Recent Advances in Understanding the Molecular Mechanisms of SGLT2 Inhibitors in Atrial Remodeling
Abstract
1. Introduction
2. Methods
3. Results and Discussions
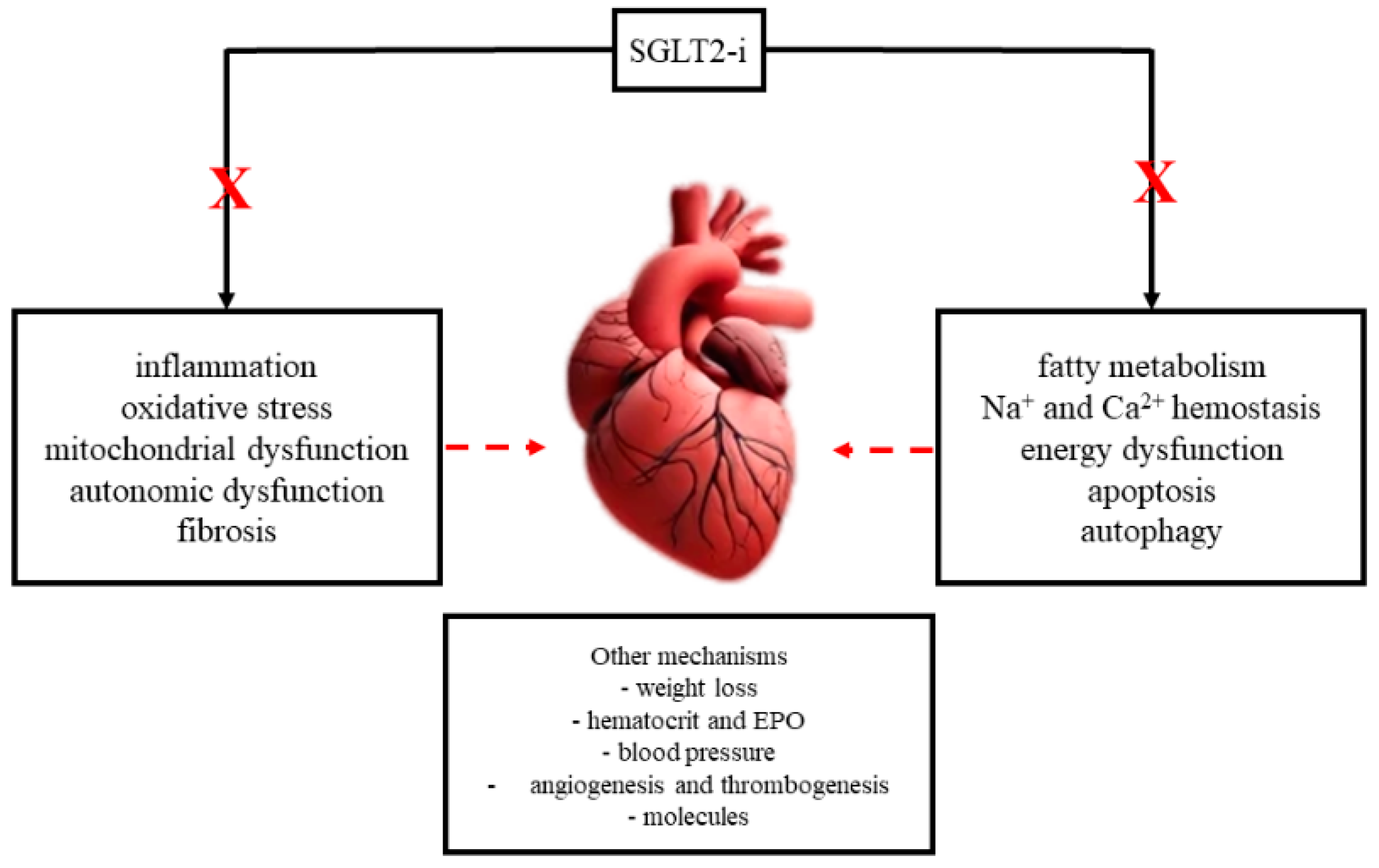
3.1. Molecular Mechanisms of SGLT2is and Atrial Remodeling
3.1.1. Inflammation
3.1.2. Oxidative Stress and Mitochondrial Dysfunction
3.1.3. Autonomic Dysfunction
3.1.4. Fibrosis
3.1.5. Myocardial Energy Metabolism
3.1.6. Sodium and Calcium Metabolism
3.1.7. Other Mechanisms
Weight Loss
Hematocrit and Erythropoietin
Blood Pressure
Angio- and Thrombogenesis
Molecules
Epigenetic Effects
4. Clinical Implications
4.1. Atrial Fibrillation
4.2. Other Cardiac Conditions
4.3. Other Non-Cardiac Conditions
4.4. Cardiovascular Aging
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Zelniker, T.A.; Bonaca, M.P.; Furtado, R.H.; Mosenzon, O.; Kuder, J.F.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; McGuire, D.K.; Wilding, J.P.; et al. Effect of Dapagliflozin on Atrial Fibrillation in Patients with Type 2 Diabetes Mellitus: Insights from the DECLARE-TIMI 58 Trial. Circulation 2020, 141, 1227–1234. [Google Scholar] [CrossRef]
- von Lewinski, D.; Tripolt, N.J.; Sourij, H.; Pferschy, P.N.; Oulhaj, A.; Alber, H.; Gwechenberger, M.; Martinek, M.; Seidl, S.; Moertl, D.; et al. Ertugliflozin to reduce arrhythmic burden in ICD/CRT patients (ERASe-trial)—A phase III study. Am. Heart J. 2022, 246, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Manolis, T.A.; Melita, H.; Manolis, A.S. Sodium-glucose cotransporter type 2 inhibitors and cardiac arrhythmias. J. Clin. Med. 2023, 12, 2868. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Almorós, A.; Cerrada, J.C.; Walther, L.-A.-S.; Bailón, M.M.; González, L. Atrial Fibrillation and Diabetes Mellitus: Dangerous Liaisons or Innocent Bystanders? J. Clin. Med. 2023, 12, 2868. [Google Scholar] [CrossRef] [PubMed]
- Al Ghamdi, B.; Hassan, W. Atrial Remodeling and Atrial Fibrillation: Mechanistic Interactions And Clinical Implications. J. Atr. Fibrillation 2009, 2, 125. [Google Scholar] [PubMed] [PubMed Central]
- Hoit, B.D. Left Atrial Remodeling: More Than Just Left Atrial Enlargement. Circ. Cardiovasc. Imaging 2017, 10, e006036. [Google Scholar] [CrossRef] [PubMed]
- Sabouret, P.; Attias, D.; Beauvais, C.; Berthelot, E.; Bouleti, C.; Genty, G.G.; Galat, A.; Hanon, O.; Hulot, J.; Isnard, R.; et al. Diagnosis and management of heart failure from hospital admission to discharge: A practical expert guidance. Ann. Cardiol. d’Angéiologie 2022, 71, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; Mc-Guire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin effects on biomarkers, symptoms, and functional status in patients with heart failure with reduced ejection fraction. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 inhibitor dapagliflozin in heart failure with preserved ejection fraction: A multicenter randomized trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner–La Rocca, H.-P.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2022. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: Executive Summary: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2022. [Google Scholar] [CrossRef]
- Lee, H.-C.; Shiou, Y.-L.; Jhuo, S.-J.; Chang, C.-Y.; Liu, P.-L.; Jhuang, W.-J.; Dai, Z.-K.; Chen, W.-Y.; Chen, Y.-F.; Lee, A.-S. The sodium–glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol. 2019, 18, 45. [Google Scholar] [CrossRef]
