CX3CL1/Fractalkine: A Potential Biomarker for Liver Fibrosis in Chronic HBV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Cytokine Analysis
2.3. Statistical Analysis
2.4. Visualization
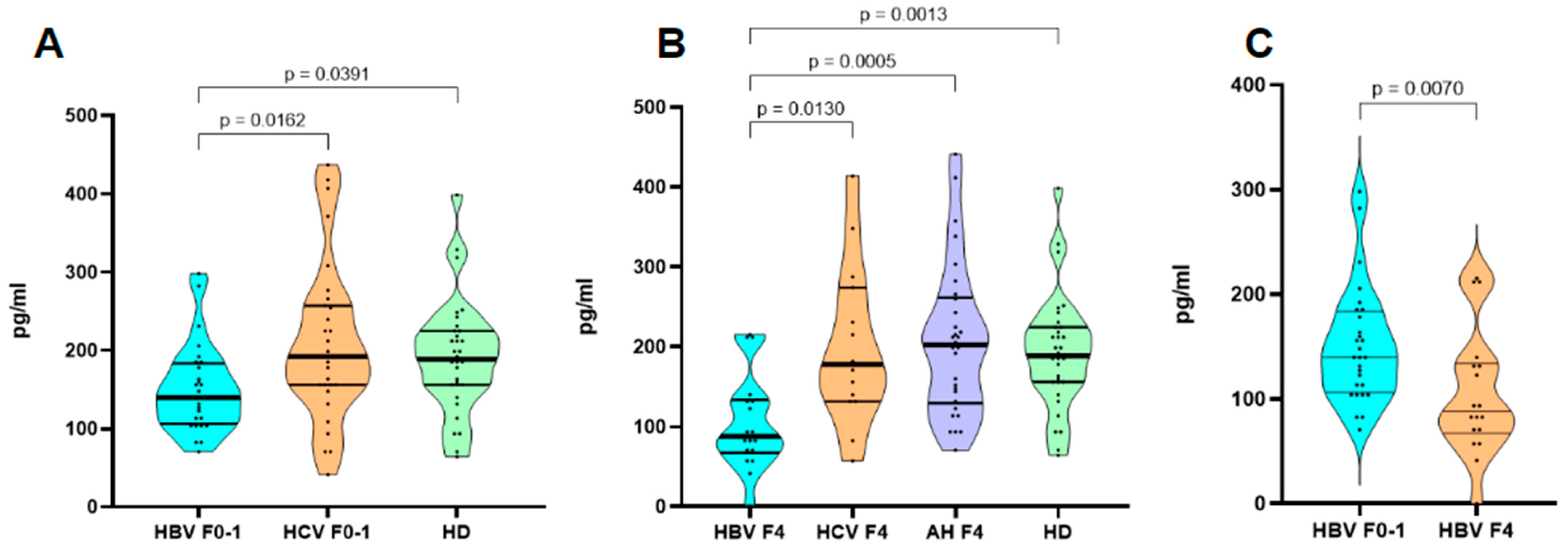
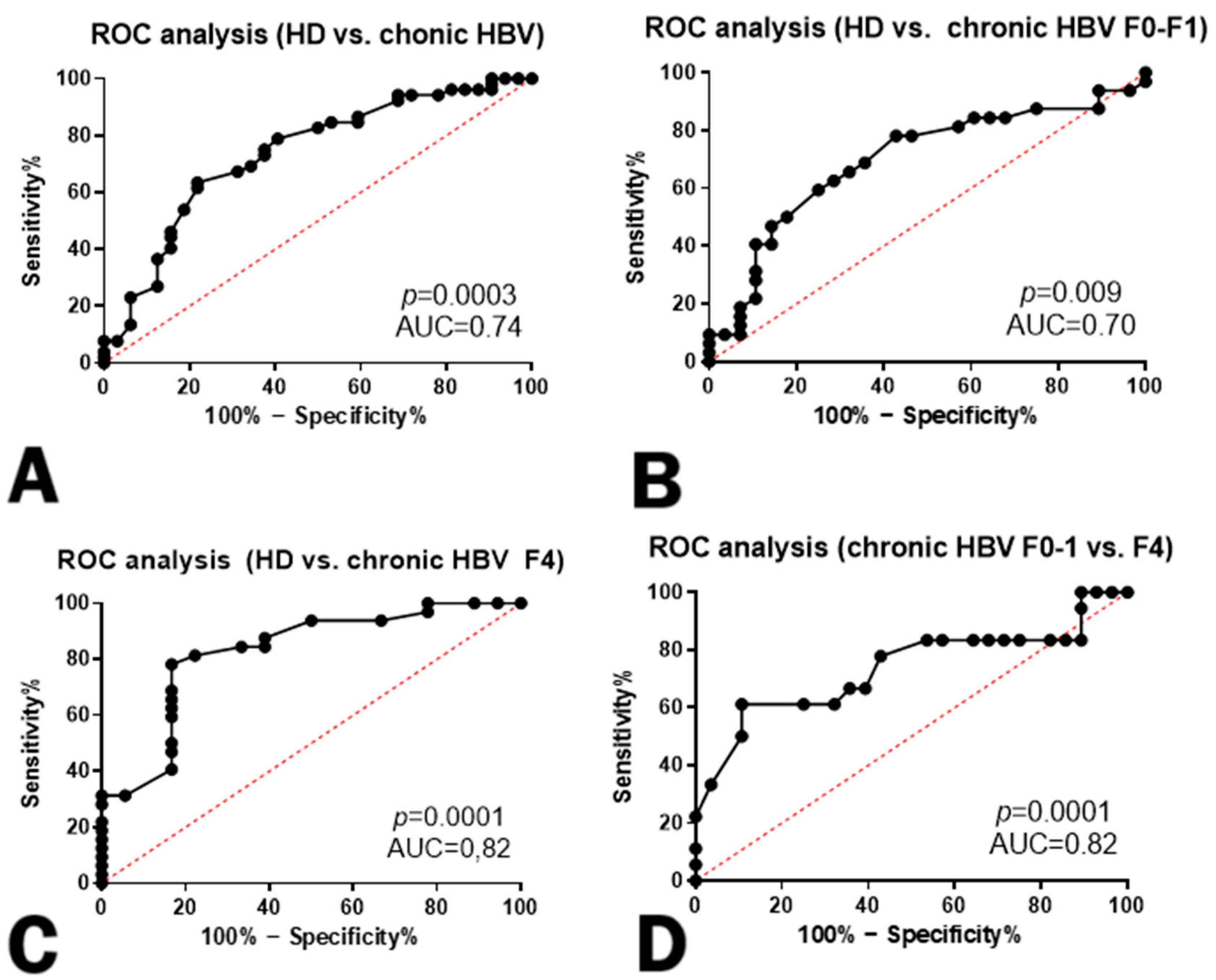
3. Results
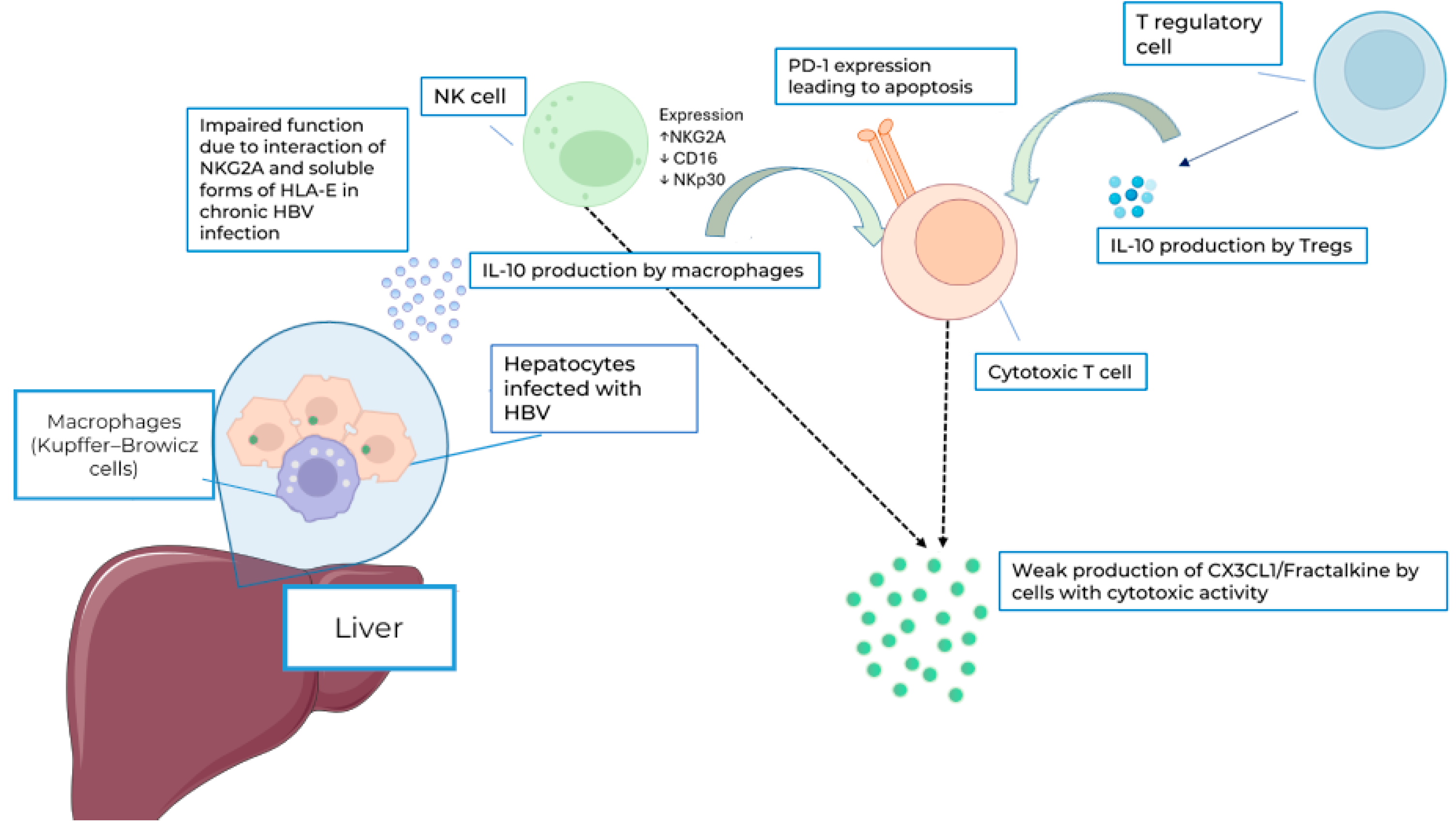
4. Discussion
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Batsunov, O.K.; Arsentieva, N.A.; Lyubimova, N.E.; Esaulenko, E.V.; Semenov, A.V.; Totolyan, A.A. Content of certain cytokines and chemokines in blood of patients with chronic hepatitis B in the early stages of liver fibrosis. Med. Immunol. 2020, 22, 291–300. [Google Scholar] [CrossRef]
- Semenov, A.V.; Arsentieva, N.A.; Lubimova, N.E.; Tulienev, S.V.; Basina, V.V.; Esaulenko, E.V.; Totolyan, A.A. The role of cytokines and сhemokines in laboratory diagnostic of chronic viral hepatitis C. Klin. Lab. Diagn. 2015, 60, 45–51. [Google Scholar] [PubMed]
- Available online: https://www.who.int/ru/news-room/fact-sheets/detail/hepatitis-b (accessed on 24 July 2024).
