Ferredoxins: Functions, Evolution, Potential Applications, and Challenges of Subtype Classification
Abstract
:1. Introduction
2. Ferredoxin Functions
2.1. Pyruvate Synthesis/CO2 Fixation
2.2. Role in Photosynthesis
2.3. Role in the Production of Hydrogen
2.4. Transfer of Electrons to Cytochrome P450 Monooxygenases
2.5. Role in Nitrogen Fixation
2.6. Role in Sulfur Metabolism
2.7. Role in Iron-Sulfur Cluster Biosynthesis
2.8. Role in Lipid Metabolism
3. Classification of Ferredoxins-Types
4. Evolution of Ferredoxins
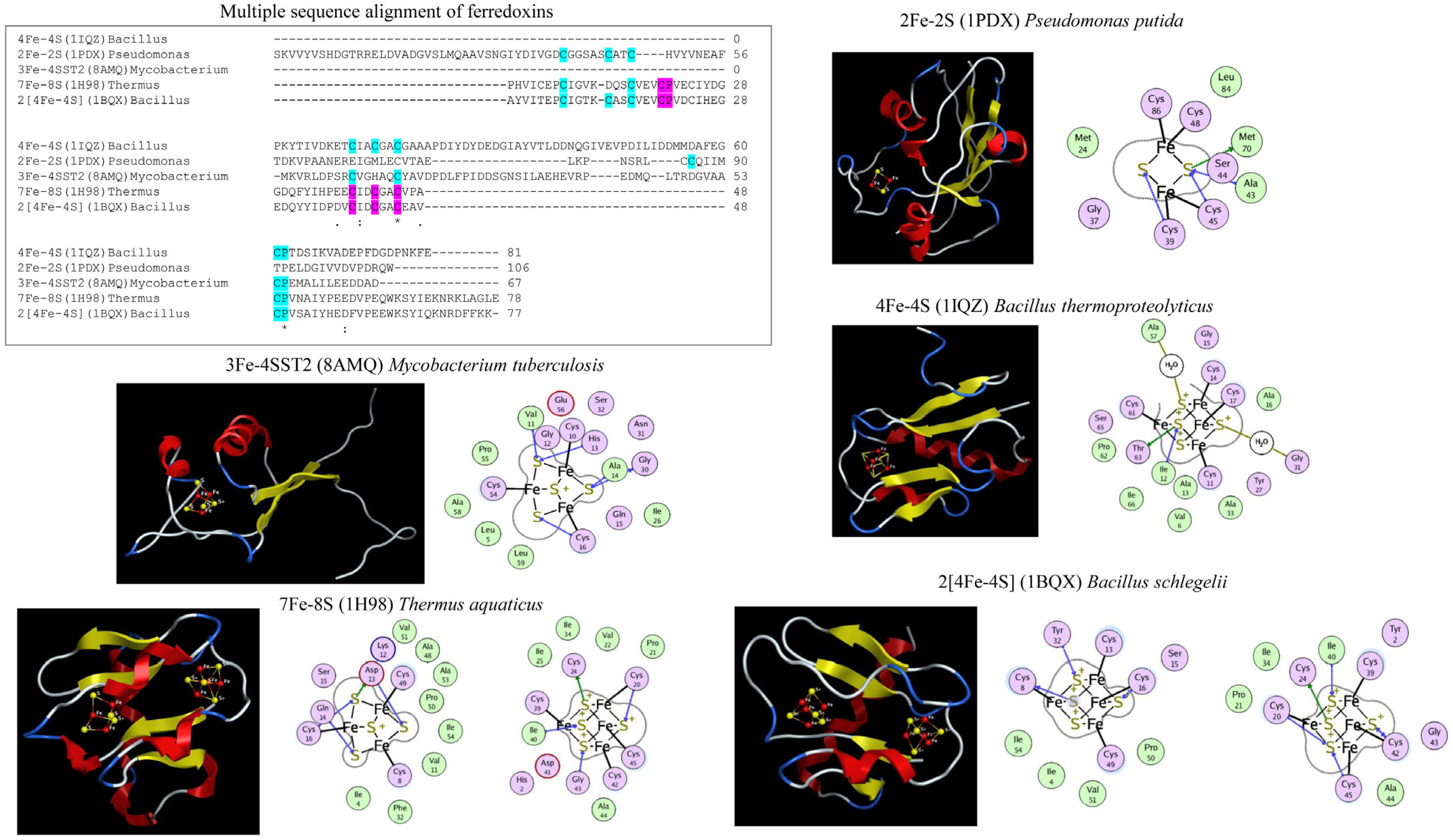
5. Ferredoxin Subtype Classification and Nomenclature
6. Applications of Subtype Classification
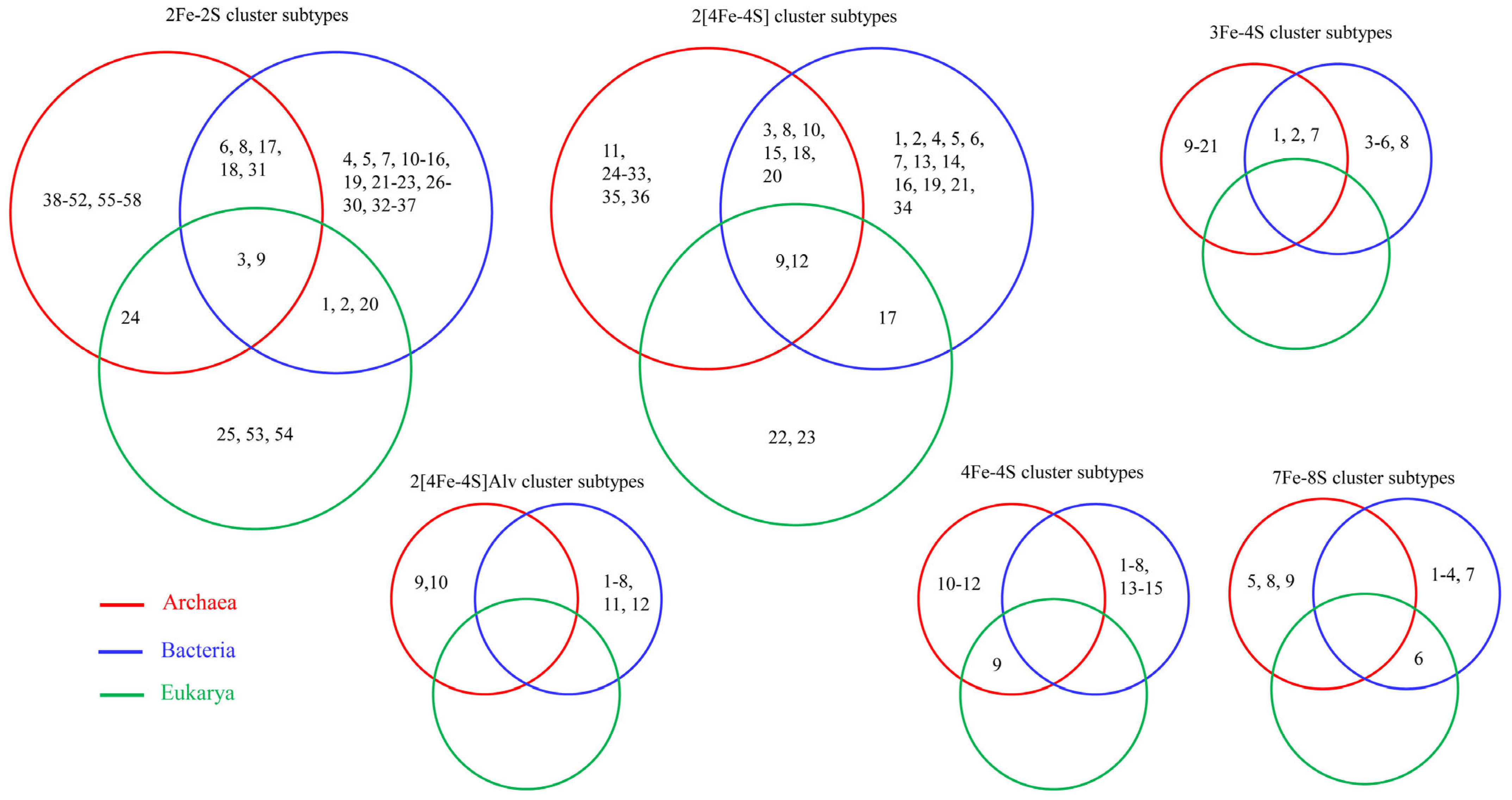
6.1. Diversity Analysis/Enrichment/Evolutionary Linkage of Ferredoxins in Organisms
6.2. Lateral/Horizontal Gene Transfer (LGT/HGT) of Ferredoxins
7. Challenges of Assigning Ferredoxins to Different Fe-S Cluster Types and Subtypes
8. Conclusions and Future Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Nzuza, N.; Padayachee, T.; Chen, W.; Gront, D.; Nelson, D.R.; Syed, K. Diversification of ferredoxins across living organisms. Curr. Issues Mol. Biol. 2021, 43, 1374–1390. [Google Scholar] [CrossRef]
- Hall, D.; Cammack, R.; Rao, K. Role for ferredoxins in the origin of life and biological evolution. Nature 1971, 233, 136–138. [Google Scholar] [CrossRef]
- Mondal, J.; Bruce, B. Ferredoxin: The central hub connecting photosystem I to cellular metabolism. Photosynthetica 2018, 56, 279–293. [Google Scholar] [CrossRef]
- Braymer, J.J.; Freibert, S.A.; Rakwalska-Bange, M.; Lill, R. Mechanistic concepts of iron-sulfur protein biogenesis in Biology. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118863. [Google Scholar] [CrossRef] [PubMed]
- Ewen, K.M.; Ringle, M.; Bernhardt, R. Adrenodoxin—A versatile ferredoxin. IUBMB Life 2012, 64, 506–512. [Google Scholar] [CrossRef]
- Mohibi, S.; Zhang, Y.; Perng, V.; Chen, M.; Zhang, J.; Chen, X. Ferredoxin 1 is essential for embryonic development and lipid homeostasis. Elife 2024, 13, e91656. [Google Scholar] [CrossRef] [PubMed]
- Mustila, H.; Allahverdiyeva, Y.; Isojärvi, J.; Aro, E.; Eisenhut, M. The bacterial-type [4Fe–4S] ferredoxin 7 has a regulatory function under photooxidative stress conditions in the cyanobacterium Synechocystis sp. PCC 6803. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1837, 1293–1304. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, C.; Moreira, D.; López-García, P.; Brochier-Armanet, C. Horizontal gene transfer of a chloroplast DnaJ-Fer protein to Thaumarchaeota and the evolutionary history of the DnaK chaperone system in Archaea. BMC Evol. Biol. 2012, 12, 226. [Google Scholar] [CrossRef]
