Immunolocalization of Aquaporin 1, 2, and 9 in Anuran Testis of the Neotropical Pointedbelly Frog Leptodactylus podicipinus
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Location
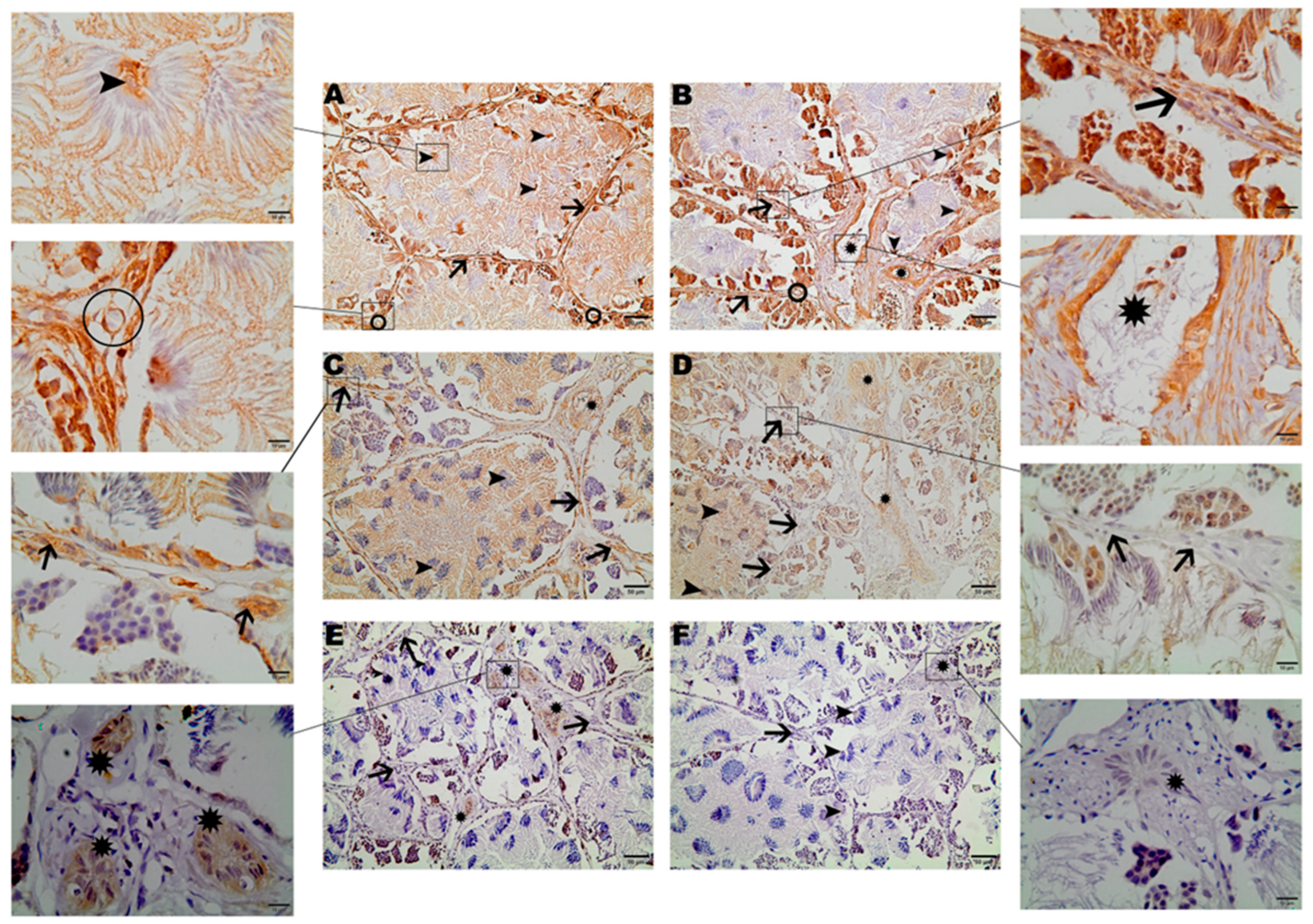
2.2. Immunohistochemistry
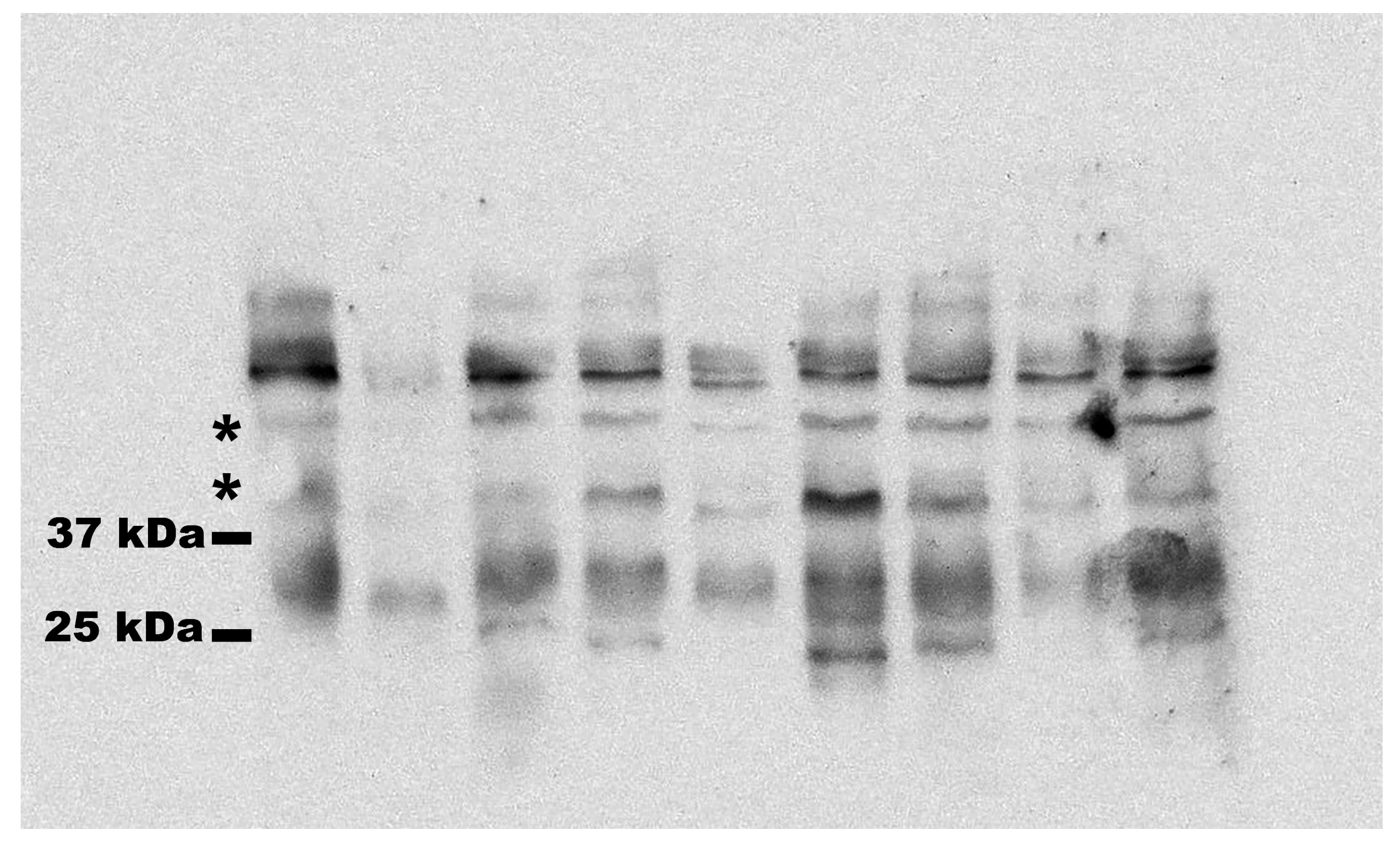
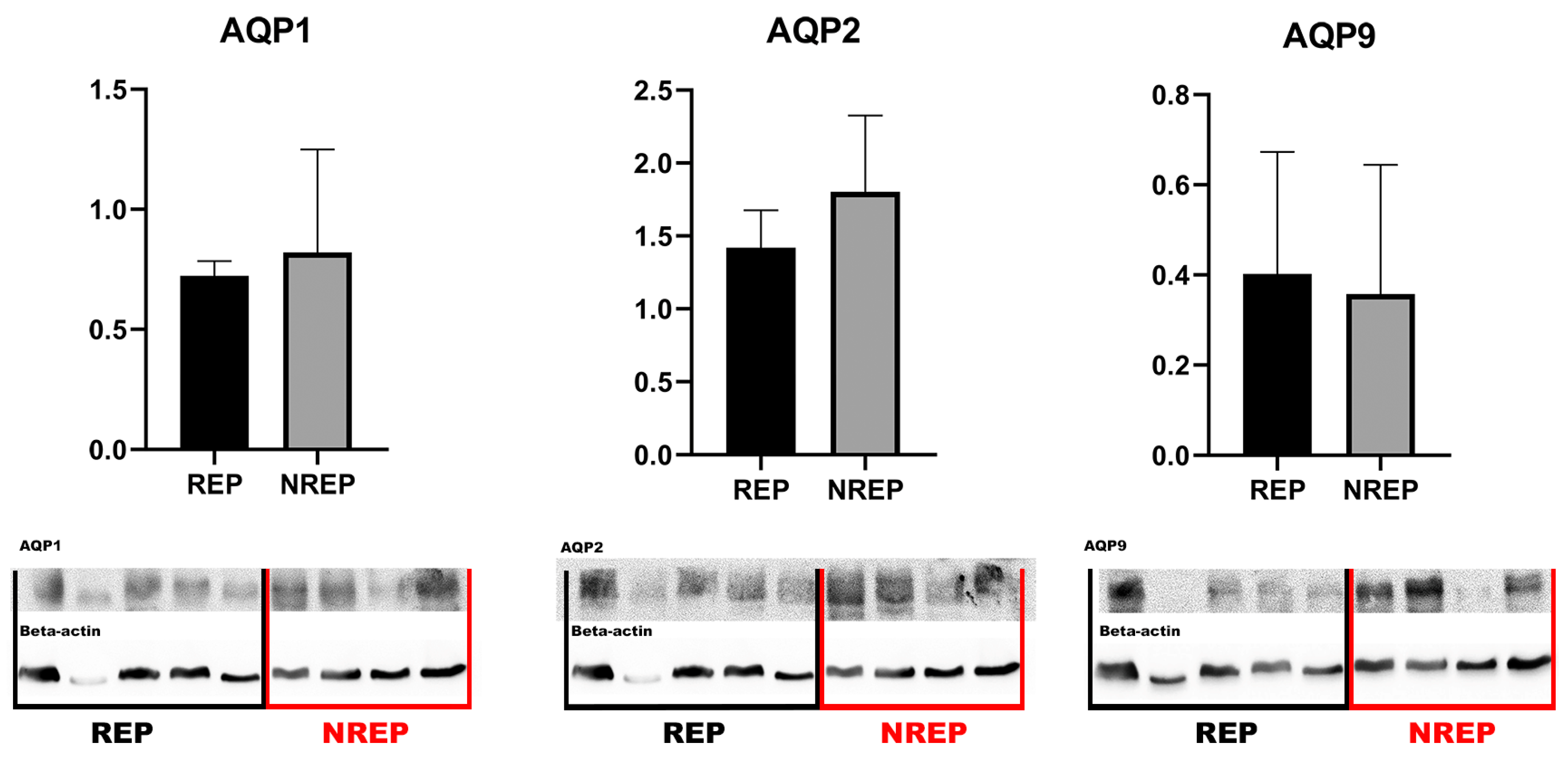
2.3. Western Blotting
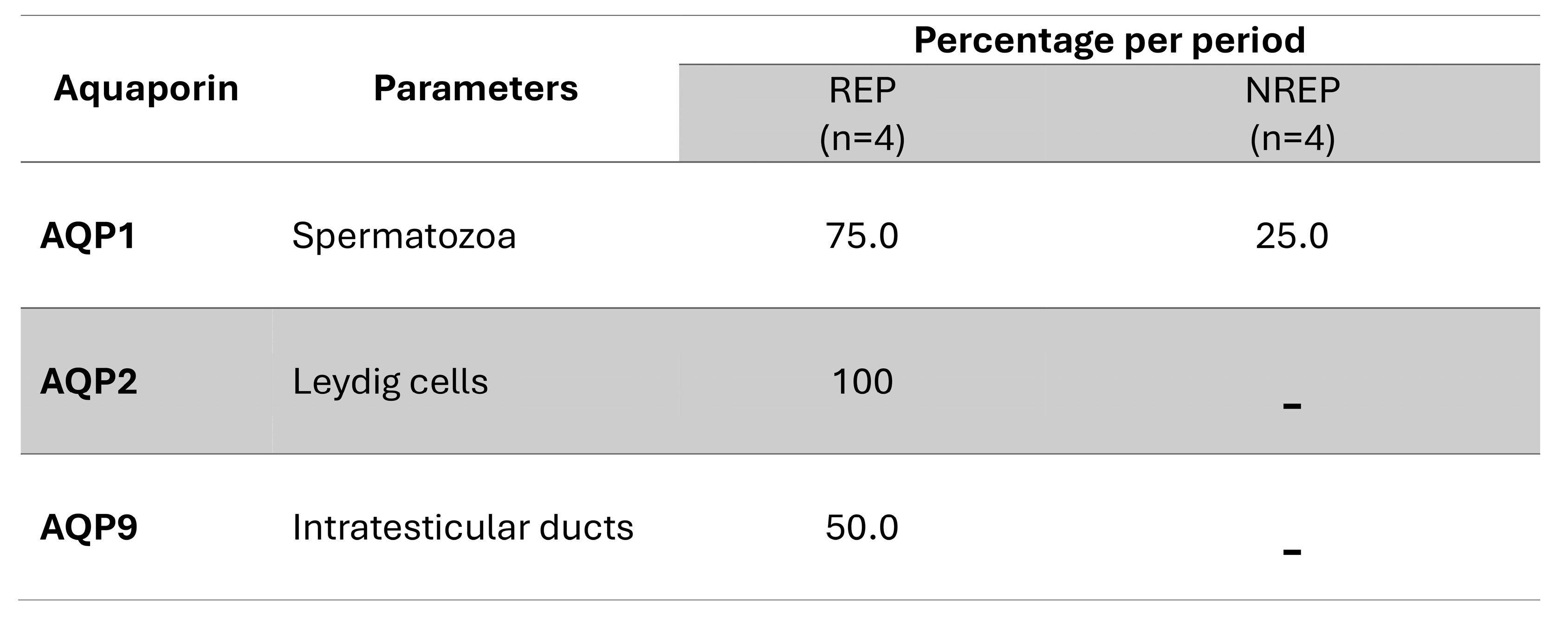
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Vitt, L.J.; Caldwell, J.P. Amphibians and Reptiles Herpetology; Academic Press: Cambridge, MA, USA, 2014; ISBN 978-0-12-386919-7. [Google Scholar]
- Haddad, C.; Prado, C.P.A. Reproductive Modes in Frogs and Their Unexpected Diversity in the Atlantic Forest of Brazil. Bioscience 2005, 55, 207–217. [Google Scholar] [CrossRef]
- Altig, R.; McDiarmid, R.W. Morphological Diversity and Evolution of Egg and Clutch Structure in Amphibians. Herpetol. Monogr. 2007, 21, 1–32. [Google Scholar] [CrossRef]
- Pereira, E.B.; Collevatti, R.G.; Kokubum, M.N.D.C.; Miranda, N.E.D.O.; Maciel, N.M. Ancestral Reconstruction of Reproductive Traits Shows No Tendency toward Terrestriality in Leptodactyline Frogs. BMC Evol. Biol. 2015, 15, 91. [Google Scholar] [CrossRef] [PubMed]
- Pucci Alcaide, A.; Pucci Alcaide, F.; Michel, A.A.; Ponssa, M.L. Testicular Histology of Anurans That Deposit Eggs out of Water. Acta Zoológica Lilloana 2020, 64, 84–115. [Google Scholar] [CrossRef]
- Gomez-Mestre, I.; Pyron, R.A.; Wiens, J.J. Phylogenetic analyses reveal unexpected patterns in the evolution of reproductive modes in frogs. Evolution 2012, 66, 3687–3700. [Google Scholar] [CrossRef] [PubMed]
