Expression of Tenascin-C Is Upregulated in the Early Stages of Radiation Pneumonitis/Fibrosis in a Novel Mouse Model
Abstract
1. Introduction
2. Materials and Methods
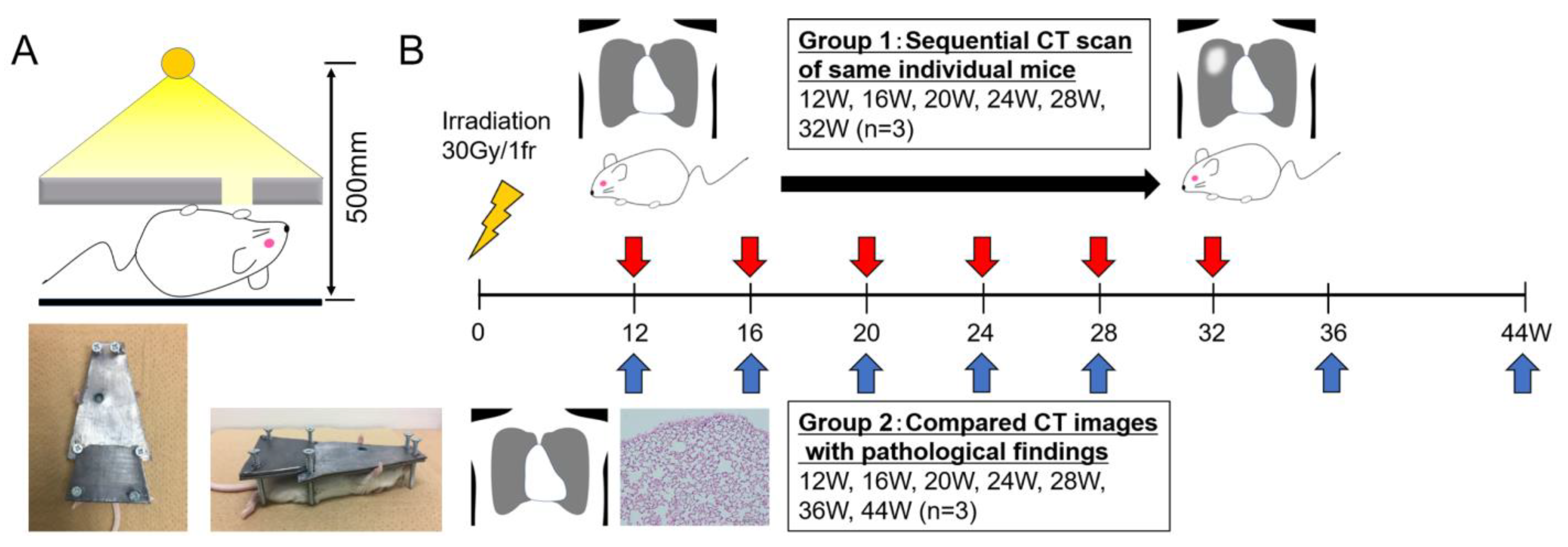
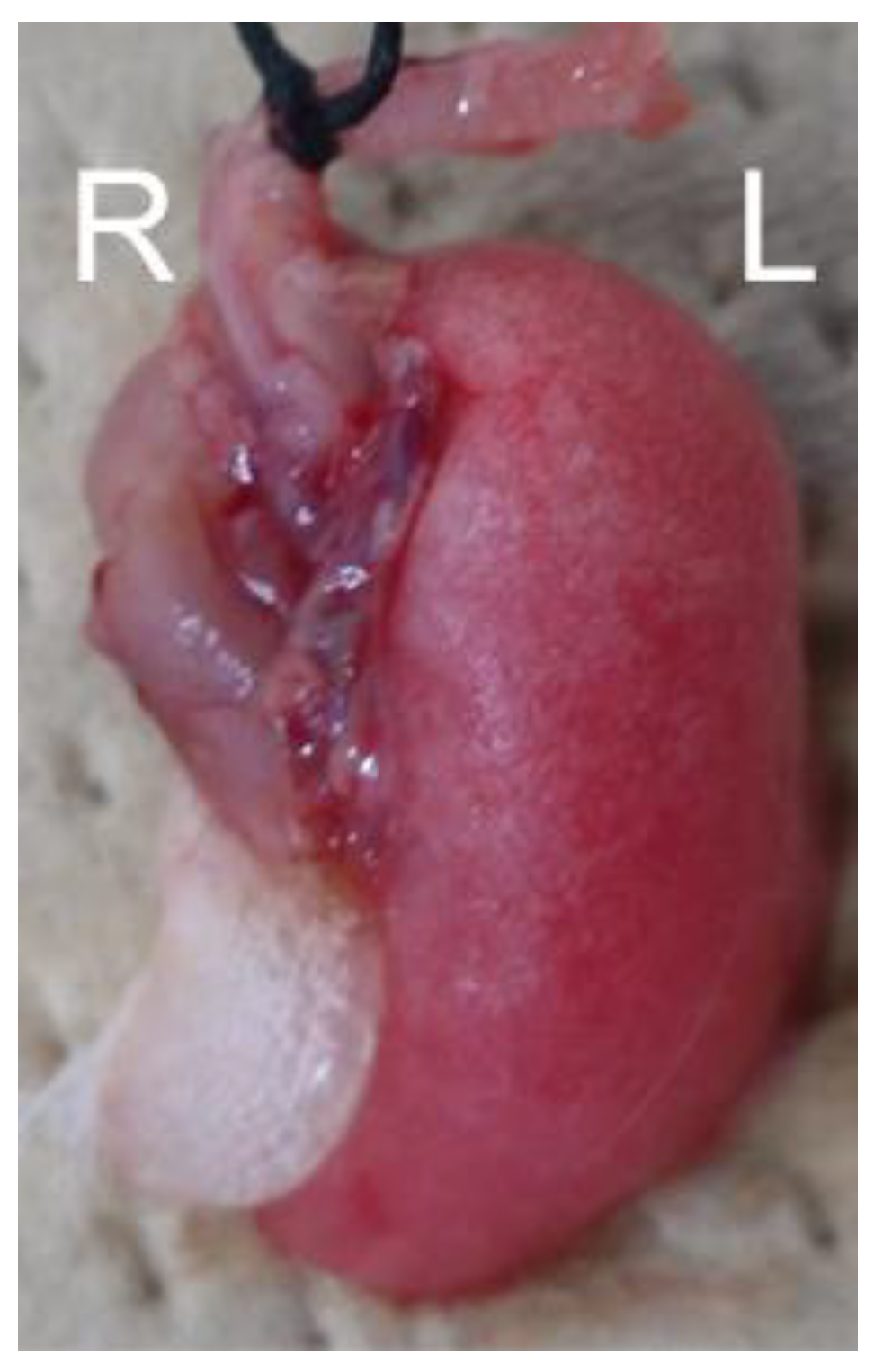
2.1. Mice and Pb Shielding Device
2.2. Irradiation and CT Scans
2.3. Histopathological Analysis of Lung Tissue
2.4. Quantification of TNC-Expressing Regions
3. Results
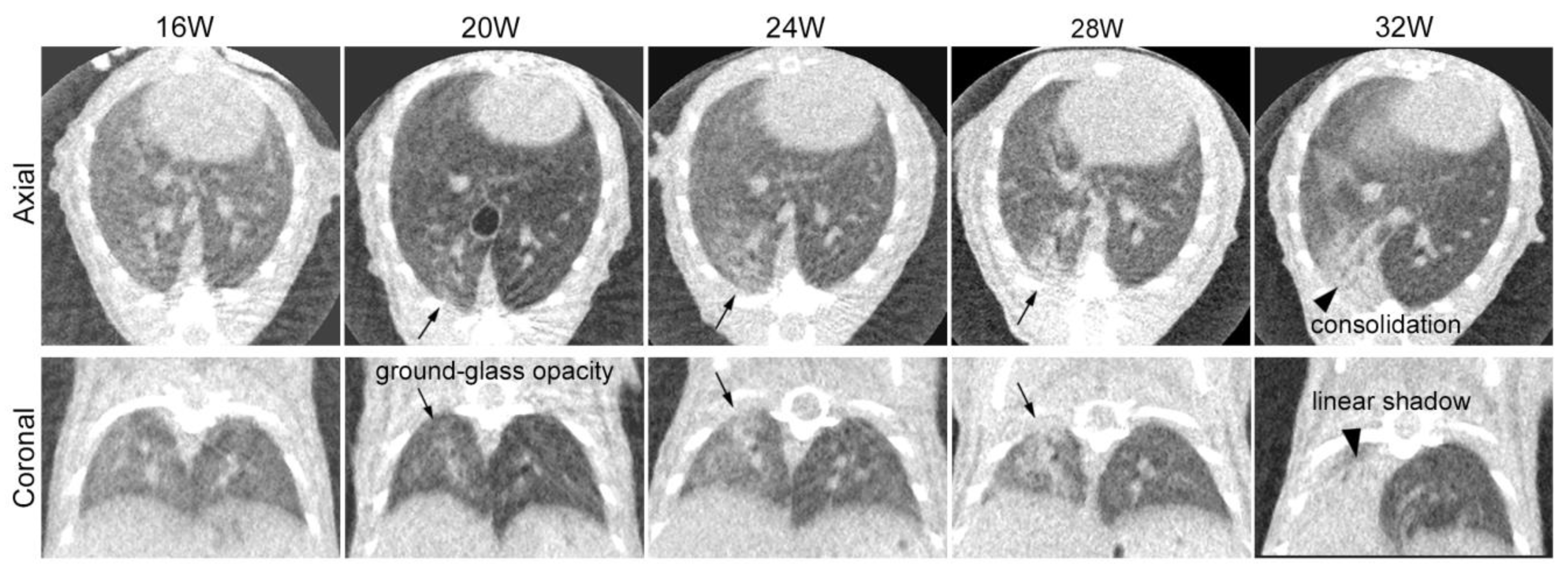
3.1. Sequential CT Image Changes in Focally Irradiated Lung of Mouse with 30 Gy/1fr
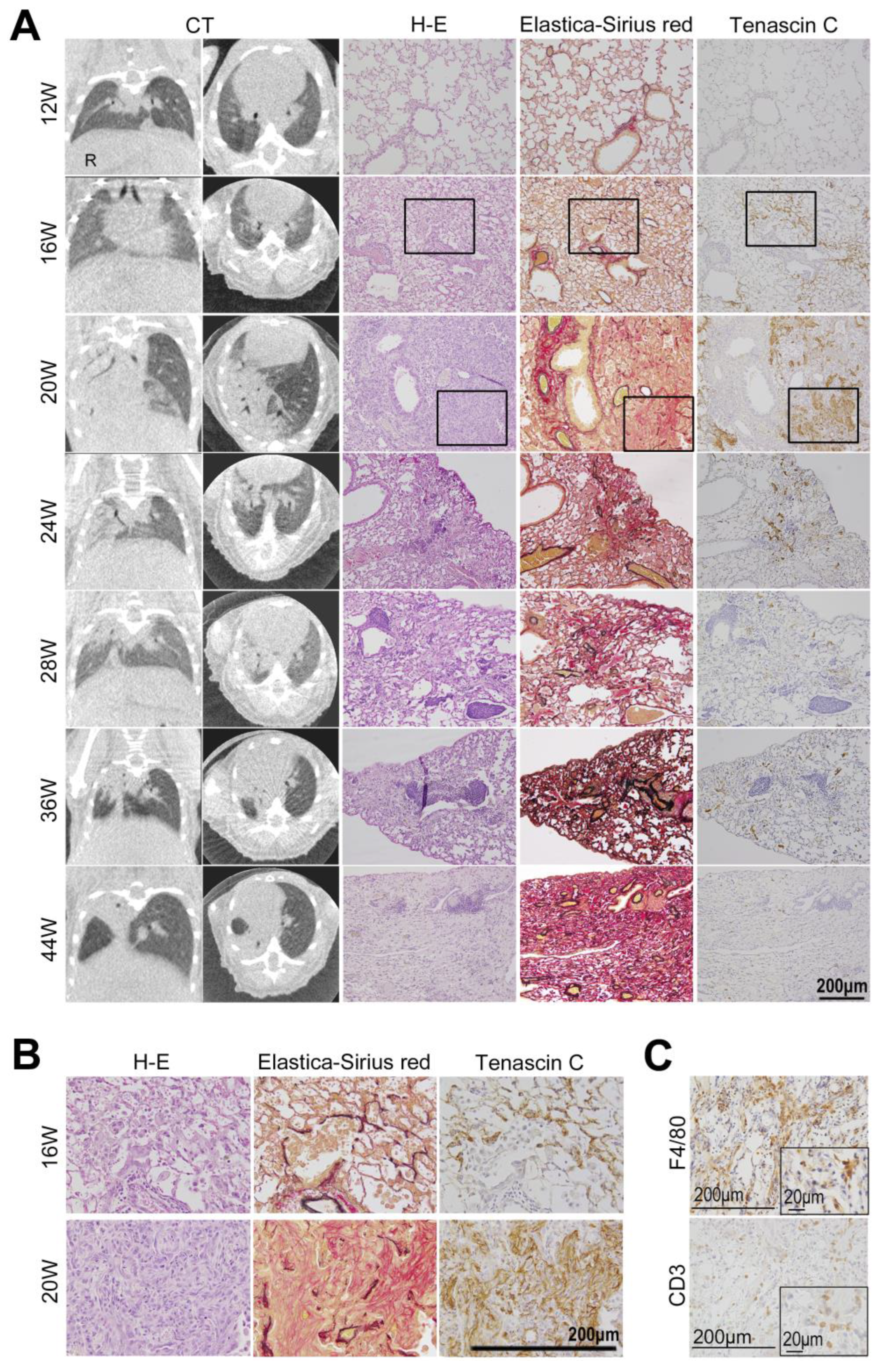
3.2. Comparison of CT Image Findings with Pathological Findings
4. Discussion
4.1. Partial Lung Irradiation Model for Mice
4.2. Sequential Changes in CT Images after Irradiation
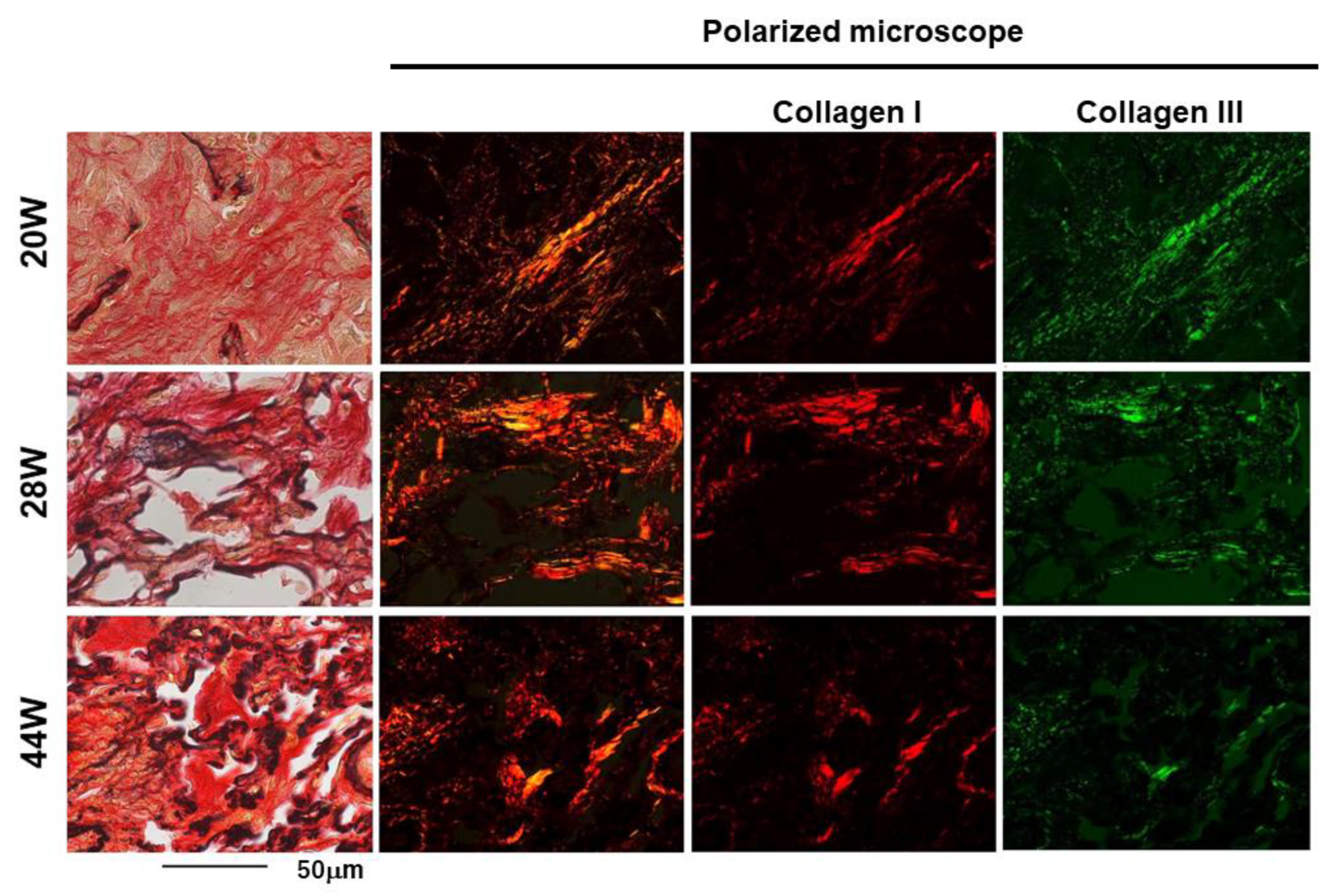
4.3. Comparison of CT Images and Histological Changes
4.4. TNC Is Upregulated at the Early Stage of Radiation Pneumonitis
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marks, L.; Yu, X.; Vujaskovic, Z.; Smalljr, W.; Folz, R.; Anscher, M. Radiation-induced lung injury. Semin. Radiat. Oncol. 2003, 13, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Rube, C.E.; Uthe, D.; Schmid, K.W.; Richter, K.D.; Wessel, J.; Schuck, A.; Willich, N.; Rube, C. Dose-dependent induction of transforming growth factor beta (TGF-beta) in the lung tissue of fibrosis-prone mice after thoracic irradiation. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Chen, Y.H.; Hatabu, H.; Mak, R.H.; Nishino, M. Radiographic patterns of symptomatic radiation pneumonitis in lung cancer patients: Imaging predictors for clinical severity and outcome. Lung Cancer 2020, 145, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Anscher, M.S.; Thrasher, B.; Zgonjanin, L.; Rabbani, Z.N.; Corbley, M.J.; Fu, K.; Sun, L.; Lee, W.C.; Ling, L.E.; Vujaskovic, Z. Small molecular inhibitor of transforming growth factor-beta protects against development of radiation-induced lung injury. Int. J. Radiat. Oncol. Biol. Phys. 2008, 71, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Beach, T.A.; Groves, A.M.; Williams, J.P.; Finkelstein, J.N. Modeling radiation-induced lung injury: Lessons learned from whole thorax irradiation. Int. J. Radiat. Biol. 2020, 96, 129–144. [Google Scholar] [CrossRef]
- Haston, C.K.; Travis, E.L. Murine susceptibility to radiation-induced pulmonary fibrosis is influenced by a genetic factor implicated in susceptibility to bleomycin-induced pulmonary fibrosis. Cancer Res. 1997, 57, 5286–5291. [Google Scholar] [PubMed]
- McDonald, S.; Rubin, P.; Chang, A.Y.; Penney, D.P.; Finkelstein, J.N.; Grossberg, S.; Feins, R.; Gregory, P.K. Pulmonary changes induced by combined mouse beta-interferon (rMuIFN-beta) and irradiation in normal mice—Toxic versus protective effects. Radiother. Oncol. 1993, 26, 212–218. [Google Scholar] [CrossRef]
- Stone, H.B.; Coleman, C.N.; Anscher, M.S.; McBride, W.H. Effects of radiation on normal tissue: Consequences and mechanisms. Lancet Oncol. 2003, 4, 529–536. [Google Scholar] [CrossRef]