- Schulz, V.; Basu, S.; Freibert, S.-A.; Webert, H.; Boss, L.; Mühlenhoff, U.; Pierrel, F.; Essen, L.-O.; Warui, D.M.; Booker, S.J. Functional spectrum and specificity of mitochondrial ferredoxins FDX1 and FDX2. Nat. Chem. Biol. 2023, 19, 206–217. [Google Scholar] [CrossRef]
- Tsvetkov, P.; Coy, S.; Petrova, B.; Dreishpoon, M.; Verma, A.; Abdusamad, M.; Rossen, J.; Joesch-Cohen, L.; Humeidi, R.; Spangler, R.D. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science 2022, 375, 1254–1261. [Google Scholar] [CrossRef]
- Mortenson, L.; Valentine, R.; Carnahan, J. An electron transport factor from Clostridium pasteurianum. Biochem. Biophys. Res. Commun. 1962, 7, 448–452. [Google Scholar] [CrossRef]
- Venkateswara Rao, P.; Holm, R. Synthetic analogues of the active sites of iron—Sulfur proteins. Chem. Rev. 2004, 104, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Cammack, R. Evolution and diversity in the iron-sulphur proteins. Chem. Scr. 1983, 21, 87–95. [Google Scholar]
- Hall, D.O.; Cammack, R.; Rao, K.K. The iron-sulphur proteins: Evolution of a ubiquitous protein from model systems to higher organisms. Orig. Life 1974, 5, 363–386. [Google Scholar] [CrossRef]
- Romero Romero, M.L.; Rabin, A.; Tawfik, D.S. Functional proteins from short peptides: Dayhoff’s hypothesis turns 50. Angew. Chem. Int. Ed. 2016, 55, 15966–15971. [Google Scholar] [CrossRef] [PubMed]
- Eck, R.V.; Dayhoff, M.O. Evolution of the structure of ferredoxin based on living relics of primitive amino acid sequences. Science 1966, 152, 363–366. [Google Scholar] [CrossRef]
- Brabender, M.; Henriques Pereira, D.P.; Mrnjavac, N.; Schlikker, M.L.; Kimura, Z.I.; Sucharitakul, J.; Kleinermanns, K.; Tüysüz, H.; Buckel, W.; Preiner, M.; et al. Ferredoxin reduction by hydrogen with iron functions as an evolutionary precursor of flavin-based electron bifurcation. Proc. Natl. Acad. Sci. USA 2024, 121, e2318969121. [Google Scholar] [CrossRef] [PubMed]
- King, S.J.; Jerkovic, A.; Brown, L.J.; Petroll, K.; Willows, R.D. Synthetic biology for improved hydrogen production in Chlamydomonas reinhardtii. Microb. Biotechnol. 2022, 15, 1946–1965. [Google Scholar] [CrossRef]
- Koo, J.; Cha, Y. Investigation of the ferredoxin’s influence on the anaerobic and aerobic, enzymatic H2 production. Front. Bioeng. Biotechnol. 2021, 9, 641305. [Google Scholar] [CrossRef]
- Chiliza, Z.E.; Martínez-Oyanedel, J.; Syed, K. An overview of the factors playing a role in cytochrome P450 monooxygenase and ferredoxin interactions. Biophys. Rev. 2020, 12, 1217–1222. [Google Scholar] [CrossRef]
