The Neuroanatomy of Induced Pluripotent Stem Cells: In Vitro Models of Subcortical Nuclei in Neurodegenerative Disorders
Abstract
1. Introduction
2. The Neuromodulatory Subcortical Systems: Elements of Functional Anatomy
2.1. The Raphe Nuclei and the Serotoninergic System
2.2. The Locus Coeruleus and the Noradrenergic System
2.3. The Dopaminergic Nuclei: The Substantia Nigra and the Ventral Tegmental Area
2.4. The Cholinergic Nuclei
3. Methodology of the Literature Search
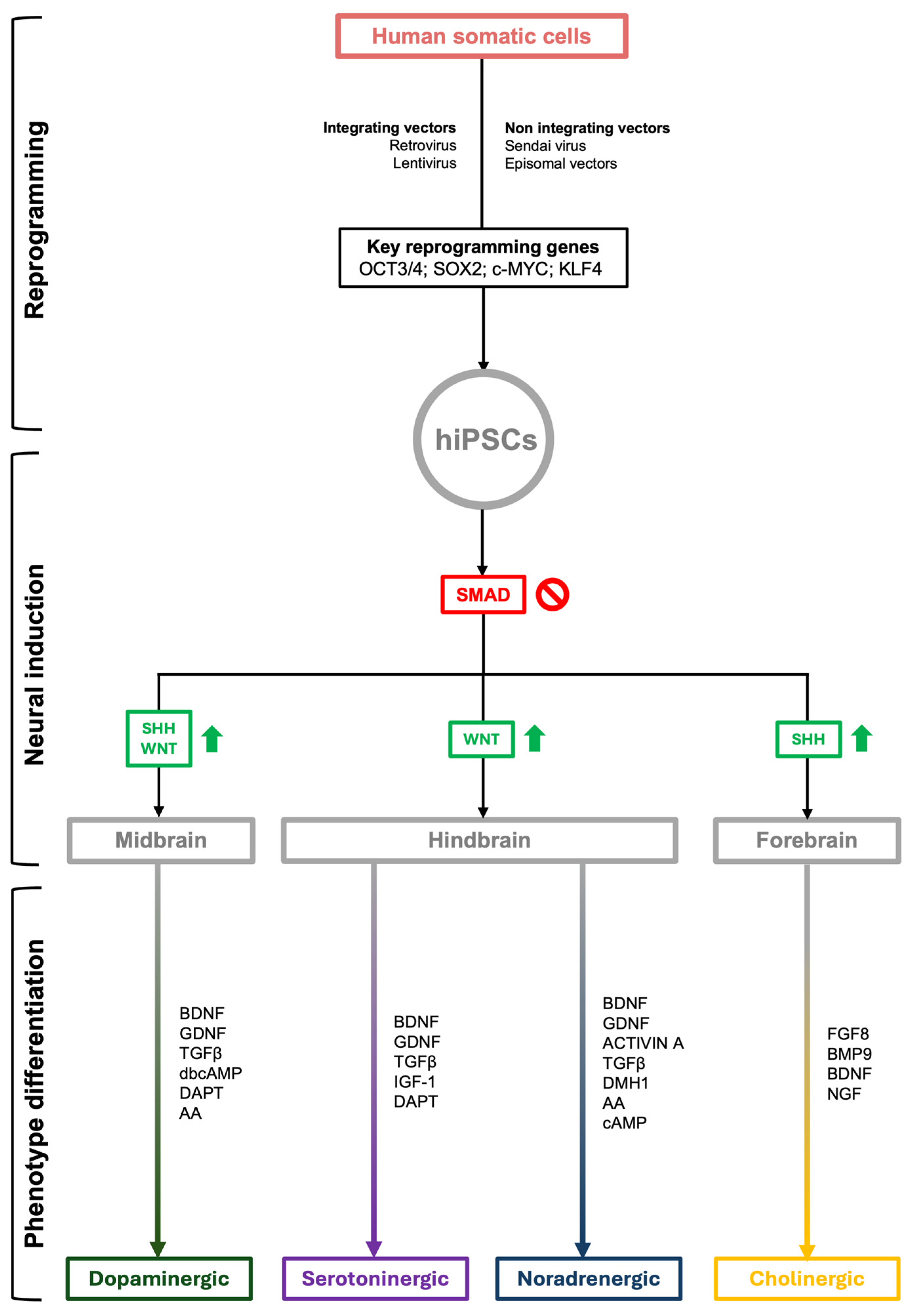
4. NSS iPSCs: Differentiation Protocols
4.1. Reprogramming of Somatic Cells
4.2. Induction of Neural Precursors
4.3. Dopaminergic Differentiation
4.4. Noradrenergic Differentiation
4.5. Serotoninergic Differentiation
4.6. Cholinergic Differentiation
5. Experimental Findings and Insight into NDDs’ Pathophysiology
5.1. Dopaminergic IPSC Models
5.2. Other NSS iPSC Models
6. Drug Testing and Therapy Development
6.1. In Vitro Evaluation of Drug Safety and Pharmacodynamics
6.2. In Vivo Transplantation of iPSCs
7. Discussion and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Dorsey, E.R.; Sherer, T.; Okun, M.S.; Bloemd, B.R. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- John Wiley & Sons, Ltd. 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2023, 19, 1598–1695. [Google Scholar] [CrossRef] [PubMed]
- Ehrenberg, A.J.; Kelberman, M.A.; Liu, K.Y.; Dahl, M.J.; Weinshenker, D.; Falgàs, N.; Dutt, S.; Mather, M.; Ludwig, M.; Betts, M.J.; et al. Priorities for research on neuromodulatory subcortical systems in Alzheimer’s disease: Position paper from the NSS PIA of ISTAART. Alzheimer’s Dement. 2023, 8, 21. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Mai, J.K. The Human Nervous System, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2003. [Google Scholar] [CrossRef]
- Lees, A.J.; Hardy, J.; Revesz, T. Parkinson’s disease. Lancet 2009, 373, 2055–2066. [Google Scholar] [CrossRef]
- Hampel, H.; Mesulam, M.M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141, 1917–1933. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.-M. Cholinergic circuitry of the human nucleus basalis and its fate in Alzheimer’s disease. J. Comp. Neurol. 2013, 521, 4124–4144. [Google Scholar] [CrossRef]
- Theofilas, P.; Ehrenberg, A.J.; Dunlop, S.; Di Lorenzo Alho, A.T.; Nguy, A.; Leite, R.E.P.; Rodriguez, R.D.; Mejia, M.B.; Suemoto, C.K.; Ferretti-Rebustini, R.E.; et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimers Dement. 2017, 13, 236–246. [Google Scholar] [CrossRef]
- Kelly, S.C.; He, B.; Perez, S.E.; Ginsberg, S.D.; Mufson, E.J.; Counts, S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 8. [Google Scholar] [CrossRef]
- Galgani, A.; Giorgi, F.S. Exploring the Role of Locus Coeruleus in Alzheimer’s Disease: A Comprehensive Update on MRI Studies and Implications. Curr. Neurol. Neurosci. Rep. 2023, 23, 925–936. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Ryskalin, L.; Ruffoli, R.; Biagioni, F.; Limanaqi, F.; Ferrucci, M.; Busceti, C.L.; Bonuccelli, U.; Fornai, F. The Neuroanatomy of the Reticular Nucleus Locus Coeruleus in Alzheimer’s Disease. Front. Neuroanat. 2017, 11, 80. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Galgani, A.; Puglisi-Allegra, S.; Busceti, C.L.; Fornai, F. The connections of Locus Coeruleus with hypothalamus: Potential involvement in Alzheimer’s disease. J. Neural Transm. 2021, 128, 589–613. [Google Scholar] [CrossRef] [PubMed]