- Jegaskanda, S.; Ahn, S.H.; Skinner, N.; Thompson, A.J.; Ngyuen, T.; Holmes, J.; De Rose, R.; Navis, M.; Winnall, W.R.; Kramski, M.; et al. Downregulation of interleukin-18-mediated cell signaling and interferon gamma expression by the hepatitis B virus e antigen. J. Virol. 2014, 88, 10412–10420. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Qian, Y.; Yan, F.; Tu, J.; Yang, X.; Xing, Y.; Chen, Z. 5′-triphosphate-siRNA activates RIG-I-dependent type I interferon production and enhances inhibition of hepatitis B virus replication in HepG2.2.15 cells. Eur. J. Pharmacol. 2013, 721, 86–95. [Google Scholar] [CrossRef]
- Kakimi, K.; Guidotti, L.G.; Koezuka, Y.; Chisari, F.V. Natural killer T cell activation inhibits hepatitis B virus replication in vivo. J. Exp. Med. 2000, 192, 921–930. [Google Scholar] [CrossRef]
- Sun, C.; Fu, B.; Gao, Y.; Liao, X.; Sun, R.; Tian, Z.; Wei, H. TGF-β1 down-regulation of NKG2D/DAP10 and 2B4/SAP expression on human NK cells contributes to HBV persistence. PLoS Pathog. 2012, 8, e1002594. [Google Scholar] [CrossRef]
- Capone, F.; Costantini, S.; Guerriero, E.; Calemma, R.; Napolitano, M.; Scala, S.; Izzo, F.; Castello, G. Serum cytokine levels in patients with hepatocellular carcinoma. Eur. Cytokine Netw. 2010, 21, 99–104. [Google Scholar]
- Hassanshahi, G.; Jafarzadeh, A.; James Dickson, A. Expression of stromal derived factor alpha (SDF-1 alpha) by primary hepatocytes following isolation and heat shock stimulation. Iran. J. Allergy Asthma Immunol. 2008, 7, 61–68. [Google Scholar]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef]
- Tang, L.; Hu, H.D.; Hu, P.; Lan, Y.H.; Peng, M.L.; Chen, M.; Ren, H. Gene therapy with CX3CL1/Fractalkine induces antitumor immunity to regress effectively mouse hepatocellular carcinoma. Gene Ther. 2007, 14, 1226–1234. [Google Scholar] [CrossRef]
- Jones, B.A.; Beamer, M.; Ahmed, S. Fractalkine/CX3CL1: A potential new target for inflammatory diseases. Mol. Interv. 2010, 10, 263–270. [Google Scholar] [CrossRef]
- Matsubara, T.; Ono, T.; Yamanoi, A.; Tachibana, M.; Nagasue, N. Fractalkine-CX3CR1 axis regulates tumor cell cycle and deteriorates prognosis after radical resection for hepatocellular carcinoma. J. Surg. Oncol. 2007, 95, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.; et al. Identification and molecular characterization of Fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Hu, A.; Wang, S.; Tian, B.; Jiang, L.; Liang, Y.; Wang, H.; Dong, J. ADAM17-regulated CX3CL1 expression produced by bone marrow endothelial cells promotes spinal metastasis from hepatocellular carcinoma. Int. J. Oncol. 2020, 57, 249–263. [Google Scholar] [CrossRef]
- Faure, S.; Meyer, L.; Costagliola, D.; Vaneensberghe, C.; Genin, E.; Autran, B.; Delfraissy, J.; McDermott, D.; Murphy, P.; Debré, P.; et al. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science 2000, 287, 2274–2277. [Google Scholar] [CrossRef]
- Meucci, O.; Fatatis, A.; Simen, A.; Miller, R. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. USA 2000, 97, 8075–8080. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Zhuang, R.; Yang, K.; Chen, L.; Jin, B.; Ma, Y.; Zhang, Y.; Tang, K. Alterations in CX3CL1 Levels and Its Role in Viral Pathogenesis. Int. J. Mol. Sci. 2024, 25, 4451. [Google Scholar] [CrossRef] [PubMed]
- Efsen, E.; Grappone, C.; DeFranco, R.; Milani, S.; Romanelli, R.; Bonacchi, A.; Caligiuri, A.; Failli, P.; Annunziato, F.; Pagliai, G.; et al. Up-regulated expression of Fractalkine and its receptor CX3CR1 during liver injury in humans. J. Hepatol. 2002, 37, 39–47. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for the prevention, diagnosis, care and treatment for people with chronic hepatitis b infection (text extract): Executive summary. Infect. Dis. Immun. 2024, 4, 103–105. [Google Scholar] [CrossRef]
- Abraham, G.M.; Obley, A.J.; Humphrey, L.; Qaseem, A. World Health Organization Guidelines on Treatment of Hepatitis C Virus Infection: Best Practice Advice From the American College of Physicians. Ann. Intern. Med. 2021, 174, 98–100. [Google Scholar] [CrossRef]
- Weerakkody, Y.; Bell, D.; Saber, M. METAVIR Score. 2022, p. 51855. Available online: https://radiopaedia.org/articles/metavir-score-1?lang=us (accessed on 26 July 2024).