- Giuranno, L.; Ient, J.; De Ruysscher, D.; Vooijs, M.A. Radiation-Induced Lung Injury (RILI). Front. Oncol. 2019, 9, 877. [Google Scholar] [CrossRef]
- Kasmann, L.; Dietrich, A.; Staab-Weijnitz, C.A.; Manapov, F.; Behr, J.; Rimner, A.; Jeremic, B.; Senan, S.; De Ruysscher, D.; Lauber, K.; et al. Radiation-induced lung toxicity-cellular and molecular mechanisms of pathogenesis, management, and literature review. Radiat. Oncol. 2020, 15, 214. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Tawara, I.; Yoshida, T. Tenascin-C in cardiac disease: A sophisticated controller of inflammation, repair, and fibrosis. Am. J. Physiol. Cell Physiol. 2020, 319, C781–C796. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.S.; Hussenet, T.; Langlois, B.; Orend, G. Advances in tenascin-C biology. Cell Mol. Life Sci. 2011, 68, 3175–3199. [Google Scholar] [CrossRef]
- Yonebayashi, S.; Tajiri, K.; Hara, M.; Saito, H.; Suzuki, N.; Sakai, S.; Kimura, T.; Sato, A.; Sekimoto, A.; Fujita, S.; et al. Generation of Transgenic Mice that Conditionally Overexpress Tenascin-C. Front. Immunol. 2021, 12, 620541. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T.; Tajiri, K.; Sato, A.; Sakai, S.; Wang, Z.; Yoshida, T.; Uede, T.; Hiroe, M.; Aonuma, K.; Ieda, M.; et al. Tenascin-C accelerates adverse ventricular remodelling after myocardial infarction by modulating macrophage polarization. Cardiovasc. Res. 2019, 115, 614–624. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ospelt, C.; Gay, S.; Midwood, K.S. Location, location, location: How the tissue microenvironment affects inflammation in RA. Nat. Rev. Rheumatol. 