- Lion, M.B.; Mazzochini, G.G.; Garda, A.A.; Lee, T.M.; Bickford, D.; Costa, G.C.; Fonseca, C.R. Global Patterns of Terrestriality in Amphibian Reproduction. Glob. Ecol. Biogeogr. 2018, 28, 744–756. [Google Scholar] [CrossRef]
- Nakhoul, N.L.; Davis, B.A.; Romero, M.F.; Boron, W.F. Effect of Expressing the Water Channel Aquaporin-1 on the CO2 Permeability of Xenopus Oocytes. Am. J. Physiol. Physiol. 1998, 274, C543–C548. [Google Scholar] [CrossRef]
- Székely, P.; Tudor, M.; Cogǎlniceanu, D. Effect of Habitat Drying on the Development of the Eastern Spadefoot Toad (Pelobates syriacus) Tadpoles. Amphib. Reptil. 2010, 31, 425–434. [Google Scholar] [CrossRef]
- Venturelli, D.P.; Klein, W. Effect of Hydric Stress on Locomotion and Morphology of Tadpoles from Temporary Ponds. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2019, 331, 175–184. [Google Scholar] [CrossRef]
- Sasso-Cerri, E.; De Faria, F.P.; Freymüller, E.; Miraglia, S.M. Testicular Morphological Changes during the Seasonal Reproductive Cycle in the Bullfrog Rana Catesbeiana. J. Exp. Zool. Part A Comp. Exp. Biol. 2004, 301, 249–260. [Google Scholar] [CrossRef]
- Chaves, M.F.; de Moura, G.J.B.; Tenório, F.D.C.M.A.; Baptista, J.D.S.; Lapa Neto, C.J.C.; Texeira, V.W.; Texeira, Á.A.C. Influence of Rainfall and Temperature on the Spermatogenesis of Leptodactylus macrosternum (Anura: Leptodactylidae). Zoologia 2017, 34, 1–7. [Google Scholar] [CrossRef]
- Jakob, C.; Poizat, G.; Veith, M.; Seitz, A.; Crivelli, A.J. Breeding Phenology and Larval Distribution of Amphibians in a Mediterranean Pond Network with Unpredictable Hydrology. Ann. Oper. Res. 2003, 97, 131–141. [Google Scholar] [CrossRef]