- Shao, Q.; Meng, L.; Lee, S.; Tse, G.; Gong, M.; Zhang, Z.; Zhao, J.; Zhao, Y.; Li, G.; Liu, T. Empagliflozin, a sodium glucose co-transporter-2 inhibitor, alleviates atrial remodeling and improves mitochondrial function in high-fat diet/streptozotocin-induced diabetic rats. Cardiovasc. Diabetol. 2019, 18, 165. [Google Scholar] [CrossRef]
- Ferrini, M.; Johansson, I.; Aboyans, V. Heart failure and its complications in patients with diabetes: Mounting evidence for a growing burden. Eur. J. Prev. Cardiol. 2019, 26, 106–113. [Google Scholar] [CrossRef]
- Natali, A.; Nesti, L.; Fabiani, I.; Calogero, E.; Di Bello, V. Impact of empagliflozin on subclinical left ventricular dysfunctions and on the mechanisms involved in myocardial disease progression in type 2 diabetes: Rationale and design of the EMPA-HEART trial. Cardiovasc. Diabetol. 2017, 16, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, H.; Wang, J. Metabolism and Chronic Inflammation: The Links Between Chronic Heart Failure and Comorbidities. Front. Cardiovasc. Med. 2021, 8, 650278. [Google Scholar] [CrossRef] [PubMed]
- Patoulias, D.; Fragakis, N.; Rizzo, M. The Therapeutic Role of SGLT-2 Inhibitors in Acute Heart Failure: From Pathophysiologic Mechanisms to Clinical Evidence with Pooled Analysis of Relevant Studies across Safety and Efficacy Endpoints of Interest. Life 2022, 12, 2062. [Google Scholar] [CrossRef] [PubMed]
- Bode, D.; Semmler, L.; Wakula, P.; Hegemann, N.; Primessnig, U.; Beindorff, N.; Powell, D.; Dahmen, R.; Ruetten, H.; Oeing, C.; et al. Dual SGLT-1 and SGLT-2 inhibition improves left atrial dysfunction in HFpEF. Cardiovasc. Diabetol. 2021, 20, 7. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.A.; Obradovic, Z.; Fushtey, I.M.; Berezina, T.A.; Novikov, E.V.; Schmidbauer, L.; Lichtenauer, M.; Berezin, A.E. The Impact of SGLT2 Inhibitor Dapagliflozin on Adropin Serum Levels in Men and Women with Type 2 Diabetes Mellitus and Chronic Heart Failure. Biomedicines 2023, 11, 457. [Google Scholar] [CrossRef]
- Chen, Y.; Peng, D. New insights into the molecular mechanisms of SGLT2 inhibitors on ventricular remodeling. Int. Immunopharmacol. 2023, 118, 110072. [Google Scholar] [CrossRef]
- Persson, F.; Nyström, T.; Jørgensen, M.E.; Carstensen, B.; Gulseth, H.L.; Thuresson, M.; Fenici, P.; Nathanson, D.; Eriksson, J.W.; Norhammar, A.; et al. Dapagliflozin is associated with lower risk of cardiovascular events and all-cause mortality in people with type 2 diabetes (CVD-REAL Nordic) when compared with dipeptidyl peptidase-4 inhibitor therapy: A multinational observational study. Diabetes Obes. Metab. 2018, 20, 344–351. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Goncalves, E. Atrial fibrillation and type 2 diabetes: Prevalence, etiology, pathophysiology and effect of anti-diabetic therapies. Diabetes Obes. Metab. 2019, 21, 210–217. [Google Scholar] [CrossRef]
- Bell, D.S.H.; Goncalves, E. Heart failure in the patient with diabetes: Epidemiology, aetiology, prognosis, therapy and the effect of glucose-lowering medications. Diabetes Obes. Metab. 2019, 21, 1277–1290. [Google Scholar] [CrossRef]
- Iwakura, K. Heart failure in patients with type 2 diabetes mellitus: Assessment with echocardiography and effects of antihyperglycemic treatments. J. Echocardiogr. 2019, 17, 177–186. [Google Scholar] [CrossRef]