2021, 17, 195–212. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, N.; Hashizume, R.; Kanayama, K.; Hara, M.; Suzuki, Y.; Nishioka, T.; Hiroe, M.; Yoshida, T.; Imanaka-Yoshida, K. Tenascin-C may accelerate cardiac fibrosis by activating macrophages via the integrin αVβ3/nuclear factor-κB/interleukin-6 axis. Hypertension 2015, 66, 757–766. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Wang, W.; Morales-Nebreda, L.; Feng, G.; Wu, M.; Zhou, X.; Lafyatis, R.; Lee, J.; Hinchcliff, M.; Feghali-Bostwick, C.; et al. Tenascin-C drives persistence of organ fibrosis. Nat. Commun. 2016, 7, 11703. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K. Tenascin-C in Heart Diseases-The Role of Inflammation. Int. J. Mol. Sci. 2021, 22, 5828. [Google Scholar] [CrossRef]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal. 2009, 3, 287–310. [Google Scholar] [CrossRef]
- Carey, W.A.; Taylor, G.D.; Dean, W.B.; Bristow, J.D. Tenascin-C deficiency attenuates TGF-ss-mediated fibrosis following murine lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L785–L793. [Google Scholar] [CrossRef]
- Estany, S.; Vicens-Zygmunt, V.; Llatjós, R.; Montes, A.; Penín, R.; Escobar, I.; Xaubet, A.; Santos, S.; Manresa, F.; Dorca, J.; et al. Lung fibrotic tenascin-C upregulation is associated with other extracellular matrix proteins and induced by TGFβ1. BMC Pulm. Med. 2014, 14, 120. [Google Scholar] [CrossRef] [PubMed]
