Promising Support Coming from Nature: Antioxidant and Anti-Inflammatory Potential of Castanea sativa Wood Distillate on Skin Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Cell Cultures
2.3. Western Blot for Cytotoxicity Analysis
2.4. Alkaline Comet Assay
2.5. Permeability Assay
2.6. Immunofluorescence of Inflammatory and Adhesion Markers on HUVEC
2.7. ROS Measurements
2.8. Scratch Assay
2.9. Statistical Analysis
3. Results
3.1. Characterization of the Safety Profile of WD on Skin Cell Types and the Endothelium
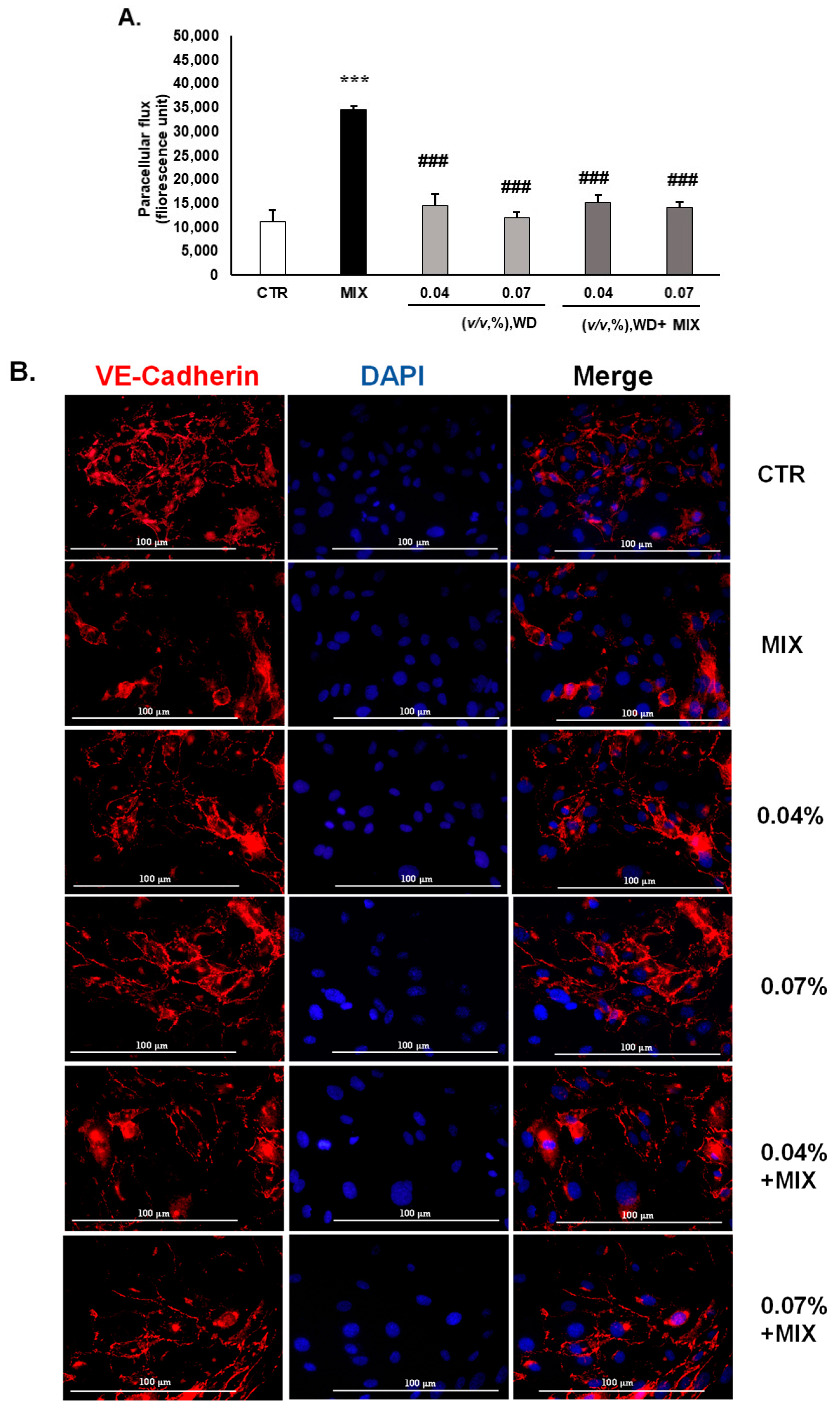
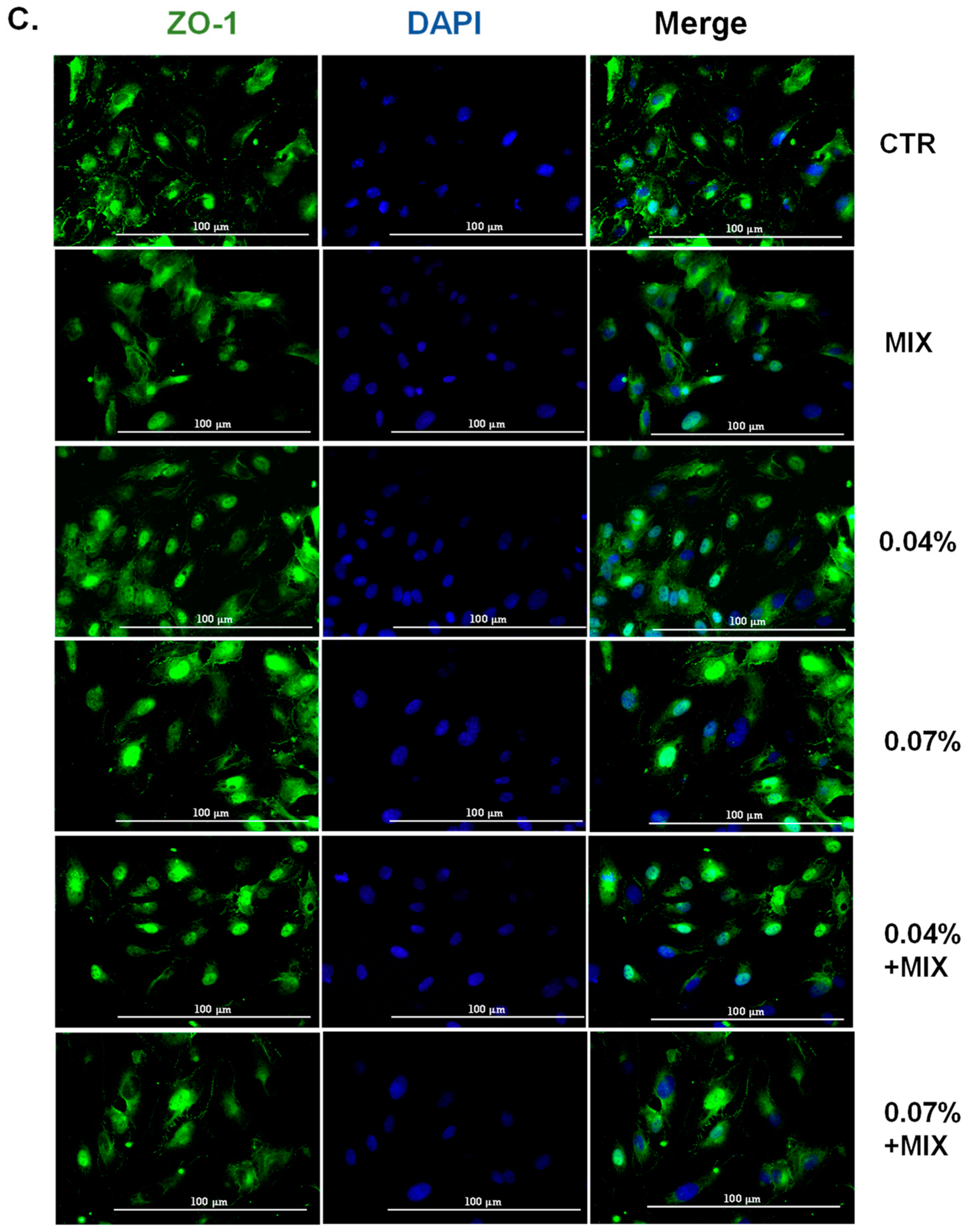
3.2. WD Did Not Affect the Permeability of HUVEC and Decreased the Inflammation-Mediated Permeability, Retaining the Integrity of Tight Junctions
3.3. WD Interfered with the Prostanoid Enzymatic Cascade and Adhesion Molecules in the Endothelium
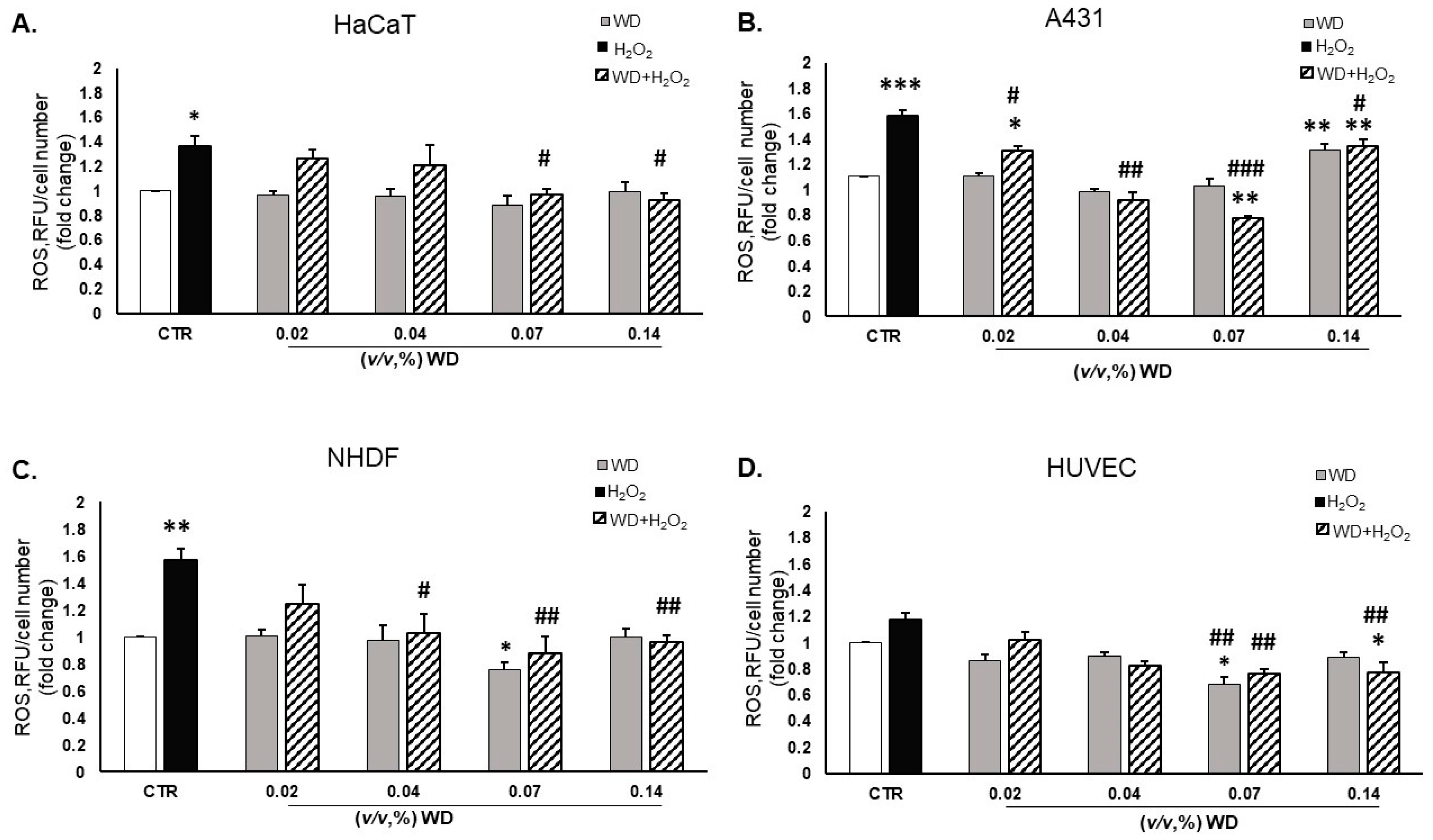
3.4. Oxidative Stress Was Reduced by Exposure to WD