- Qiao, G.; Men, X.; Yang, L.; Han, E.; Liu, L.; Han, C. Serum Fractalkine Levels Were Positively Associated with Disease Severity of Liver Cirrhosis. Ann. Clin. Lab. Sci. 2021, 51, 844–851. [Google Scholar]
- García-Álvarez, M.; Berenguer, J.; Guzmán-Fulgencio, M.; Micheloud, D.; Catalán, P.; Muñoz-Fernandez, M.; Alvarez, E.; Resino, S. High plasma Fractalkine (CX3CL1) levels are associated with severe liver disease in HIV/HCV co-infected patients with HCV genotype 1. Cytokine 2011, 54, 244–248. [Google Scholar] [CrossRef]
- Karlmark, K.R.; Zimmermann, H.W.; Roderburg, C.; Gassler, N.; Wasmuth, H.E.; Luedde, T.; Trautwein, C.; Tacke, F. The Fractalkine receptor CX₃CR1 protects against liver fibrosis by controlling differentiation and survival of infiltrating hepatic monocytes. Hepatology 2010, 52, 1769–1782. [Google Scholar] [CrossRef]
- Shimoda, S.; Selmi, C.; Gershwin, M.E. Fractalkine and other chemokines in primary biliary cirrhosis. Int. J. Hepatol. 2012, 2012, 102839. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Gao, F.; Li, Y.; Shi, K.; Hou, Y.; Chen, J.; Zhang, Q.; Wang, X. Serum cytokine and chemokine profiles and disease prognosis in hepatitis B virus-related acute-on-chronic liver failure. Front. Immunol. 2023, 27, 1133656. [Google Scholar]
- Kondo, Y.; Kimura, O.; Tanaka, Y.; Ninomiya, M.; Iwata, T.; Kogure, T.; Inoue, J.; Sugiyama, M.; Morosawa, T.; Fujisaka, Y.; et al. Differential Expression of CX3CL1 in Hepatitis B Virus-Replicating Hepatoma Cells Can Affect the Migration Activity of CX3CR1+ Immune Cells. J. Virol. 2015, 89, 7016–7027. [Google Scholar] [CrossRef]
- Kramvis, A.; Arakawa, K.; Yu, M.C.; Nogueira, R.; Stram, D.O.; Kew, M.C. Relationship of serological subtype, basic core promoter and precore mutations to genotypes/subgenotypes of hepatitis B virus. J. Med. Virol. 2008, 80, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-L.; Kao, J.-H. Hepatitis B Virus Genotypes and Variants. Cold Spring Harb. Perspect. Med. 2015, 5, 5. [Google Scholar] [CrossRef]
- Norder, H.; Couroucé, A.-M.; Coursaget, P.; Echevarria, J.M.; Lee, S.-D.; Mushahwar, I.K.; Robertson, B.H.; Locarnini, S.; Magnius, L.O. Genetic diversity of hepatitis B virus strains derived worldwide: Genotypes, subgenotypes, and HBsAg subtypes. Intervirology 2004, 47, 289–309. [Google Scholar] [CrossRef]
- Ostankova, Y.V.; Semenov, A.V.; Zueva, E.B.; Gabdrakhmanov, I.A.; Kozlov, K.V.; Zhdanov, K.V.; Totolian, A.A. Variety of the hepatitis B virus genovariants in the military. J. Infectol. 2019, 11, 46–53. [Google Scholar] [CrossRef]
- Schuch, A.; Hoh, A.; Thimme, R. The role of natural killer cells and CD8(+) T cells in hepatitis B virus infection. Front. Immunol. 2014, 5, 258. [Google Scholar] [CrossRef]
- Kondo, Y.; Ueno, Y.; Kobayashi, K.; Kakazu, E.; Shiina, M.; Inoue, J.; Tamai, K.; Wakui, Y.; Tanaka, Y.; Ninomiya, M.; et al. Hepatitis B virus replication could enhance regulatory T cell activity by producing soluble heat shock protein 60 from hepatocytes. J. Inf. Dis. 2010, 202, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Stoop, J.N.; van der Molen, R.G.; Baan, C.C.; van der Laan, L.J.; Kuipers, E.J.; Kusters, J.G.; Janssen, H.L. Regulatory T cells contribute to the impaired immune response in patients with chronic hepatitis B virus infection. Hepatology 2005, 41, 771–778. [Google Scholar] [CrossRef] [PubMed]