- He, J.; Liu, X.; Li, C. Engineering Electron Transfer Pathway of Cytochrome P450s. Molecules 2024, 29, 2480. [Google Scholar] [CrossRef] [PubMed]
- Sepp, S.-K.; Vasar, M.; Davison, J.; Oja, J.; Anslan, S.; Al-Quraishy, S.; Bahram, M.; Bueno, C.G.; Cantero, J.J.; Fabiano, E.C. Global diversity and distribution of nitrogen-fixing bacteria in the soil. Front. Plant Sci. 2023, 14, 1100235. [Google Scholar] [CrossRef] [PubMed]
- Addison, H.; Glatter, T.; KA Hochberg, G.; Rebelein, J.G. Two distinct ferredoxins are essential for nitrogen fixation by the iron nitrogenase in Rhodobacter capsulatus. mBio 2024, 15, e03314–e03323. [Google Scholar] [CrossRef]
- Takahashi, H.; Kopriva, S.; Giordano, M.; Saito, K.; Hell, R. Sulfur assimilation in photosynthetic organisms: Molecular functions and regulations of transporters and assimilatory enzymes. Annu. Rev. Plant Biol. 2011, 62, 157–184. [Google Scholar] [CrossRef]
- Kim, J.Y.; Nakayama, M.; Toyota, H.; Kurisu, G.; Hase, T. Structural and mutational studies of an electron transfer complex of maize sulfite reductase and ferredoxin. J. Biochem. 2016, 160, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Cai, K.; Tonelli, M.; Frederick, R.O.; Markley, J.L. Human mitochondrial ferredoxin 1 (FDX1) and ferredoxin 2 (FDX2) both bind cysteine desulfurase and donate electrons for iron–sulfur cluster biosynthesis. Biochemistry 2017, 56, 487–499. [Google Scholar] [CrossRef]
- Gao, F. Iron–sulfur cluster biogenesis and iron homeostasis in cyanobacteria. Front. Microbiol. 2020, 11, 165. [Google Scholar] [CrossRef]
- Mutter, A.C.; Tyryshkin, A.M.; Campbell, I.J.; Poudel, S.; Bennett, G.N.; Silberg, J.J.; Nanda, V.; Falkowski, P.G. De novo design of symmetric ferredoxins that shuttle electrons in vivo. Proc. Natl. Acad. Sci. USA 2019, 116, 14557–14562. [Google Scholar] [CrossRef] [PubMed]
- Cerone, M.; Smith, T.K. Desaturases: Structural and mechanistic insights into the biosynthesis of unsaturated fatty acids. Iubmb. Life 2022, 74, 1036–1051. [Google Scholar] [CrossRef]
- Hannemann, F.; Bichet, A.; Ewen, K.M.; Bernhardt, R. Cytochrome P450 systems--biological variations of electron transport chains. Biochim. Biophys. Acta 2007, 1770, 330–344. [Google Scholar] [CrossRef]
- Saridakis, E.; Giastas, P.; Efthymiou, G.; Thoma, V.; Moulis, J.-M.; Kyritsis, P.; Mavridis, I.M. Insight into the protein and solvent contributions to the reduction potentials of [4Fe–4S] 2+/+ clusters: Crystal structures of the Allochromatium vinosum ferredoxin variants C57A and V13G and the homologous Escherichia coli ferredoxin. JBIC J. Biol. Inorg. Chem. 2009, 14, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Pochapsky, T.C.; Jain, N.U.; Kuti, M.; Lyons, T.A.; Heymont, J. A refined model for the solution structure of oxidized putidaredoxin. Biochemistry 1999, 38, 4681–4690. [Google Scholar] [CrossRef]