- Eser, R.A.; Ehrenberg, A.J.; Petersen, C.; Dunlop, S.; Mejia, M.B.; Suemoto, C.K.; Walsh, C.M.; Rajana, H.; Oh, J.; Theofilas, P.; et al. Selective Vulnerability of Brainstem Nuclei in Distinct Tauopathies: A Postmortem Study. J. Neuropathol. Exp. Neurol. 2018, 77, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Eser, R.A.; Ehrenberg, A.J.; Morales, D.; Petersen, C.; Kudlacek, J.; Dunlop, S.R.; Theofilas, P.; Resende, E.D.P.F.; Cosme, C.; et al. Profound degeneration of wake-promoting neurons in Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 1253–1263. [Google Scholar] [CrossRef]
- Pavese, N.; Tai, Y.F. Nigrosome Imaging and Neuromelanin Sensitive MRI in Diagnostic Evaluation of Parkinsonism. Mov. Disord. Clin. Pract. 2018, 5, 131–140. [Google Scholar] [CrossRef]
- Galgani, A.; Lombardo, F.; Della Latta, D.; Martini, N.; Bonuccelli, U.; Fornai, F.; Giorgi, F.S. Locus Coeruleus Magnetic Resonance Imaging in Neurological Diseases. Curr. Neurol. Neurosci. Rep. 2020, 21, 2. [Google Scholar] [CrossRef]
- Vadodaria, K.C.; Jones, J.R.; Linker, S.; Gage, F.H. Modeling Brain Disorders Using Induced Pluripotent Stem Cells. Cold Spring Harb. Perspect. Biol. 2020, 12, a035659. [Google Scholar] [CrossRef]
- Malik, N.; Rao, M.S. A Review of the Methods for Human iPSC Derivation. Methods Mol. Biol. 2013, 997, 23–33. [Google Scholar]
- Beghini, D.G.; Kasai-Brunswick, T.H.; Henriques-Pons, A. Induced Pluripotent Stem Cells in Drug Discovery and Neurodegenerative Disease Modelling. Int. J. Mol. Sci. 2024, 25, 2392. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Li, S.; He, X.B.; Cheng, C.; Le, W. Induced pluripotent stem cells in Alzheimer’s disease: Applications for disease modeling and cell-replacement therapy. Mol. Neurodegener. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Xu, X.; Huang, J.; Li, J.; Liu, L.; Han, C.; Shen, Y.; Zhang, G.; Jiang, H.; Lin, Z.; Xiong, N.; et al. Induced pluripotent stem cells and Parkinson’s disease: Modelling and treatment. Cell Prolif. 2016, 49, 14–26. [Google Scholar] [CrossRef]
- Jungverdorben, J.; Till, A.; Brüstle, O. Induced pluripotent stem cell-based modeling of neurodegenerative diseases: A focus on autophagy. J. Mol. Med. 2017, 95, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Raab, S.; Klingenstein, M.; Liebau, S.; Linta, L. A Comparative View on Human Somatic Cell Sources for iPSC Generation. Stem Cells Int. 2014, 2014, 768391. [Google Scholar] [CrossRef] [PubMed]
- Theofilas, P.; Dunlop, S.; Heinsen, H.; Grinberg, L.T. Turning on the Light Within: Subcortical Nuclei of the Isodentritic Core and their Role in Alzheimer’s Disease Pathogenesis. J. Alzheimers Dis. 2015, 46, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Lenzi, P. Sistema Nervoso Vegetativo: Anatomia Funzionale Della Vita Vegetativa, 1st ed.; Pisa University Press: Pisa, Italy, 2020. [Google Scholar]
- Moruzzi, G.; Magoun, H.W. Brain stem reticular formation and activation of the EEG. Electroencephalogr. Clin. Neurophysiol. 1949, 1, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Mesulam, M.-M. The cholinergic innervation of the human cerebral cortex. Prog. Brain Res. 2004, 145, 67–78. [Google Scholar]