3.5. WD Did Not Affect Cell Migration
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.K.; Chan, F.K.-M. Inflammasome, Inflammation, and Tissue Homeostasis. Trends Mol. Med. 2018, 24, 304–318. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The Role of Antioxidants on Wound Healing: A Review of the Current Evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Ridiandries, A.; Tan, J.T.M.; Bursill, C.A. The Role of Chemokines in Wound Healing. Int. J. Mol. Sci. 2018, 19, 3217. [Google Scholar] [CrossRef]
- Gonçalves, R.V.; Costa, A.M.A.; Grzeskowiak, L. Oxidative Stress and Tissue Repair: Mechanism, Biomarkers, and Therapeutics. Oxid. Med. Cell. Longev. 2021, 2021, 6204096. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Bulfone-Paus, S.; Griffiths, C.E.M.; Watson, R.E.B. Inflammaging and the Skin. J. Investig. Dermatol. 2021, 141, 1087–1095. [Google Scholar] [CrossRef]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef]
- Lee, Y.I.; Choi, S.; Roh, W.S.; Lee, J.H.; Kim, T.-G. Cellular Senescence and Inflammaging in the Skin Microenvironment. Int. J. Mol. Sci. 2021, 22, 3849. [Google Scholar] [CrossRef]
- Tong, Z.; He, W.; Fan, X.; Guo, A. Biological Function of Plant Tannin and Its Application in Animal Health. Front. Vet. Sci. 2022, 8, 803657. [Google Scholar] [CrossRef]
- Gülçin, İ.; Huyut, Z.; Elmastaş, M.; Aboul-Enein, H.Y. Radical Scavenging and Antioxidant Activity of Tannic Acid. Arab. J. Chem. 2010, 3, 43–53. [Google Scholar] [CrossRef]
- Mishra, A.; Sharma, A.K.; Kumar, S.; Saxena, A.K.; Pandey, A.K. Bauhinia Variegata Leaf Extracts Exhibit Considerable Antibacterial, Antioxidant, and Anticancer Activities. BioMed Res. Int. 2013, 2013, e915436. [Google Scholar] [CrossRef] [PubMed]
- Wijesinghe, W.A.J.P.; Ahn, G.; Lee, W.-W.; Kang, M.-C.; Kim, E.-A.; Jeon, Y.-J. Anti-Inflammatory Activity of Phlorotannin-Rich Fermented Ecklonia Cava Processing by-Product Extract in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. J. Appl. Phycol. 2013, 25, 1207–1213. [Google Scholar] [CrossRef]
- Morbidelli, L. Polyphenol-Based Nutraceuticals for the Control of Angiogenesis: Analysis of the Critical Issues for Human Use. Pharmacol. Res. 2016, 111, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Jia, Z.; Anandh Babu, P.V.; Chen, W.; Sun, X. Natural Products Targeting on Oxidative Stress and Inflammation: Mechanisms, Therapies, and Safety Assessment. Oxid. Med. Cell. Longev. 2018, 2018, e6576093. [Google Scholar] [CrossRef]
- Tabolacci, C.; Forni, C.; Jadeja, R.N.; Facchiano, F. Natural Compounds against Cancer, Inflammation, and Oxidative Stress. BioMed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Le Proprietà del Distillato di Legno—BioDea 2020. BioDea. Datasheet—Distillato di Legno. 2020. Available online: https://biodea.bio/wp-content/uploads/2024/02/catalogo-biodea-2024DISTILLATO-DI-LEGNO.pdf (accessed on 1 January 2020).
- Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Characterization of the Safety Profile of Sweet Chestnut Wood Distillate Employed in Agriculture. Safety 2021, 7, 79. [Google Scholar] [CrossRef]
- Maresca, V.; Fedeli, R.; Vannini, A.; Munzi, S.; Corrêa, A.; Cruz, C.; Loppi, S. Wood Distillate Enhances Seed Germination of Chickpea, Lettuce, and Basil. Appl. Sci. 2024, 14, 631. [Google Scholar] [CrossRef]
- Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A.; Cardelli, R.; Becagli, M.; Marchini, F.; Saviozzi, A. Soil Biochemical Activities after the Application of Pyroligneous Acid to Soil. Soil Res. 2020, 58, 461–467. [Google Scholar] [CrossRef]
- Celletti, S.; Fedeli, R.; Ghorbani, M.; Aseka, J.M.; Loppi, S. Exploring Sustainable Alternatives: Wood Distillate Alleviates the Impact of Bioplastic in Basil Plants. Sci. Total Environ. 2023, 900, 166484. [Google Scholar] [CrossRef]
- Misuri, F.; Marri, L. Antibacterial Activity of Wood Distillate from Residual Virgin Chestnut Biomass. Eur. J. Wood Prod. 2021, 79, 237–239. [Google Scholar] [CrossRef]
- Ciccone, V.; Filippelli, A.; Angeli, A.; Supuran, C.T.; Morbidelli, L. Pharmacological Inhibition of CA-IX Impairs Tumor Cell Proliferation, Migration and Invasiveness. Int. J. Mol. Sci. 2020, 21, 2983. [Google Scholar] [CrossRef]
- Bardoloi, A.; Soren, A.D. Genotoxicity Induced by Medicinal Plants. Bull. Natl. Res. Cent. 2022, 46, 119. [Google Scholar] [CrossRef]
- Ristori, E.; Cicaloni, V.; Salvini, L.; Tinti, L.; Tinti, C.; Simons, M.; Corti, F.; Donnini, S.; Ziche, M. Amyloid-β Precursor Protein APP Down-Regulation Alters Actin Cytoskeleton-Interacting Proteins in Endothelial Cells. Cells 2020, 9, 2506. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A Master Regulator of Cellular Responses in Inflammation, Injury Resolution, and Tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Free Radicals: Health Implications and Their Mitigation by Herbals. J. Adv. Med. Med. Res. 2015, 7, 438–457. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Ho, C.-H.; Huang, J.-H.; Sun, M.-S.; Tzeng, I.-S.; Hsu, Y.-C.; Kuo, C.-Y. Wild Bitter Melon Extract Regulates LPS-Induced Hepatic Stellate Cell Activation, Inflammation, Endoplasmic Reticulum Stress, and Ferroptosis. Evid.-Based Complement. Altern. Med. 2021, 2021, e6671129. [Google Scholar] [CrossRef]
- Tuyen, P.T.; Xuan, T.D.; Khang, D.T.; Ahmad, A.; Quan, N.V.; Tu Anh, T.T.; Anh, L.H.; Minh, T.N. Phenolic Compositions and Antioxidant Properties in Bark, Flower, Inner Skin, Kernel and Leaf Extracts of Castanea Crenata Sieb. et Zucc. Antioxidants 2017, 6, 31. [Google Scholar] [CrossRef]
- Taïlé, J.; Arcambal, A.; Clerc, P.; Gauvin-Bialecki, A.; Gonthier, M.-P. Medicinal Plant Polyphenols Attenuate Oxidative Stress and Improve Inflammatory and Vasoactive Markers in Cerebral Endothelial Cells during Hyperglycemic Condition. Antioxidants 2020, 9, 573. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Khanna, S.; Kaur, G.; Singh, I. Medicinal Plants and Their Components for Wound Healing Applications. Futur. J. Pharm. Sci. 2021, 7, 53. [Google Scholar] [CrossRef]