- Gilep, A.; Varaksa, T.; Bukhdruker, S.; Kavaleuski, A.; Ryzhykau, Y.; Smolskaya, S.; Sushko, T.; Tsumoto, K.; Grabovec, I.; Kapranov, I.; et al. Structural insights into 3Fe-4S ferredoxins diversity in M. tuberculosis highlighted by a first redox complex with P450. Front. Mol. Biosci. 2022, 9, 1100032. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, K.; Okada, T.; Kakuta, Y.; Takahashi, Y. Atomic resolution structures of oxidized [4Fe-4S] ferredoxin from Bacillus thermoproteolyticus in two crystal forms: Systematic distortion of [4Fe-4S] cluster in the protein. J. Mol. Biol. 2002, 315, 1155–1166. [Google Scholar] [CrossRef] [PubMed]
- Macedo-Ribeiro, S.; Martins, B.M.; Pereira, P.B.; Buse, G.; Huber, R.; Soulimane, T. New insights into the thermostability of bacterial ferredoxins: High-resolution crystal structure of the seven-iron ferredoxin from Thermus thermophilus. JBIC J. Biol. Inorg. Chem. 2001, 6, 663–674. [Google Scholar] [CrossRef] [PubMed]
- Aono, S.; Bentrop, D.; Bertini, I.; Cosenza, G.; Luchinat, C. Solution structure of an artificial Fe8S8 ferredoxin: The D13C variant of Bacillus schlegelii Fe7S8 ferredoxin. Eur. J. Biochem. 1998, 258, 502–514. [Google Scholar] [CrossRef]
- Beinert, H.; Holm, R.H.; Münck, E. Iron-sulfur clusters: Nature’s modular, multipurpose structures. Science 1997, 277, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. Iron–sulfur protein folds, iron–sulfur chemistry, and evolution. JBIC J. Biol. Inorg. Chem. 2008, 13, 157–170. [Google Scholar] [CrossRef]
- Imlay, J.A. Iron-sulphur clusters and the problem with oxygen. Mol. Microbiol. 2006, 59, 1073–1082. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, B.; Golbeck, J.H. Understanding of the binding interface between PsaC and the PsaA/PsaB heterodimer in photosystem I. Biochemistry 2009, 48, 5405–5416. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, K.-L.; Yu, L.-K.; Chen, Y.-H.; Cheng, Y.-H.; Hsieh, Y.-C.; Ke, S.-c.; Hung, K.-W.; Chen, C.-J.; Huang, T.-h. FeoC from Klebsiella pneumoniae contains a [4Fe-4S] cluster. J. Bacteriol. 2013, 195, 4726–4734. [Google Scholar] [CrossRef]
- Campbell, I.J.; Bennett, G.N.; Silberg, J.J. Evolutionary relationships between low potential ferredoxin and flavodoxin electron carriers. Front. Energy Res. 2019, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Yasunobu, K.T.; Tanaka, M. The evolution of iron-sulfur protein containing organisms. Syst. Zool. 1973, 22, 570–589. [Google Scholar] [CrossRef]
