 267, 429–439. [Google Scholar] [CrossRef]
- Saito, A.; Shinya, T.; Miyamoto, K.; Yokoyama, T.; Kaku, H.; Minami, E.; Shibuya, N.; Tsujibo, H.; Nagata, Y.; Ando, A.; et al. The DasABC Gene Cluster, Adjacent to DasR, Encodes a Novel ABC Transporter for the Uptake of N,N′-Diacetylchitobiose in Streptomyces coelicolor A3(2). Appl. Environ. Microbiol. 2007, 73, 3000–3008. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xiao, X.; Saito, A.; Schrempf, H. Streptomyces Olivaceoviridis Possesses a Phosphotransferase System That Mediates Specific, Phosphoenolpyruvate-Dependent Uptake of N-Acetylglucosamine. Mol. Genet. Genom. 2002, 268, 344–351. [Google Scholar] [CrossRef]
- Li, J.; Goddard-Borger, E.D.; Raji, O.; Saxena, H.; Solhi, L.; Mathieu, Y.; Master, E.R.; Wakarchuk, W.W.; Brumer, H. Chitin-Active Lytic Polysaccharide Monooxygenases Are Rare in Cellulomonas Species. Appl. Environ. Microbiol. 2022, 88, e0096822. [Google Scholar] [CrossRef]


| CAZy Function Class | CAZy Family (No.) |
|---|---|
| Auxiliary activity | AA0 (1), AA10 (5), AA3 (2), AA4 (1), AA5 (1), AA7 (4) |
| Carbohydrate-binding module | CBM13 (10), CBM16 (2), CBM2 (10), CBM25 (2), CBM3 (1), CBM32 (3), CBM35 (2), CBM4 (1), CBM48 (4), CBM6 (2), CBM61 (1), CBM67 (2), CBM92 (1) |
| Carbohydrate esterases | CE1 (2), CE12 (2), CE14 (3), CE19 (1), CE3 (3), CE4 (11), CE7 (2), CE8 (1), CE9 (1) |
| Glycoside hydrolases | GH0 (2), GH1 (6), GH10 (4), GH11 (3), GH12 (2), GH13 (20), GH146 (1), GH15 (4), GH16 (2), GH17 (3), GH18 (5), GH19 (1), GH2 (5), GH20 (3), GH23 (7), GH25 (3), GH27 (1), GH3 (5), GH30 (1), GH31 (2), GH35 (1), GH36 (1), GH38 (1), GH39 (1), GH4 (2), GH42 (3), GH43 (3), GH46 (3), GH5 (4),GH51 (1),GH52 (1), GH6 (4), GH64 (2), GH65 (3),GH67 (1), GH74 (1), GH75 (1), GH77 (1), GH81 (2), GH84 (1), GH87 (2), GH92 (2), GH95 (1) |
| Glycosyl transferases | GT0 (1), GT1 (3), GT2 (20), GT20 (1), GT28 (2), GT35 (1), GT39 (1), GT4 (15), GT51 (4), GT76 (2), GT81 (1), GT83 (1), GT83 (1), GT84 (1), GT87 (1), GT9 (2) |
| Gene(s) ID in PMB5 Genome | EC | Activity |
|---|---|---|
| AAEO51_08485 AAEO51_09820 AAEO51_11400 AAEO51_27560 AAEO51_20785 | EC: 3.2.1.14 | Chitinase (GH18) |
| AAEO51_29740 | EC: 3.2.1.14 | Chitinase (GH19) |
| AAEO51_02230 AAEO51_02920 AAEO51_05575 AAEO51_06195 AAEO51_20110 AAEO51_27435 AAEO51_12580 AAEO51_12585 AAEO51_21230 | EC: 3.5.1.41/EC: 3.1.1.72 | Chitin deacetylase/Acetyl xylan esterase (CE4, polysaccharide deacetylases) |
| AAEO51_01075 AAEO51_02335 AAEO51_02750 AAEO51_04200 AAEO51_04895 AAEO51_08435 AAEO51_22480 AAEO51_29405 AAEO51_31010 AAEO51_31015 AAEO51_31560 | EC: 3.2.1.23 | β-Galactosidase |
| AAEO51_00800 AAEO51_02890 AAEO51_20790 AAEO51_23445 AAEO51_31395 | EC: 3.2.1.21 | β-Glucosidase |
| AAEO51_24520 AAEO51_29530 | EC: 3.2.1.132 | Chitosanase (GH46/GH75) |
| AAEO51_26300 | EC: 2.7.1.12 | Glucokinase |
| AAEO51_12700 AAEO51_20840 | EC: 2.6.1.16 | GlcN-fructose-6-phosphate aminotransferase (isomerization) |
| AAEO51_12720 | EC: 5.4.2.10 | Phosphoglucosamine mutase |
| AAEO51_00475 AAEO51_10410 | EC: 3.5.99.6 | GlcN-6-phosphate deaminase |
| AAEO51_19315 AAEO51_19735 | EC: 2.3.1.157/EC: 2.7.7.23 | GlcN-1-phosphate N-acetyltransferase |
| AAEO51_17955 | EC 3.5.1.25 | N-acetylglucosamine-6-phosphate deacetylase |
| AAEO51_21430 AAEO51_28730 AAEO51_01145 AAEO51_17960 | EC 2.7.1.59 | N-acetylglucosamine kinase |
| AAEO51_21020 AAEO51_06825 AAEO51_12015 AAEO51_20200 AAEO51_20855 | EC 3.2.1.52 | N-acetylhexosaminidase |
| AAEO51_00940 AAEO51_20645 AAEO51_26015 AAEO51_30375 AAEO51_30765 | EC: 1.14.99.53/1.14.99.54 | Lytic polysaccharide monooxygenases (LPMOs) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baev, V.; Iliev, I.; Apostolova, E.; Gozmanova, M.; Hristova, Y.; Ilieva, Y.; Yahubyan, G.; Gochev, V. Genomic Exploration of a Chitinolytic Streptomyces albogriseolus PMB5 Strain from European mantis (Mantis religiosa). Curr. Issues Mol. Biol. 2024, 46, 9359-9375. https://doi.org/10.3390/cimb46090554
Baev V, Iliev I, Apostolova E, Gozmanova M, Hristova Y, Ilieva Y, Yahubyan G, Gochev V. Genomic Exploration of a Chitinolytic Streptomyces albogriseolus PMB5 Strain from European mantis (Mantis religiosa). Current Issues in Molecular Biology. 2024; 46(9):9359-9375. https://doi.org/10.3390/cimb46090554
Chicago/Turabian StyleBaev, Vesselin, Ivan Iliev, Elena Apostolova, Mariyana Gozmanova, Yana Hristova, Yanitsa Ilieva, Galina Yahubyan, and Velizar Gochev. 2024. "Genomic Exploration of a Chitinolytic Streptomyces albogriseolus PMB5 Strain from European mantis (Mantis religiosa)" Current Issues in Molecular Biology 46, no. 9: 9359-9375. https://doi.org/10.3390/cimb46090554