- Toyomasu, Y.; Matsui, K.; Omori, K.; Takada, A.; Imanaka-Yoshida, K.; Tawara, I.; Shimamoto, A.; Takao, M.; Kobayashi, H.; Tomaru, A.; et al. Tenascin C in radiation-induced lung damage: Pathological expression and serum level elevation. Thorac. Cancer 2022, 13, 2904–2907. [Google Scholar] [CrossRef]
- Namba, T.; Tsutsui, H.; Tagawa, H.; Takahashi, M.; Saito, K.; Kozai, T.; Usui, M.; Imanaka-Yoshida, K.; Imaizumi, T.; Takeshita, A. Regulation of fibrillar collagen gene expression and protein accumulation in volume-overloaded cardiac hypertrophy. Circulation 1997, 95, 2448–2454. [Google Scholar] [CrossRef]
- Imanaka-Yoshida, K.; Hiroe, M.; Nishikawa, T.; Ishiyama, S.; Shimojo, T.; Ohta, Y.; Sakakura, T.; Yoshida, T. Tenascin-C modulates adhesion of cardiomyocytes to extracellular matrix during tissue remodeling after myocardial infarction. Lab. Investig. A J. Tech. Methods Pathol. 2001, 81, 1015–1024. [Google Scholar] [CrossRef]
- Shaikh, P.M.; Singh, S.A.; Alite, F.; Vargo, J.A.; Emami, B.; Wu, M.J.; Jacobson, G.; Bakalov, V.; Small, W., Jr.; Dahshan, B.; et al. Radiation Toxicity in Patients With Collagen Vascular Disease: A Meta-Analysis of Case-Control Studies. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 1214–1226. [Google Scholar] [CrossRef] [PubMed]
- Arroyo-Hernández, M.; Maldonado, F.; Lozano-Ruiz, F.; Muñoz-Montaño, W.; Nuñez-Baez, M.; Arrieta, O. Radiation-induced lung injury: Current evidence. BMC Pulm. Med. 2021, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Machtay, M.; Scherpereel, A.; Santiago, J.; Lee, J.; McDonough, J.; Kinniry, P.; Arguiri, E.; Shuvaev, V.V.; Sun, J.; Cengel, K.; et al. Systemic polyethylene glycol-modified (PEGylated) superoxide dismutase and catalase mixture attenuates radiation pulmonary fibrosis in the C57/bl6 mouse. Radiother. Oncol. 