- Maier, H.; Isogawa, M.; Freeman, G.; Chisari, F. PD-1:PD-L1 interactions contribute to the functional suppression of virus-specific CD8+ T lymphocytes in the liver. J. Immunol. 2007, 178, 2714–2720. [Google Scholar] [CrossRef]
- Tjwa, E.T.; van Oord, G.W.; Hegmans, J.P.; Janssen, H.L.; Woltman, A.M. Viral load reduction improves activation and function of natural killer cells in patients with chronic hepatitis B. J. Hepatol. 2011, 54, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Zidi, I.; Laaribi, A.B.; Bortolotti, D.; Belhadj, M.; Mehri, A.; Yahia, H.B.; Babay, W.; Chaouch, H.; Zidi, N.; Letaief, A.; et al. HLA-E polymorphism and soluble HLA-E plasma levels in chronic hepatitis B patients. HLA 2016, 87, 153–159. [Google Scholar] [CrossRef]


| HBV | HCV | Autoimmune Liver Diseases | Healthy Donors | |||
|---|---|---|---|---|---|---|
| METAVIR liver fibrosis | F0–1 | F4 | F0–1 | F4 | F4 | n/a |
| n | 28 | 19 | 30 | 13 | 30 | 32 |
| Male/Female, % | 10/18 35%, 65% | 9/10 47%, 53% | 12/18 40%, 60% | 5/8 38%, 62% | 2/28 7%/93% | 15/17 46%/54% |
| Age (Mean ± SD) | 37.7 ± 8.7 | 60 ± 9.2 | 39.0 ± 10.4 | 41.0 ± 9.1 | 59.2 ± 9.1 | 32.0 ± 6.4 |
| ALT, Me (min–max) | 23.46 (7.66–72) U/L | 21 (8–286) U/L | 57.8 (7–198) U/L | 54 (50–148) U/L | 58 (21–175) U/L | 19 (12–33) U/L |
| AST, Me (min–max) | 22 (8–44) U/L | 49 (11–327) U/L | 44.4 (17–143) U/L | 36 (29–49) U/L | 68 (31–207) U/L | 20 (14–25) U/L |
| AST/ALT (De Ritis) ratio (Mean ± SD) | 0.89 ± 0.33 | 2.74 ± 3.8 | 0.87 ± 0.65 | 1.01 ± 0.65 | 1.32 ± 0.75 | 1.33 ± 0.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsentieva, N.A.; Korobova, Z.R.; Batsunov, O.K.; Lyubimova, N.E.; Basina, V.V.; Esaulenko, E.V.; Totolian, A.A. CX3CL1/Fractalkine: A Potential Biomarker for Liver Fibrosis in Chronic HBV Infection. Curr. Issues Mol. Biol. 2024, 46, 9948-9957. https://doi.org/10.3390/cimb46090593
Arsentieva NA, Korobova ZR, Batsunov OK, Lyubimova NE, Basina VV, Esaulenko EV, Totolian AA. CX3CL1/Fractalkine: A Potential Biomarker for Liver Fibrosis in Chronic HBV Infection. Current Issues in Molecular Biology. 2024; 46(9):9948-9957. https://doi.org/10.3390/cimb46090593
Chicago/Turabian StyleArsentieva, Natalia A., Zoia R. Korobova, Oleg K. Batsunov, Natalia E. Lyubimova, Valentina V. Basina, Elena V. Esaulenko, and Areg A. Totolian. 2024. "CX3CL1/Fractalkine: A Potential Biomarker for Liver Fibrosis in Chronic HBV Infection" Current Issues in Molecular Biology 46, no. 9: 9948-9957. https://doi.org/10.3390/cimb46090593
APA StyleArsentieva, N. A., Korobova, Z. R., Batsunov, O. K., Lyubimova, N. E., Basina, V. V., Esaulenko, E. V., & Totolian, A. A. (2024). CX3CL1/Fractalkine: A Potential Biomarker for Liver Fibrosis in Chronic HBV Infection. Current Issues in Molecular Biology, 46(9), 9948-9957. https://doi.org/10.3390/cimb46090593