- Harayama, S.; Polissi, A.; Rekik, M. Divergent evolution of chloroplast-type ferredoxins. FEBS Lett. 1991, 285, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, M.; Guerlesquin, F. Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol. Lett. 1988, 54, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J. The evolution of ferredoxins. Trends Ecol. Evol. 1988, 3, 222–226. [Google Scholar] [CrossRef] [PubMed]
- Ngcobo, P.E.; Nkosi, B.V.Z.; Chen, W.; Nelson, D.R.; Syed, K. Evolution of cytochrome P450 enzymes and their redox partners in Archaea. Int. J. Mol. Sci. 2023, 24, 4161. [Google Scholar] [CrossRef] [PubMed]
- Nkosi, B.V.Z.; Padayachee, T.; Gront, D.; Nelson, D.R.; Syed, K. Contrasting health effects of Bacteroidetes and Firmicutes lies in their genomes: Analysis of P450s, ferredoxins, and secondary metabolite clusters. Int. J. Mol. Sci. 2022, 23, 5057. [Google Scholar] [CrossRef]
- Nixon, J.E.; Wang, A.; Field, J.; Morrison, H.G.; McArthur, A.G.; Sogin, M.L.; Loftus, B.J.; Samuelson, J. Evidence for lateral transfer of genes encoding ferredoxins, nitroreductases, NADH oxidase, and alcohol dehydrogenase 3 from anaerobic prokaryotes to Giardia lamblia and Entamoeba histolytica. Eukaryot. Cell 2002, 1, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, F.; Griffig, J.; Oesterhelt, D. The fdx gene encoding the [2Fe-2S] ferredoxin of Halobacterium salinarium (H. halobium). Mol. Gen. Genet. MGG 1993, 239, 66–71. [Google Scholar] [CrossRef]
- Ishizaka, M.; Chen, M.; Narai, S.; Tanaka, Y.; Ose, T.; Horitani, M.; Yao, M. Quick and Spontaneous Transformation between [3Fe-4S] and [4Fe-4S] Iron-Sulfur Clusters in the tRNA-Thiolation Enzyme TtuA. Int. J. Mol. Sci. 2023, 24, 833. [Google Scholar] [CrossRef] [PubMed]
- Ricagno, S.; de Rosa, M.; Aliverti, A.; Zanetti, G.; Bolognesi, M. The crystal structure of FdxA, a 7Fe ferredoxin from Mycobacterium smegmatis. Biochem. Biophys. Res. Commun. 2007, 360, 97–102. [Google Scholar] [CrossRef]
- Beinert, H.; Emptage, M.H.; Dreyer, J.-L.; Scott, R.A.; Hahn, J.E.; Hodgson, K.O.; Thomson, A.J. Iron-sulfur stoichiometry and structure of iron-sulfur clusters in three-iron proteins: Evidence for [3Fe-4S] clusters. Proc. Natl. Acad. Sci. USA 1983, 80, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Armengaud, J.; Gaillard, J.; Forest, E.; Jouanneau, Y. Characterization of a 2[4Fe-4S] ferredoxin obtained by chemical insertion of the Fe-S clusters into the apoferredoxin II from Rhodobacter capsulatus. Eur. J. Biochem. 1995, 231, 396–404. [Google Scholar] [CrossRef] [PubMed]



| Subtype Number | Nomenclature | Cysteine Spacing Signature (CSS) |