- Kang, S.; Verma, S.; Hassanabad, A.F.; Teng, G.; Belke, D.D.; Dundas, J.A.; Guzzardi, D.G.; Svystonyuk, D.A.; Pattar, S.S.; Park, D.S.; et al. Direct Effects of Empagliflozin on Extracellular Matrix Remodelling in Human Cardiac Myofibroblasts: Novel Translational Clues to Explain EMPA-REG OUTCOME Results. Can. J. Cardiol. 2020, 36, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Lan, N.S.R.; Yeap, B.B.; Fegan, P.G.; Green, G.; Rankin, J.M.; Dwivedi, G. Empagliflozin and left ventricular diastolic function following an acute coronary syndrome in patients with type 2 diabetes. Int. J. Cardiovasc. Imaging 2021, 37, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-W.; Lin, Y.-K.; Chen, Y.-C.; Kao, Y.-H.; Chen, Y.-J. Effect of antidiabetic drugs on the risk of atrial fibrillation: Mechanistic insights from clinical evidence and translational studies. Cell. Mol. Life Sci. 2021, 78, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.E.; Januzzi, J.L. Sodium-Glucose Co-Transporter 2 Inhibitors and Insights from Biomarker Measurement in Heart Failure Patients. Clin. Chem. 2021, 67, 79–86. [Google Scholar] [CrossRef]
- Tanaka, H.; Tatsumi, K.; Matsuzoe, H.; Soga, F.; Matsumoto, K.; Hirata, K.-I. Association of type 2 diabetes mellitus with the development of new-onset atrial fibrillation in patients with non-ischemic dilated cardiomyopathy: Impact of SGLT2 inhibitors. Int. J. Cardiovasc. Imaging 2021, 37, 1333–1341. [Google Scholar] [CrossRef]
- Nishinarita, R.; Niwano, S.; Niwano, H.; Nakamura, H.; Saito, D.; Sato, T.; Matsuura, G.; Arakawa, Y.; Kobayashi, S.; Shirakawa, Y.; et al. Canagliflozin suppresses atrial remodeling in a canine atrial fibrillation model. J. Am. Heart Assoc. 2021, 10, e017483. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Chen, C.; Shih, J.; Cheng, B.; Chang, C.; Lin, M.; Ho, C.; Chen, Z.; Fisch, S.; Chang, W. Dapagliflozin improves cardiac hemodynamics and mitigates arrhythmogenesis in mitral regurgitation-induced myocardial dysfunction. J. Am. Heart Assoc. 2021, 10, e019274. [Google Scholar] [CrossRef]
- Kearney, A.; Linden, K.; Savage, P.; Menown, I.B.A. Advances in Clinical Cardiology 2020: A Summary of Key Clinical Trials. Adv. Ther. 2021, 38, 2170–2200. [Google Scholar] [CrossRef]
- Kondo, H.; Akoumianakis, I.; Badi, I.; Akawi, N.; Kotanidis, C.P.; Polkinghorne, M.; Stadiotti, I.; Sommariva, E.; Antonopoulos, A.S.; Carena, M.C.; et al. Effects of canagliflozin on human myocardial redox signalling: Clinical implications. Eur. Heart J. 2021, 42, 4947–4960. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vrachatis, D.A.; Papathanasiou, K.A.; Iliodromitis, K.E.; Giotaki, S.G.; Kossyvakis, C.; Raisakis, K.; Kaoukis, A.; Lambadiari, V.; Avramides, D.; Reimers, B.; et al. Could Sodium/Glucose Co-Transporter-2 Inhibitors Have Antiarrhythmic Potential in Atrial Fibrillation? Literature Review and Future Considerations. Drugs 2021, 81, 1381–1395. [Google Scholar] [CrossRef]
- Tschöpe, C.; Elsanhoury, A.; Nelki, V.; Van Linthout, S.; Kelle, S.; Remppis, A. Heart failure with preserved ejection fraction as a model disease for the cardio-pulmonary-renal syndrome: Importance of visceral fat expansion as central pathomechanism. Die Inn. Med. 2021, 62, 1141–1152. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Benfari, G.; Bonapace, S.; Simon, T.G.; Targher, G. Risk of Heart Failure in Patients With Nonalcoholic Fatty Liver Disease. J. Am. Coll. Cardiol. 2022, 79, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Savage, P.; Cox, B.; Linden, K.; Coburn, J.; Shahmohammadi, M.; Menown, I. Advances in Clinical Cardiology 2021: A Summary of Key Clinical Trials. Adv. Ther. 2022, 39, 2398–2437. [Google Scholar] [CrossRef]