2006, 81, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Hong, Z.Y.; Nam, J.K.; Lee, H.J.; Jang, J.; Yoo, R.J.; Lee, Y.J.; Lee, C.Y.; Kim, K.H.; Park, S.; et al. A Hypoxia-Induced Vascular Endothelial-to-Mesenchymal Transition in Development of Radiation-Induced Pulmonary Fibrosis. Clin. Cancer Res. 2015, 21, 3716–3726. [Google Scholar] [CrossRef]
- Granton, P.V.; Dubois, L.; van Elmpt, W.; van Hoof, S.J.; Lieuwes, N.G.; De Ruysscher, D.; Verhaegen, F. A longitudinal evaluation of partial lung irradiation in mice by using a dedicated image-guided small animal irradiator. Int. J. Radiat. Oncol. Biol. Phys. 2014, 90, 696–704. [Google Scholar] [CrossRef]
- Hong, Z.Y.; Lee, C.G.; Shim, H.S.; Lee, E.J.; Song, K.H.; Choi, B.W.; Cho, J.; Story, M.D. Time, Dose, and Volume Responses in a Mouse Pulmonary Injury Model Following Ablative Irradiation. Lung 2016, 194, 81–90. [Google Scholar] [CrossRef]
- Lavigne, J.; Suissa, A.; Verger, N.; Dos Santos, M.; Benadjaoud, M.; Mille-Hamard, L.; Momken, I.; Soysouvanh, F.; Buard, V.; Guipaud, O.; et al. Lung Stereotactic Arc Therapy in Mice: Development of Radiation Pneumopathy and Influence of HIF-1alpha Endothelial Deletion. Int. J. Radiat. Oncol. Biol. Phys. 2019, 104, 279–290. [Google Scholar] [CrossRef] [PubMed]