- Cayuela, H.; Besnard, A.; Béchet, A.; Devictor, V.; Olivier, A. Reproductive Dynamics of Three Amphibian Species in Mediterranean Wetlands: The Role of Local Precipitation and Hydrological Regimes. Freshw. Biol. 2012, 57, 2629–2640. [Google Scholar] [CrossRef]
- Lemenager, L.A.; Tracy, C.R.; Christian, K.A.; Tracy, C.R. Physiological Control of Water Exchange in Anurans. Ecol. Evol. 2022, 12, 8597. [Google Scholar] [CrossRef] [PubMed]
- Krane, C.M.; Goldstein, D.L. Comparative Functional Analysis of Aquaporins/Glyceroporins in Mammals and Anurans. Mamm. Genome 2007, 18, 452–462. [Google Scholar] [CrossRef]
- Miller, D. Osmotic Activation of Sperm Motility via Water Flow through Aquaporins in the Freeze-Tolerant Cope’s Gray Treefrog, Dryophytes chrysoscelis. Master’s Thesis, Wright State University, Dayton, OH, USA, 2018. [Google Scholar]
- Yang, B.; Verkman, A.S. Water and Glycerol Permeabilities of Aquaporins 1-5 and MIP Determined Quantitatively by Expression of Epitope-Tagged Constructs in Xenopus Oocytes. J. Biol. Chem. 1997, 272, 16140–16146. [Google Scholar] [CrossRef]
- Pastor-Soler, N.; Bagnis, C.; Sabolic, I.; Tyszkowski, R.; McKee, M.; Van Hoek, A.; Breton, S.; Brown, D. Aquaporin 9 Expression along the Male Reproductive Tract. Biol. Reprod. 2001, 65, 384–393. [Google Scholar] [CrossRef]
- Verkman, A.S.; Mitra, A.K. Structure and Function of Aquaporin Water Channels. Am. J. Physiol. Physiol. 2000, 278, F13–F28. [Google Scholar] [CrossRef]
- Nozaki, K.; Ishii, D.; Ishibashi, K. Intracellular Aquaporins: Clues for Intracellular Water Transport? Pflügers Arch.-Eur. J. Physiol. 2008, 456, 701–707. [Google Scholar] [CrossRef]
- Verkman, A.S. Aquaporins: Translating Bench Research to Human Disease. J. Exp. Biol. 2009, 212, 1707–1715. [Google Scholar] [CrossRef]