- Theofilis, P.; Antonopoulos, A.S.; Katsimichas, T.; Oikonomou, E.; Siasos, G.; Aggeli, C.; Tsioufis, K.; Tousoulis, D. The impact of SGLT2 inhibition on imaging markers of cardiac function: A systematic review and meta-analysis. Pharmacol. Res. 2022, 180, 106243. [Google Scholar] [CrossRef]
- Thirumathyam, R.; Richter, E.A.; Goetze, J.P.; Fenger, M.; Van Hall, G.; Dixen, U.; Holst, J.J.; Madsbad, S.; Vejlstrup, N.; Madsen, P.L.; et al. Investigating the roles of hyperglycaemia, hyperinsulinaemia and elevated free fatty acids in cardiac function in patients with type 2 diabetes via treatment with insulin compared with empagliflozin: Protocol for the HyperCarD2 randomised, crossover trial. BMJ Open 2022, 12, e054100. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Wen, S.; Zhou, L. The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obesity 2022, 15, 2583–2597. [Google Scholar] [CrossRef]
- Subramanian, M.; Sravani, V.; Krishna, S.P.; Bijjam, S.; Sunehra, C.; Yalagudri, S.; Saggu, D.K.; Narasimhan, C. Efficacy of SGLT2 Inhibitors in Patients With Diabetes and Nonobstructive Hypertrophic Cardiomyopathy. Am. J. Cardiol. 2021, 188, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Watanabe, M.; Yokota, T.; Tsuda, M.; Handa, H.; Koya, J.; Nishino, K.; Tatsuta, D.; Natsui, H.; Kadosaka, T.; et al. Empagliflozin suppresses mitochondrial reactive oxygen species generation and mitigates the inducibility of atrial fibrillation in diabetic rats. Front. Cardiovasc. Med. 2023, 10, 1005408. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nassif, M.E.; Windsor, S.L.; Gosch, K.; Borlaug, B.A.; Husain, M.; Inzucchi, S.E.; Kitzman, D.W.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; et al. Dapagliflozin Improves Heart Failure Symptoms and Physical Limitations Across the Full Range of Ejection Fraction: Pooled Patient-Level Analysis From DEFINE-HF and PRESERVED-HF Trials. Circ. Heart Fail. 2023, 16, e009837. [Google Scholar] [CrossRef] [PubMed]
- Scatularo, C.E.; Martínez, E.L.P.; Alba, A.C.; Renedo, M.F.; Llober, M.N.; Elfman, M.; de Arenaza, D.P.; Diez, M.; Saldarriaga, C.; Cingolani, E.; et al. Endomyocardiofibrosis in the Americas Collaborative Study: The EMF-SIAC Registry. Curr. Probl. Cardiol. 2023, 48, 101995. [Google Scholar] [CrossRef]
- Avula, V.; Sharma, G.; Kosiborod, M.N.; Vaduganathan, M.; Neilan, T.G.; Lopez, T.; Dent, S.; Baldassarre, L.; Scherrer-Crosbie, M.; Barac, A.; et al. SGLT2 Inhibitor Use and Risk of Clinical Events in Patients With Cancer Therapy–Related Cardiac Dysfunction. JACC Heart Fail. 2024, 12, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Escobar, C.; Pascual-Figal, D.; Manzano, L.; Nuñez, J.; Camafort, M. Current Role of SLGT2 Inhibitors in the Management of the Whole Spectrum of Heart Failure: Focus on Dapagliflozin. J. Clin. Med. 2023, 12, 6798. [Google Scholar] [CrossRef]
- El-Saied, S.B.; El-Sherbeny, W.S.; El-Sharkawy, S.I. Impact of sodium glucose co-transporter-2 inhibitors on left atrial functions in patients with type-2 diabetes and heart failure with mildly reduced ejection fraction. IJC Heart Vasc. 2024, 50, 101329. [Google Scholar] [CrossRef]
- Carberry, J.; Petrie, M.C.; Lee, M.M.; Brooksbank, K.; Campbell, R.T.; Good, R.; Jhund, P.S.; Kellman, P.; Lang, N.N.; Mangion, K.; et al. Empagliflozin to prevent progressive adverse remodelling after myocardial infarction (EMPRESS-MI): Rationale and design. ESC Heart Fail. 2024, 11, 2001–2012. [Google Scholar] [CrossRef] [PubMed]