- Soysouvanh, F.; Benadjaoud, M.A.; Dos Santos, M.; Mondini, M.; Lavigne, J.; Bertho, A.; Buard, V.; Tarlet, G.; Adnot, S.; Deutsch, E.; et al. Stereotactic Lung Irradiation in Mice Promotes Long-Term Senescence and Lung Injury. Int. J. Radiat. Oncol. Biol. Phys. 2020, 106, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Kang, G.Y.; Jeon, S.; Kim, J.M.; Park, Y.N.; Cho, J.; Lee, Y.S. Identification of molecular signatures involved in radiation-induced lung fibrosis. J. Mol. Med. 2019, 97, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Videtic, G.M.M.; Reddy, C.A.; Woody, N.M.; Stephans, K.L. Ten-Year Experience in Implementing Single-Fraction Lung SBRT for Medically Inoperable Early-Stage Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2021, 111, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Berman, A.T. Radiation Pneumonitis: Old Problem, New Tricks. Cancers 2018, 10, 222. [Google Scholar] [CrossRef]
- Keffer, S.; Guy, C.L.; Weiss, E. Fatal Radiation Pneumonitis: Literature Review and Case Series. Adv. Radiat. Oncol. 2020, 5, 238–249. [Google Scholar] [CrossRef]
- Fajardo, L.F. The pathology of ionizing radiation as defined by morphologic patterns. Acta Oncol. 2005, 44, 13–22. [Google Scholar] [CrossRef]
- Jin, H.; Yoo, Y.; Kim, Y.; Kim, Y.; Cho, J.; Lee, Y.S. Radiation-Induced Lung Fibrosis: Preclinical Animal Models and Therapeutic Strategies. Cancers 2020, 12, 1561. [Google Scholar] [CrossRef]
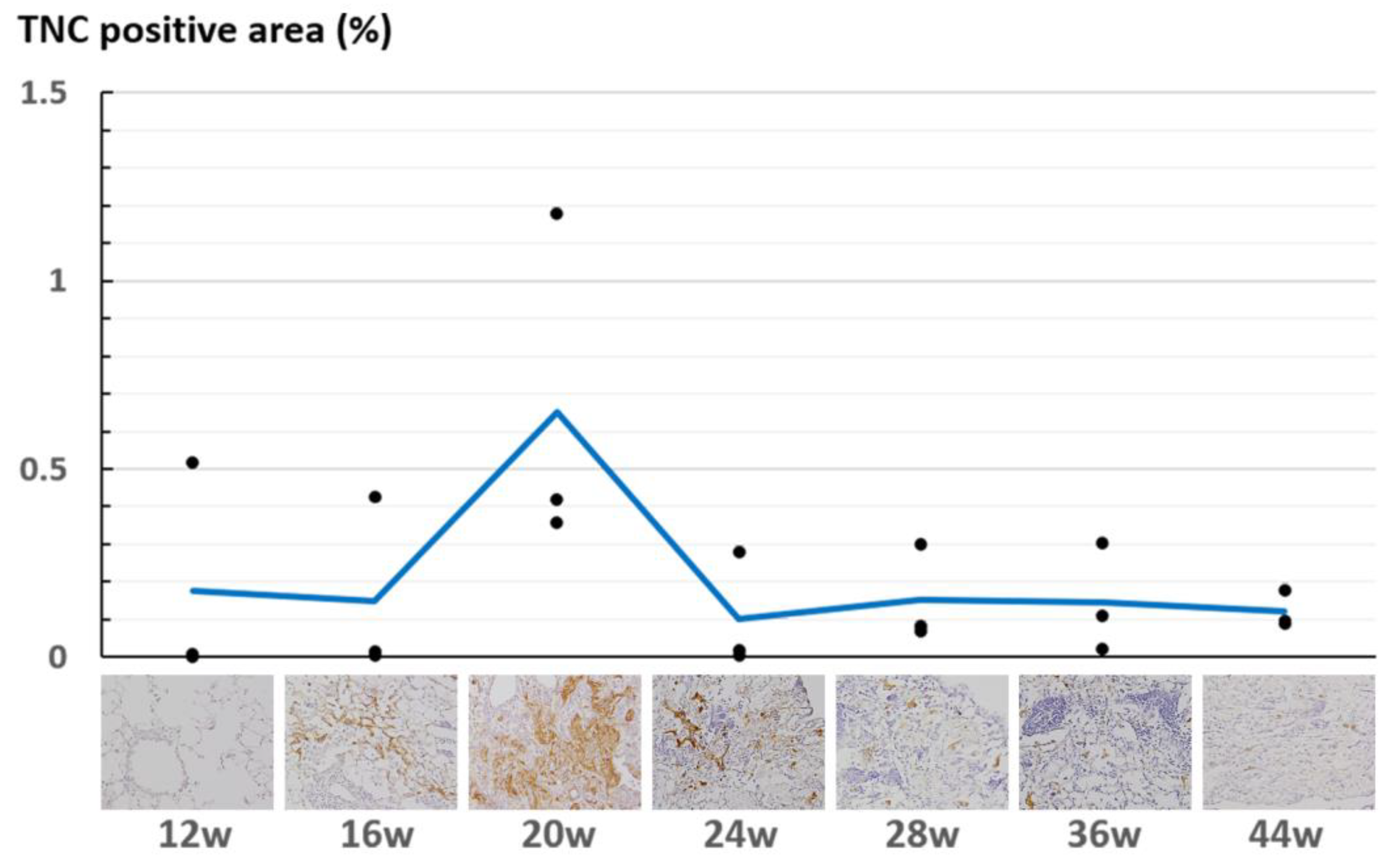
| Post Irradiation | 12 w | 16 w | 20 w | 24 w | 28 w | 36 w | 44 w | ||
|---|---|---|---|---|---|---|---|---|---|
| Computed Tomography | − | − | Radiation pneumonia | Radiation fibrsis | |||||