- Verkman, A.S. More than Just Water Channels: Unexpected Cellular Roles of Aquaporins. J. Cell Sci. 2005, 118, 3225–3232. [Google Scholar] [CrossRef] [PubMed]
- Hermo, L.; Smith, C.E. Thirsty Business: Cell, Region, and Review Membrane Specificity of Aquaporins in the Testis, Efferent Ducts, and Epididymis and Factors Regulating Their Expression. J. Androl. 2011, 32, 565–575. [Google Scholar] [CrossRef]
- Brown, D.; Katsura, T.; Kawashima, M.; Verkman, A.S.; Sabolic, I. Cellular Distribution of the Aquaporins: A Family of Water Channel Proteins. Histochem. Cell Biol. 1995, 104, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Katsura, T.; Gustafson, C.E. Cellular Mechanisms of Aquaporin Trafficking. Am. J. Physiol.-Ren. Physiol. 1998, 275, 328–331. [Google Scholar] [CrossRef] [PubMed]
- Byrne, P.G.; Dunne, C.; Munn, A.J.; Silla, A.J. Environmental Osmolality Influences Sperm Motility Activation in an Anuran Amphibian. J. Evol. Biol. 2015, 28, 521–534. [Google Scholar] [CrossRef]
- Deen, P.M.; Van Os, C.H. Epithelial Aquaporins. Curr. Opin. Cell Biol. 1998, 10, 435–442. [Google Scholar] [CrossRef]
- Echevarría, M.; Ilundáin, A.A. Aquaporins. J. Physiol. Biochem. 1998, 54, 107–118. [Google Scholar]
- Engel, A.; Fujiyoshi, Y.; Agre, P. The Importance of Aquaporin Water Channel Protein Structures. EMBO J. 2000, 19, 800–806. [Google Scholar] [CrossRef]
- Suzuki, M.; Hasegawa, T.; Ogushi, Y.; Tanaka, S. Amphibian Aquaporins and Adaptation to Terrestrial Environments: A Review. Comp. Biochem. Physiol.-A Mol. Integr. Physiol. 2007, 148, 72–81. [Google Scholar] [CrossRef]