- Fakih, W.; Mroueh, A.; Gong, D.-S.; Kikuchi, S.; Pieper, M.P.; Kindo, M.; Mazzucottelli, J.-P.; Mommerot, A.; Kanso, M.; Ohlmann, P.; et al. Activated factor X stimulates atrial endothelial cells and tissues to promote remodelling responses through AT1R/NADPH oxidases/SGLT1/2. Cardiovasc. Res. 2024, 0, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Žlahtič, T.; Mrak, M.; Žižek, D. Complexities of treating co-morbidities in heart failure with preserved ejection fraction. ESC Heart Fail. 2024. [Google Scholar] [CrossRef]
- Kodur, N.; Tang, W.H.W. Non-cardiac comorbidities in heart failure: An update on diagnostic and management strategies. Minerva Med. 2024, 115, 337–353. [Google Scholar] [CrossRef]
- Connelly, K.; Cai, S. Softening the Stiff Heart. JACC Cardiovasc. Imaging 2021, 14, 408–410. [Google Scholar] [CrossRef]
- Hitzeroth, J.; Mpe, M.; Klug, E.; Ranjith, N.; Sliwa, K.; Steingo, L.; Lachman, L.; Tsabedze, N.; Ntusi, N.A.B.; Society of South Africa HF. 2020 Heart Failure Society of South Africa perspective on the 2016 Eu-ropean Society of Cardiology Chronic Heart Failure Guidelines. S. Afr. Med. J. 2020, 110, 13057. [Google Scholar] [PubMed]
| No. | Year | Study | Ref. No. | No. of Subjects | Subject Type | Comparison | Endpoints | Conclusions |
|---|---|---|---|---|---|---|---|---|
| 1 | 2017 | Persson F et al. | [29] | 40.980 | Human, type 2 diabetes | Dapagliflozin vs. DPP-4 inhibitors (dose not specified) | Major adverse cardiovascular events; non-fatal MI, non-fatal stroke, cardiovascular mortality, all-cause mortality, hospitalization for HF, atrial fibrillation, and severe hypoglycemia | Lower risk of major adverse cardiovascular events, hospitalization for HF and all-cause mortality |
| 2 | 2017 | Natali A et al. | [23] | 75 | Human, type 2 diabetes | Empagliflozin 10 mg/day vs. Satagliptin 100 mg/day | Global longitudinal strain, left ventricle ejection fraction, left atrial volume, E/E′, VO2max, cardiac autonomic function tests, and plasma biomarkers | |
| 3 | 2018 | David S et al. | [30] | review | v | GLP-1 agonists vs. SGLT2i vs. DPP-4 inhibitors | Structural and electrical atrial remodeling | GLP-1 agonists and SGLT2 and DPP-4 inhibitors do not accelerate or decelerate the development of new-onset AF |
| 4 | 2018 | David S et al. | [31] | review | Human | Several antidiabetic drugs | Reversal of cardiac remodeling | Metformin and SGLT2is can prevent or ameliorate HF |
| 5 | 2019 | Lee HC et al. | [20] | 13 | Spontaneous hypertensive rats | Empagliflozin 20 mg/day vs. control | Electrocardiography, echocardiography, invasive hemodynamic study, biomarkers, and tissue analyses | Beneficial effects on systemic blood pressure, renal function, ameliorated left atrial dilatation, intra-cardiac fibrosis, contraction, and relaxation dysfunction |
| 6 | 2019 | Iwakura K et al. | [32] | review | Human | Several antidiabetic drugs | Left ventricle hypertrophy, left atrium dilatation, systolic and diastolic function | SGLT2is decrease the incidence of hospitalization for HF, cardiovascular death, and left ventricle mass |
| 7 | 2019 | Ferrini M et al. | [22] | review | Human | Several antidiabetic drugs | Major adverse cardiovascular events, HF outcome, sudden cardiac death, AF | Glucose-lowering drugs may reduce the progression and avoid the onset of HF |
| 8 | 2019 | Shao Q et al. | [21] | 96 | Diabetic rats | Empagliflozin 10 mg/kg/day vs. empagliflozin 30 mg/kg/day vs. control | Left atrium diameter, interstitial fibrosis AF, atrial mitochondrial respiratory function, mitochondrial membrane potential, and mitochondrial biogenesis | Empagliflozin can ameliorate atrial structural and electrical remodeling and improve mitochondrial function and mitochondrial biogenesis in type 2 DM |