- Babu, M.; Prasad, O.; Murthy, T.E.G.K. Comparison of the Dermal Wound Healing of Centella Asiatica Extract Impregnated Collagen and Crosslinked Collagen Scaffolds. J. Chem. Pharm. Res. 2011, 3, 353–362. [Google Scholar]
- Xiong, Y.; Chen, L.; Man, J.; Hu, Y.; Cui, X. Chemical and Bioactive Comparison of Panax Notoginseng Root and Rhizome in Raw and Steamed Forms. J. Ginseng Res. 2019, 43, 385–393. [Google Scholar] [CrossRef]
- Oryan, A.; Naeini, A.T.; Nikahval, B.; Gorjian, E. Effect of Aqueous Extract of Aloe Vera on Experimental Cutaneous Wound Healing in Rat. Vet. Arh. 2010, 80, 509–522. [Google Scholar]
- Akhma Mat Yasin, Z.; Ibrahim, F.; Nor Rashid, N.; Mohamad Razif, M.; Yusof, R. The Importance of Some Plant Extracts as Skin Anti-Aging Resources: A Review. Curr. Pharm. Biotechnol. 2017, 19, 864–876. [Google Scholar] [CrossRef]


| Parameter/Chemical Element | Value |
|---|---|
| TOC (% DW) | 58.03 |
| TN (% DW) | 1.06 |
| H (% DW) | 7.27 |
| S (% DW) | 0.07 |
| pH | 4 |
| Density (g mL−1) | 1.05 |
| Flash point (°C) | >60 |
| Total organic compounds (g L−1) | 33.8 |
| Acidity (mg L−1) | 1289 |
| Organic acids (mg L−1) | 32.3 |
| Acetic acid (mg L−1) | 21.5 |
| Polyphenols (g L−1) | 24.5 |
| Phenols (g L−1) | 3 |
| PCBs (mg L−1) | <0.2 |
| Hydrocarbons C < 12 (mg L−1) | <0.1 |
| Hydrocarbons C10–C40 (mg L−1) | <0.1 |
| Elements (mg L−1) | |
| Ca | 325.50 |
| Na | 103.59 |
| K | 23.49 |
| Fe | 21.16 |
| P | 7.28 |
| Mg | 6.79 |
| Zn | 3.22 |
| Al | 1.96 |
| Mn | 0.58 |
| Cu | 0.18 |
| Ba | 0.06 |
| Cr | 0.03 |
| Mo | 0.0007 |
| Inflammatory Markers | CTR | MIX | 0.04% (v/v, WD) | 0.07% (v/v, WD) | MIX + 0.04% (v/v, WD) | MIX + 0.07% (v/v, WD) |
|---|---|---|---|---|---|---|
| COX-2 | 0.07 ± 0.006 | 0.38 ± 0.027 ***↑↑ | 0.06 ± 0.023 ###↓ | 0.8 ± 0.005 ###↓↓ | 0.10 ± 0.007 ##↓ | 0.07 ± 0.024 ###↓↓ |
| mPGES-1 | 0.33 ± 0.023 | 0.68 ± 0.039 ***↑↑ | 0.50 ± 0.010 *#↓↑ | 0.26 ± 0.006 ###↓↓ | 0.34 ± 0.015 ##↓ | 0.37 ± 0.025 ##↓ |
| VCAM-1 | 0.11 ± 0.021 | 0.52 ± 0.072 ***↑↑ | 0.20 ± 0.056 ##↓ | 0.13 ± 0.022 ###↓↓ | 0.26 ± 0.014 *##↓↑ | 0.16 ± 0.063 ###↓↓ |
| ICAM-1 | 0.10 ± 0.039 | 1.08 ± 0.041 ***↑↑ | 0.27 ± 0.072 *###↓↓↑ | 0.12 ± 0.040 ###↓↓ | 0.10 ± 0.008 ###↓↓↑ | 0.09 ± 0.016 ###↓↓ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filippelli, A.; Ciccone, V.; Loppi, S.; Morbidelli, L. Promising Support Coming from Nature: Antioxidant and Anti-Inflammatory Potential of Castanea sativa Wood Distillate on Skin Cells. Curr. Issues Mol. Biol. 2024, 46, 9386-9400. https://doi.org/10.3390/cimb46090556
Filippelli A, Ciccone V, Loppi S, Morbidelli L. Promising Support Coming from Nature: Antioxidant and Anti-Inflammatory Potential of Castanea sativa Wood Distillate on Skin Cells. Current Issues in Molecular Biology. 2024; 46(9):9386-9400. https://doi.org/10.3390/cimb46090556
Chicago/Turabian StyleFilippelli, Arianna, Valerio Ciccone, Stefano Loppi, and Lucia Morbidelli. 2024. "Promising Support Coming from Nature: Antioxidant and Anti-Inflammatory Potential of Castanea sativa Wood Distillate on Skin Cells" Current Issues in Molecular Biology 46, no. 9: 9386-9400. https://doi.org/10.3390/cimb46090556