- Castellar, A.; Bertoli, P.C.; Galdino, L.H.; Domeniconi, R.F.; Cruz-Neto, A.P. Differences in Physiological Traits Associated with Water Balance among Rodents, And Their Relationship to Tolerance of Habitat Fragmentation. J. Exp. Zool. Part A Ecol. Genet. Physiol. 2015, 323, 731–744. [Google Scholar] [CrossRef]
- Huang, H.F.; He, R.H.; Sun, C.C.; Zhang, Y.; Meng, Q.X.; Ma, Y.Y. Function of Aquaporins in Female and Male Reproductive Systems. Hum. Reprod. Update 2006, 12, 785–795. [Google Scholar] [CrossRef] [PubMed]
- Belleannée, C.; Thimon, V.; Sullivan, R. Region-Specific Gene Expression in the Epididymis. Cell Tissue Res. 2012, 349, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Domeniconi, R.F.; Orsi, A.M.; Justulin, L.A.; Leme Beu, C.C.; Felisbino, S.L. Immunolocalization of Aquaporins 1, 2 and 7 in Rete Testis, Efferent Ducts, Epididymis and Vas Deferens of Adult Dog. Cell Tissue Res. 2008, 332, 329–335. [Google Scholar] [CrossRef]
- Klein, C.; Troedsson, M.H.T.; Rutllant, J. Region-Specific Expression of Aquaporin Subtypes in Equine Testis, Epididymis, and Ductus Deferens. Anat. Rec. 2013, 296, 1115–1126. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Prado, C.P.; Uetanabaro, M.; Lopes, F.S. Reproductive Strategies of Leptodactylus chaquensis and L. Podicipinus in the Pantanal, Brazil. J. Herpetol. 2000, 34, 135–139. [Google Scholar] [CrossRef]
- Jorgewich-Cohen, G.; Paredero, R.C.B.; Kanasiro, A.; Seiko, V. Herpetofauna da Cuesta Paulista, 1st ed.; Anolis Books: São Paulo, Brazil, 2020; ISBN 978-65-992458-0-0. [Google Scholar]
- Hasegawa, T.; Tanii, H.; Suzuki, M.; Tanaka, S. Regulation of Water Absorption in the Frog Skins by Two Vasotocin-Dependent Water-Channel Aquaporins, AQP-H2 and AQP-H3. Endocrinology 2003, 144, 4087–4096. [Google Scholar] [CrossRef][Green Version]