| 9 | 2020 | Kang S et al. | [33] | 11 | Cardiac fibroblasts from human atrial tissue | Empagliflozin 10/25 mg/day vs. control | Myofibroblast activity, cell morphology, and cell-mediated extracellular matrix remodeling | Empagliflozin significantly attenuated TGF-b1e-induced fibroblast activation |
| 10 | 2020 | Lan NSR et al. | [34] | 44 | Human, acute coronary syndrome, and type 2 diabetes | Empagliflozin 10/25 mg/day vs. control | E/e′ ratio, left ventricle mass index, and left ventricle diastolic function | Empagliflozin is associated with a reduction in left ventricle mass and favorable changes in diastolic function |
| 11 | 2021 | Lee TW et al. | [35] | review | Human | Several antidiabetic drugs | New-onset AF, atrial dilatation and fibrosis, mitochondrial function, and mitochondrial biogenesis | Empagliflozin and canagliflozin—no effect on the incidence of AF in patients with DM, dapagliflozin reduced AF risk and atrial flutter events |
| 12 | 2021 | Ibrahim NE et al. | [36] | review | Human | SGLT2is vs. control | Concentrations of atrial natriuretic peptide, B-type natriuretic peptide, and N-terminal pro-B-type natriuretic peptide, and reduction in high-sensitivity troponin | Reduction in concentrations of atrial natriuretic peptide, B-type natriuretic peptide, and N-terminal pro-B-type natriuretic peptide and high-sensitivity Troponin C in patients receiving SLGT2is |
| 13 | 2021 | Tanaka H et al. | [37] | 210 | Human, non-ischemic diabetic cardiomyopathy | SGLT2is vs. control | New-onset AF | SGLT2is can significantly reduce new-onset AF |
| 14 | 2021 | Nishinarita R et al. | [38] | 12 | Beagle dogs, induced AF | Canagliflozin (3 mg/kg/day) vs. control | Atrial effective refractory period, conduction velocity, and AF inducibility | Canagliflozin and possibly other SGLT2is might be useful for preventing AF and suppressing the promotion of atrial remodeling |
| 15 | 2021 | Bode D et al. | [26] | - | Obese rats | Dual SGLT-1&2 inhibitors (sotagliflozin 30 mg/day) vs. control | LA remodeling | Dual SGLT-1&2 inhibitors ameliorated left atrial remodeling and exerted an anti-arrhythmic effect on left atrial cardiomyocytes |
| 16 | 2021 | Lin YW et al. | [39] | 32 | Rats, induced left heart dilatation | Dapagliflozin 10 mg/kg/day vs. control | Electrocardiography and echocardiography, hemodynamic studies, and postmortem tissue analyses | Dapagliflozin suppresses cardiac fibrosis and endoplasmic reticulum stress |
| 17 | 2021 | Kearney A et al. | [40] | review | Human | SGLT2is | - | Reduction in left ventricle end-systolic volume, left ventricle end-diastolic volume, and N-terminal pro-B-type natriuretic peptide, suggesting reverse left ventricle remodeling |
| 18 | 2021 | Lee Z et al. | [24] | review | Human | SGLT2is | - | SGLT2is are beneficial for HF regardless of diabetic status |
| 19 | 2021 | Damy T et al. | [7] | review | Human | SGLT2is | - | SGLT2is are beneficial for HF regardless of diabetic status |
| 20 | 2021 | Kondo H et al. | [41] | 364 | Human | Canagliflozin 10 µmol/L (cell culture) vs control | Superoxide sources and the expression of inflammation, fibrosis, and myocardial stretch genes | Canagliflozin has global anti-inflammatory and anti-apoptotic effects in the human myocardium |