|---|---|---|
| 2Fe-2S | ||
| Subtype 1 | 2Fe-2SST1 | CX5CX2CX36C |
| Subtype 2 | 2Fe-2SST2 | CX5CX2CX37C |
| Subtype 3 | 2Fe-2SST3 | CX4CX2CX29C |
| Subtype 4 | 2Fe-2SST4 | CX5CX2CX35C |
| Subtype 5 | 2Fe-2SST5 | CX5CX2CX38C |
| Subtype 6 | 2Fe-2SST6 | CX4CX2CX34C |
| Subtype 7 | 2Fe-2SST7 | CX4CX2CX51C |
| Subtype 8 | 2Fe-2SST8 | CX4CX2CX31C |
| Subtype 9 | 2Fe-2SST9 | CX4CX2CX33C |
| Subtype 10 | 2Fe-2SST10 | CX5CX2CX39C |
| Subtype 11 | 2Fe-2SST11 | CX4CX2CX35C |
| Subtype 12 | 2Fe-2SST12 | CX4CX2CX25C |
| Subtype 13 | 2Fe-2SST13 | CX7CX34CX3C |
| Subtype 14 | 2Fe-2SST14 | CX4CX29C |
| Subtype 15 | 2Fe-2SST15 | CX7CX29C |
| Subtype 16 | 2Fe-2SST16 | CX7CX35C |
| Subtype 17 | 2Fe-2SST17 | CX5CX2CX33C |
| Subtype 18 | 2Fe-2SST18 | CX5CX2CX34C |
| Subtype 19 | 2Fe-2SST19 | CX10CX31CX3C |
| Subtype 20 | 2Fe-2SST20 | CX5CX2CX32C |
| Subtype 21 | 2Fe-2SST21 | CX4CX31CX3C |
| Subtype 22 | 2Fe-2SST22 | CX2CX41CX3C |
| Subtype 23 | 2Fe-2SST23 | CX7CX38CX3C |
| Subtype 24 | 2Fe-2SST24 | CX4CX2CX30C |
| Subtype 25 | 2Fe-2SST25 | CX5CX2CX30C |
| Subtype 26 | 2Fe-2SST26 | CX12CX30CX3C |
| Subtype 27 | 2Fe-2SST27 | CX12CX31CX3C |
| Subtype 28 | 2Fe-2SST28 | CX8CX44CX3C |
| Subtype 29 | 2Fe-2SST29 | CX8CX33CX3C |
| Subtype 30 | 2Fe-2SST30 | CX8CX32CX3C |
| Subtype 31 | 2Fe-2SST31 | CX4CX36CX3C |
| Subtype 32 | 2Fe-2SST32 | CX8CX38CX3C |
| Subtype 33 | 2Fe-2SST33 | CX8CX39CX3C |
| Subtype 34 | 2Fe-2SST34 | CX9CX33CX3C |
| Subtype 35 | 2Fe-2SST35 | CX12CX33CX3C |
| Subtype 36 | 2Fe-2SST36 | CX3CX1CX38C |
| Subtype 37 | 2Fe-2SST37 | CX12CX32CX3C |
| Subtype 38 | 2Fe-2SST38 | CX4CX2CX28C |
| Subtype 39 | 2Fe-2SST39 | CX4CX2CX46C |
| Subtype 40 | 2Fe-2SST40 | CX4CX2CX49C |
| Subtype 41 | 2Fe-2SST41 | CX4CX2CX45C |
| Subtype 42 | 2Fe-2SST42 | CX4CX2CX65C |
| Subtype 43 | 2Fe-2SST43 | CX4CX2CX50C |
| Subtype 44 | 2Fe-2SST44 | CX4CX2CX47C |
| Subtype 45 | 2Fe-2SST45 | CX4CX2CX48C |
| Subtype 46 | 2Fe-2SST46 | CX5CX2CX52C |
| Subtype 47 | 2Fe-2SST47 | CX5CX2CX31C |
| Subtype 48 | 2Fe-2SST48 | CX5CX2CX28C |
| Subtype 49 | 2Fe-2SST49 | CX5CX2CX27C |
| Subtype 50 | 2Fe-2SST50 | CX5CX2CX82C |
| Subtype 51 | 2Fe-2SST51 | CX5CX2CX29C |
| Subtype 52 | 2Fe-2SST52 | CX4CX2CX32C |
| Subtype 53 | 2Fe-2SST53 | CX5CX2CX42C |
| Subtype 54 | 2Fe-2SST54 | CX4CX2CX22C |
| Subtype 55 | 2Fe-2SST55 | CX4CX2CX27C |
| Subtype 56 | 2Fe-2SST56 | CX4CX2CX64C |
| Subtype 57 | 2Fe-2SST57 | CX4CX2CX39C |
| Subtype 58 | 2Fe-2SST58 | CX4CX2CX53C |
| 3Fe-4S | ||
| Subtype 1 | 3Fe-4SST1 | CX5CX38CP |
| Subtype 2 | 3Fe-4SST2 | CX5CX37CP |
| Subtype 3 | 3Fe-4SST3 | CX5CX36CP |
| Subtype 4 | 3Fe-4SST4 | CX5CX40CP |