- Zimmerman, S.L.; Frisbie, J.; Goldstein, D.L.; West, J.; Rivera, K.; Krane, C.M. Excretion and Conservation of Glycerol, and Expression of Aquaporins and Glyceroporins, during Cold Acclimation in Cope’s Gray Tree Frog Hyla chrysoscelis. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2007, 292, 544–555. [Google Scholar] [CrossRef]
- Ogushi, Y.; Mochida, H.; Nakakura, T.; Suzuki, M.; Tanaka, S. Immunocytochemical and Phylogenetic Analyses of an Arginine Vasotocin-Dependent Aquaporin, AQP-H2K, Specifically Expressed in the Kidney of the Tree Frog, Hyla japonica. Endocrinology 2007, 148, 5891–5901. [Google Scholar] [CrossRef]
- Domeniconi, R.F.; Orsi, A.M.; Justulin, L.A.; Beu, C.C.L.; Felisbino, S.L. Aquaporin 9 (AQP9) Localization in the Adult Dog Testis Excurrent Ducts by Immunohistochemistry. Anat. Rec. 2007, 290, 1519–1525. [Google Scholar] [CrossRef]
- Skowronski, M.T.; Leska, A.; Robak, A.; Nielsen, S. Immunolocalization of Aquaporin-1, -5, and -7 in the Avian Testis and Vas Deferens. J. Histochem. Cytochem. 2009, 57, 915–922. [Google Scholar] [CrossRef]
- Chauvigné, F.; Boj, M.; Vilella, S.; Finn, R.N.; Cerdà, J. Subcellular Localization of Selectively Permeable Aquaporins in the Male Germ Line of a Marine Teleost Reveals Spatial Redistribution in Activated Spermatozoa. Biol. Reprod. 2013, 89, 1–17. [Google Scholar] [CrossRef]
- Zaniboni, L.; Akuffo, V.; Bakst, M.R. Aquaporins Are Observed in the Duct Epithelia of the Epididymal Region of the Large White Turkey. Poult. Sci. 2004, 83, 1917–1920. [Google Scholar] [CrossRef] [PubMed]