| 21 | 2021 | Vrachatis DA et al. | [42] | review | Human | SGLT2is | - | Pathophysiologic mechanisms involved in AF seem to be favorably affected by SGLT2 inhibition |
| 22 | 2021 | Tschope C et al. | [43] | review | Human | Several antidiabetic drugs | - | SGLT2is are beneficial for HF regardless of diabetic status and LVEF |
| 23 | 2022 | Mantovani A et al. | [44] | review | Human | Several antidiabetic drugs | - | SGLT2is reduce major adverse cardiovascular events, all-cause mortality, and hospitalization for HF regardless of the presence or absence of type 2 DM and left ventricle ejection fraction |
| 24 | 2022 | Von Lewinski D et al. | [2] | 402 | Human, HF, with reduced or midrange ejection fraction | Ertugliflozin 5 mg/day vs. control | Total burden of ventricular arrhythmias | Trial design |
| 25 | 2023 | Manolis AA et al. | [3] | review | Human | SGLT2is | - | SGLT2is can reverse atrial and ventricular remodeling and ameliorate mitochondrial dysfunction |
| 26 | 2022 | Savage P et al. | [45] | review | Human | SGLT2is | - | SGLT2is are associated with a greater incidence of clinical benefits (as defined by rates of death, HF events, time to first HF event) |
| 27 | 2022 | Theofilis P et al. | [46] | review | Human | SGLT2is | - | SGLT2ihave beneficial effects on cardiac remodeling and attenuate HF progression |
| 28 | 2022 | Thirumathyam R et al. | [47] | 20 | Human, type 2 diabetes, body mass index ≥ 28 kg/m2, glycated hemoglobin ≤ 9%, fasting C-peptide > 500 pmol/L | Empagliflozin 25 mg/day vs. insulin | Change in myocardial diastolic function | Study protocol |
| 29 | 2022 | Dong M et al. | [48] | review | Human | SGLT2is vs. GLP-1 agonists | - | GLP-1RAs and SGLT2is cross the blood–brain barrier and can be applied in the treatment of central nervous system diseases |
| 30 | 20222 | Subramanian M et al. | [49] | 48 | Human, nonobstructive hypertrophic cardiomyopathy | Empagliflozin 10/25 mg/day or dapagliflozin 5/10 mg/day vs. control | Improvement of at least 1.5 in the E/e ratio and a reduction of >1 NYHA functional class | SGLT2is have favorable effects on diastolic function and functional capacity |
| 31 | 2022 | Patoulias D et al. | [25] | review | Human | SGLT2is | Role of SGLT2i in acute HF | Insufficient data to substantiate the use of SGLT2is in acute HF |
| 32 | 2023 | Koizumi T et al. | [50] | 48 | Diabetic rats | Empagliflozin 30 mg/kg/day vs. control | Examine whether empagliflozin suppresses mitochondrial-ROS generation and mitigates fibrosis. | Empagliflozin reduced mitochondrial oxidative stress and prevented atrial remodeling in a murine model of type 2 DM |
| 33 | 2023 | Berezin AA et al. | [27] | 417 | Human, HF with preserved, midrange, and reduced ejection fraction | Dapagliflozin 10 mg/day vs. control | Modulation of adropin levels | The levels of adropin seem to be a predictor for the favorable modification of hemodynamic performances during SGLT2 inhibition |
| 34 | 2023 | Chen Y et al. | [28] | review | Human | SGLT2i | Molecular mechanisms of SGLT2i on ventricular remodeling | SGLT2 receptor is almost not expressed in the heart, so its target is difficult to determine at present |
| 35 | 2023 | Lorenzo-Almorós A et al. | [4] | review | Human | Several antidiabetic drugs | - | Structural, electrical, and autonomic remodeling may lead to AF |
| 36 | 2023 | Nassif ME et al. | [51] | 587 | Human, HF with preserved or reduced ejection fraction | Dapagliflozin 10 mg vs. control | Change in the Kansas City Cardiomyopathy Questionnaire Clinical Summary Score at 12 weeks | Dapagliflozin improved Kansas City Cardiomyopathy Questionnaire Clinical Summary Score at 12 weeks by 5.0 points |