| Subtype 5 | 3Fe-4SST5 | CX5CX36CP |
| Subtype 6 | 3Fe-4SST6 | CX5CX35CP |
| Subtype 7 | 3Fe-4SST7 | CX5CX49CP |
| Subtype 8 | 3Fe-4SST8 | CX5CX32CP |
| Subtype 9 | 3Fe-4SST9 | CX5CX53CP |
| Subtype 10 | 3Fe-4SST10 | CX5CX54CP |
| Subtype 11 | 3Fe-4SST11 | CX5CX47CP |
| Subtype 12 | 3Fe-4SST12 | CX5CX46CP |
| Subtype 13 | 3Fe-4SST13 | CX5CX43CP |
| Subtype 14 | 3Fe-4SST14 | CX5CX56CP |
| Subtype 15 | 3Fe-4SST15 | CX5CX50CP |
| Subtype 16 | 3Fe-4SST16 | CX5CX52CP |
| Subtype 17 | 3Fe-4SST17 | CX7CX53CP |
| Subtype 18 | 3Fe-4SST18 | CX5CX48CP |
| Subtype 19 | 3Fe-4SST19 | CX5CX66CP |
| Subtype 20 | 3Fe-4SST20 | CX5CX58CP |
| Subtype 21 | 3Fe-4SST21 | CX5CX59CP |
| 4Fe-4S | ||
| Subtype 1 | 4Fe-4SST1 | CX5CX3CX33CP |
| Subtype 2 | 4Fe-4SST2 | CX2CX2CX43CP |
| Subtype 3 | 4Fe-4SST3 | CX2CX2CX45CP |
| Subtype 4 | 4Fe-4SST4 | CX2CX2CX37CP |
| Subtype 5 | 4Fe-4SST5 | CX2CX2CX44CP |
| Subtype 6 | 4Fe-4SST6 | CX2CX2CX39CP |
| Subtype 7 | 4Fe-4SST7 | CX2CX2CX36CP |
| Subtype 8 | 4Fe-4SST8 | CX2CX2CX34CP |
| Subtype 9 | 4Fe-4SST9 | CX2CX2CX38CP |
| Subtype 10 | 4Fe-4SST10 | CX5CX3CX32CP |
| Subtype 11 | 4Fe-4SST11 | CX5CX3CX30CP |
| Subtype 12 | 4Fe-4SST12 | CX5CX3CX31CP |
| Subtype 13 | 4Fe-4SST13 | CX2CX2CX48CP |
| Subtype 14 | 4Fe-4SST14 | CX2CX2CX47CP |
| Subtype 15 | 4Fe-4SST15 | CX3CX5CX32CP |
| 7Fe-8S | ||
| Subtype 1 | 7Fe-8SST1 | CX7CX3CPX17CX2CX2CX3CP * |
| Subtype 2 | 7Fe-8SST2 | CX5CX3CPX40CX2CX2CX3CP |
| Subtype 3 | 7Fe-8SST10 | CX3CX3CPX22CX2CX2CX3CP |
| Subtype 4 | 7Fe-8SST4 | CX10CX3CPX22CX2CX2CX3CP |
| Subtype 5 | 7Fe-8SST5 | CX5CX3CPX26CX2CX2CX3CP |
| Subtype 6 | 7Fe-8SST6 | CX5CX3CPX24CX2CX2CX3CP |
| Subtype 7 | 7Fe-8SST7 | CX10CX3CPX17CX2CX2CX3CP |
| Subtype 8 | 7Fe-8SST8 | CX5CX3CPX22CX2CX2CX3CP |
| Subtype 9 | 7Fe-8SST9 | CX5CX3CPX18CX2CX2CX3CP |
| 2[4Fe-4S] | ||
| Subtype 1 | 2[4Fe-4S]ST1 | CX2CX4CX3CX18CX2CX2CX3C |
| Subtype 2 | 2[4Fe-4S]ST2 | CX2CX2CX3CX18CX2CX8CX3C |
| Subtype 3 | 2[4Fe-4S]ST3 | CX2CX2CX3CX20CX2CX2CX3C |
| Subtype 4 | 2[4Fe-4S]ST4 | CX7CX2CX3CX23CX2CX2CX3C |
| Subtype 5 | 2[4Fe-4S]ST5 | CX2CX2CX3CX42CX2CX2CX3C |
| Subtype 6 | 2[4Fe-4S]ST6 | CX2CX2CX3CX18CX2CX7CX3C |
| Subtype 7 | 2[4Fe-4S]ST7 | CX2CX2CX3CX18CX2CX6CX3C |
| Subtype 8 | 2[4Fe-4S]ST8 | CX2CX2CX3CX24CX2CX2CX3C |
| Subtype 9 | 2[4Fe-4S]ST9 | CX2CX2CX3CX18CX2CX2CX3C |
| Subtype 10 | 2[4Fe-4S]ST10 | CX2CX2CX3CX21CX2CX2CX3C |
| Subtype 11 | 2[4Fe-4S]ST11 | CX2CX2CX3CX18CX3CX2CX3C |
| Subtype 12 | 2[4Fe-4S]ST12 | CX2CX2CX3CX28CX2CX2CX3C |
| Subtype 13 | 2[4Fe-4S]ST13 | CX2CX2CX3CX27CX2CX2CX3C |
| Subtype 14 | 2[4Fe-4S]ST14 | CX5CX2CX3CX20CX2CX2CX3C |
| Subtype 15 | 2[4Fe-4S]ST15 | CX2CX2CX3CX19CX2CX2CX3C |