| Histology | Inflammation | − | + | + + + | + + | ± | − | − | |
| Fibrosis | − | − | + | + | + + | + + + | + + + | ||
| Tenascin-C | − | + | + + + | + + | ± | − | − | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omori, K.; Takada, A.; Toyomasu, Y.; Tawara, I.; Shintoku, C.; Imanaka-Yoshida, K.; Sakuma, H.; Nomoto, Y. Expression of Tenascin-C Is Upregulated in the Early Stages of Radiation Pneumonitis/Fibrosis in a Novel Mouse Model. Curr. Issues Mol. Biol. 2024, 46, 9674-9685. https://doi.org/10.3390/cimb46090575
Omori K, Takada A, Toyomasu Y, Tawara I, Shintoku C, Imanaka-Yoshida K, Sakuma H, Nomoto Y. Expression of Tenascin-C Is Upregulated in the Early Stages of Radiation Pneumonitis/Fibrosis in a Novel Mouse Model. Current Issues in Molecular Biology. 2024; 46(9):9674-9685. https://doi.org/10.3390/cimb46090575
Chicago/Turabian StyleOmori, Kazuki, Akinori Takada, Yutaka Toyomasu, Isao Tawara, Chihiro Shintoku, Kyoko Imanaka-Yoshida, Hajime Sakuma, and Yoshihito Nomoto. 2024. "Expression of Tenascin-C Is Upregulated in the Early Stages of Radiation Pneumonitis/Fibrosis in a Novel Mouse Model" Current Issues in Molecular Biology 46, no. 9: 9674-9685. https://doi.org/10.3390/cimb46090575
APA StyleOmori, K., Takada, A., Toyomasu, Y., Tawara, I., Shintoku, C., Imanaka-Yoshida, K., Sakuma, H., & Nomoto, Y. (2024). Expression of Tenascin-C Is Upregulated in the Early Stages of Radiation Pneumonitis/Fibrosis in a Novel Mouse Model. Current Issues in Molecular Biology, 46(9), 9674-9685. https://doi.org/10.3390/cimb46090575