| 37 | 2023 | Scatularo CE et al. | [52] | 54 | Human, endomyocardial fibrosis | - | Demographic, clinical, biochemical, and imaging variables | 7.4% of patients with endomyocardiofibrosis were treated with SGLT2is |
| 38 | 2024 | Avula V et al. | [53] | 1280 | Human, cancer therapy-related cardiac dysfunction | SGLT2is vs. control | Acute HF exacerbation, All-cause mortality | Patients on SGLT2is had a lower risk of acute HF exacerbation |
| 39 | 2023 | Escobar C et al. | [54] | review | Human | Dapagliflozin 10 mg/day | - | Dapagliflozin has effects on reversing cardiac remodeling, reducing cardiac fibrosis and inflammation, and improving endothelial dysfunction |
| 40 | 2024 | El-Saied SB et al. | [55] | 70 | Human, HF with mildly reduced ejection fraction, and DM | Empagliflozin 10 mg/day or dapagliflozin 10 mg/day vs. control | Demographic, clinical, biochemical, and imaging variables | SGLT2is cause significant improvement of left atrium volume and functions, with further improvement of left ventricle diastolic and longitudinal functions |
| 41 | 2024 | Carberry J et al. | [56] | 100 | Human, left ventricular systolic dysfunction after myocardial infarction | Empagliflozin 10 mg/day vs. control | Change in left ventricle end-systolic volume indexed to body surface area over 24 weeks from randomization | Study design |
| 42 | 2024 | Fakih W et al. | [57] | 20 | Human and porcine model | SGLT2i | Examined whether activated factor X induces pro-remodeling and profibrotic responses in atrial endothelial cells | Sotagliflozin and empagliflozin prevented the FXa-induced eNOS-NO/ROS imbalance and the induction of endothelial senescence |
| 43 | 2024 | Žlahtič T et al. | [58] | case report | Human | - | - | SGLT2is are a cornerstone in the management of HF patients |
| 44 | 2024 | Kodur N et al. | [59] | review | Human | - | - | SGLT2is have shown promise in reducing HF-related morbidity and mortality |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minciună, I.-A.; Tomoaia, R.; Mihăilă, D.; Cismaru, G.; Puiu, M.; Roșu, R.; Simu, G.; Frîngu, F.; Irimie, D.A.; Caloian, B.; et al. Recent Advances in Understanding the Molecular Mechanisms of SGLT2 Inhibitors in Atrial Remodeling. Curr. Issues Mol. Biol. 2024, 46, 9607-9623. https://doi.org/10.3390/cimb46090571
Minciună I-A, Tomoaia R, Mihăilă D, Cismaru G, Puiu M, Roșu R, Simu G, Frîngu F, Irimie DA, Caloian B, et al. Recent Advances in Understanding the Molecular Mechanisms of SGLT2 Inhibitors in Atrial Remodeling. Current Issues in Molecular Biology. 2024; 46(9):9607-9623. https://doi.org/10.3390/cimb46090571
Chicago/Turabian StyleMinciună, Ioan-Alexandru, Raluca Tomoaia, Dragos Mihăilă, Gabriel Cismaru, Mihai Puiu, Radu Roșu, Gelu Simu, Florina Frîngu, Diana Andrada Irimie, Bogdan Caloian, and et al. 2024. "Recent Advances in Understanding the Molecular Mechanisms of SGLT2 Inhibitors in Atrial Remodeling" Current Issues in Molecular Biology 46, no. 9: 9607-9623. https://doi.org/10.3390/cimb46090571
APA StyleMinciună, I.-A., Tomoaia, R., Mihăilă, D., Cismaru, G., Puiu, M., Roșu, R., Simu, G., Frîngu, F., Irimie, D. A., Caloian, B., Zdrenghea, D., & Pop, D. (2024). Recent Advances in Understanding the Molecular Mechanisms of SGLT2 Inhibitors in Atrial Remodeling. Current Issues in Molecular Biology, 46(9), 9607-9623. https://doi.org/10.3390/cimb46090571


_Kim.png)