| Subtype 16 | 2[4Fe-4S]ST16 | CX2CX2CX3CX40CX2CX2CX3C |
| Subtype 17 | 2[4Fe-4S]ST17 | CX2CX2CX3CX29CX2CX2CX3C |
| Subtype 18 | 2[4Fe-4S]ST18 | CX4CX2CX3CX18CX2CX2CX3C |
| Subtype 19 | 2[4Fe-4S]ST18 | CX3CX2CX3CX20CX2CX2CX3C |
| Subtype 20 | 2[4Fe-4S]ST20 | CX2CX2CX3CX17CX2CX2CX3C |
| Subtype 21 | 2[4Fe-4S]ST21 | CX3CX3CX3CX37CX1CX3CX3C |
| Subtype 22 | 2[4Fe-4S]ST22 | CX2CX2CX3CX26CX2CX2CX3C |
| Subtype 23 | 2[4Fe-4S]ST23 | CX2CX2CX3CX30CX2CX2CX3C |
| Subtype 24 | 2[4Fe-4S]ST24 | CX2CX2CX3CX33CX2CX2CX3C |
| Subtype 25 | 2[4Fe-4S]ST25 | CX2CX2CX3CX32CX2CX2CX3C |
| Subtype 26 | 2[4Fe-4S]ST26 | CX2CX2CX3CX23CX2CX2CX3C |
| Subtype 27 | 2[4Fe-4S]ST27 | CX2CX2CX3CX34CX2CX2CX3C |
| Subtype 28 | 2[4Fe-4S]ST28 | CX2CX2CX3CX14CX2CX2CX3C |
| Subtype 29 | 2[4Fe-4S]ST29 | CX2CX2CX3CX22CX2CX2CX3C |
| Subtype 30 | 2[4Fe-4S]ST30 | CX2CX2CX2CX38CX2CX2CX3C |
| Subtype 31 | 2[4Fe-4S]ST31 | CX4CX2CX3CX19CX2CX2CX3C |
| Subtype 32 | 2[4Fe-4S]ST32 | CX5CX2CX3CX19CX2CX2CX3C |
| Subtype 33 | 2[4Fe-4S]ST33 | CX2CX2CX3CX16CX2CX2CX3C |
| Subtype 34 | 2[4Fe-4S]ST34 | CX2CX2CX3CX38CX2CX2CX3C |
| Subtype 35 | 2[4Fe-4S]ST35 | CX3CX4CX3CX20CX2CX2CX3C |
| Subtype 36 | 2[4Fe-4S]ST36 | CX2CX4CX3CX21CX2CX2CX3C |
| 2[4Fe-4S]Alv | ||
| Subtype 1 | 2[4Fe-4S]AlvST1 | CX2CX2CX3CX18CX2CX8CX3CX3C |
| Subtype 2 | 2[4Fe-4S]AlvST2 | CX2CX2CX3CX39CX2CX2CX3CX3C |
| Subtype 3 | 2[4Fe-4S]AlvST3 | CX2CX2CX3CX43CX2CX2CX3CX3C |
| Subtype 4 | 2[4Fe-4S]AlvST4 | CX2CX2CX3CX42CX2CX2CX3CX3C |
| Subtype 5 | 2[4Fe-4S]AlvST5 | CX2CX2CX3CX40CX2CX2CX3CX3C |
| Subtype 6 | 2[4Fe-4S]AlvST6 | CX2CX2CX3CX38CX2CX2CX3CX3C |
| Subtype 7 | 2[4Fe-4S]AlvST7 | CX2CX2CX3CX46CX2CX2CX3CX3C |
| Subtype 8 | 2[4Fe-4S]AlvST8 | CX2CX2CX3CX44CX2CX2CX3CX3C |
| Subtype 9 | 2[4Fe-4S]AlvST9 | CX2CX2CX3CX30CX2CX2CX3CX3C |
| Subtype 10 | 2[4Fe-4S]AlvST10 | CX2CX2CX3CX19CX2CX2CX3CX3C |
| Subtype 11 | 2[4Fe-4S]AlvST11 | CX2CX2CX3CX52CX2CX8CX3CX3C |
| Subtype 12 | 2[4Fe-4S]AlvST12 | CX2CX2CX3CX51CX2CX8CX3CX3C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syed, K. Ferredoxins: Functions, Evolution, Potential Applications, and Challenges of Subtype Classification. Curr. Issues Mol. Biol. 2024, 46, 9659-9673. https://doi.org/10.3390/cimb46090574
Syed K. Ferredoxins: Functions, Evolution, Potential Applications, and Challenges of Subtype Classification. Current Issues in Molecular Biology. 2024; 46(9):9659-9673. https://doi.org/10.3390/cimb46090574
Chicago/Turabian StyleSyed, Khajamohiddin. 2024. "Ferredoxins: Functions, Evolution, Potential Applications, and Challenges of Subtype Classification" Current Issues in Molecular Biology 46, no. 9: 9659-9673. https://doi.org/10.3390/cimb46090574


_Kim.png)