- Wilczynski, W.; Quispe, M.; Muñoz, M.I.; Penna, M. Arginine Vasotocin, the Social Neuropeptide of Amphibians and Reptiles. Front. Endocrinol. 2017, 8, 186. [Google Scholar] [CrossRef]
- Boyd, S.K. Gonadal Steroid Modulation of Vasotocin Concentrations in the Bullfrog Brain. Neuroendocrinology 1994, 60, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Tanaka, S. Molecular Diversity of Vasotocin-Dependent Aquaporins Closely Associated with Water Adaptation Strategy in Anuran Amphibians. J. Neuroendocrinol. 2010, 22, 407–412. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bordin, R.O.A.; Oliveira, C.d.; Domeniconi, R.F. Immunolocalization of Aquaporin 1, 2, and 9 in Anuran Testis of the Neotropical Pointedbelly Frog Leptodactylus podicipinus. Curr. Issues Mol. Biol. 2024, 46, 9958-9969. https://doi.org/10.3390/cimb46090594
Bordin ROA, Oliveira Cd, Domeniconi RF. Immunolocalization of Aquaporin 1, 2, and 9 in Anuran Testis of the Neotropical Pointedbelly Frog Leptodactylus podicipinus. Current Issues in Molecular Biology. 2024; 46(9):9958-9969. https://doi.org/10.3390/cimb46090594
Chicago/Turabian StyleBordin, Rafael O. A., Classius de Oliveira, and Raquel F. Domeniconi. 2024. "Immunolocalization of Aquaporin 1, 2, and 9 in Anuran Testis of the Neotropical Pointedbelly Frog Leptodactylus podicipinus" Current Issues in Molecular Biology 46, no. 9: 9958-9969. https://doi.org/10.3390/cimb46090594
APA StyleBordin, R. O. A., Oliveira, C. d., & Domeniconi, R. F. (2024). Immunolocalization of Aquaporin 1, 2, and 9 in Anuran Testis of the Neotropical Pointedbelly Frog Leptodactylus podicipinus. Current Issues in Molecular Biology, 46(9), 9958-9969. https://doi.org/10.3390/cimb46090594