- Dahlström, A.; Fuxe, K. Localization of monoamines in the lower brain stem. Experientia 1964, 20, 398–399. [Google Scholar] [CrossRef]
- Dahlstrom, A.; Fuxe, K. Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol. Scand. Suppl. 1964, 62 (Suppl. S232), 1–55. [Google Scholar]
- Mesulam, M.-M.; Geula, C. Nucleus basalis (Ch4) and cortical cholinergic innervation in the human brain: Observations based on the distribution of acetylcholinesterase and choline acetyltransferase. J. Comp. Neurol. 1988, 275, 216–240. [Google Scholar] [CrossRef]
- Hornung, J.P. The human raphe nuclei and the serotonergic system. J. Chem. Neuroanat. 2003, 26, 331–343. [Google Scholar] [CrossRef]
- Waselus, M.; Valentino, R.J.; Van Bockstaele, E.J. Collateralized dorsal raphe nucleus projections: A mechanism for the integration of diverse functions during stress. J. Chem. Neuroanat. 2011, 41, 266–280. [Google Scholar] [CrossRef]
- Benarroch, E.E. Pain-autonomic interactions. Neurol. Sci. 2006, 27 (Suppl. S2), s130–s133. [Google Scholar] [CrossRef] [PubMed]
- Monti, J.M. The structure of the dorsal raphe nucleus and its relevance to the regulation of sleep and wakefulness. Sleep. Med. Rev. 2010, 14, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; Leko, M.B.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [PubMed]
- Poe, G.R.; Foote, S.; Eschenko, O.; Johansen, J.P.; Bouret, S.; Aston-Jones, G.; Harley, C.W.; Manahan-Vaughan, D.; Weinshenker, D.; Valentino, R.; et al. Locus coeruleus: A new look at the blue spot. Nat. Rev. Neurosci. 2020, 21, 644–659. [Google Scholar] [CrossRef]
- Counts, S.E.; Mufson, E.J. Locus Coeruleus. In The Human Nervous System, 3rd ed.; Mai, J.K., Paxinos, G., Eds.; Academic press: Cambridge, MA, USA, 2012; pp. 427–440. [Google Scholar]
- Aston-Jones, G.; Waterhouse, B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 2016, 1645, 75–78. [Google Scholar] [CrossRef]
- González, M.M.C.; Aston-Jones, G. Circadian Regulation of Arousal: Role of the Noradrenergic Locus Coeruleus System and Light Exposure. Sleep 2006, 29, 1327–1336. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Saccaro, L.F.; Galgani, A.; Busceti, C.L.; Biagioni, F.; Frati, A.; Fornai, F. The role of Locus Coeruleus in neuroinflammation occurring in Alzheimer’s disease. Brain Res. Bull. 2019, 153, 47–58. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Kalinin, S.; Braun, D. Causes, consequences, and cures for neuroinflammation mediated via the locus coeruleus: Noradrenergic signaling system. J. Neurochem. 2016, 139, 154–178. [Google Scholar] [CrossRef] [PubMed]
- Cirelli, C.; Tononi, G. Locus ceruleus control of state-dependent gene expression. J. Neurosci. 2004, 24, 5410–5419. [Google Scholar] [CrossRef]
- Cirelli, C.; Pompeiano, M.; Tononi, G. Neuronal gene expression in the waking state: A role for the locus coeruleus. Science 1996, 274, 1211–1215. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the Pathologic Process in Alzheimer Disease: Age Categories From 1 to 100 Years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Del Tredici, K.; Rüb, U.; De Vos, R.A.I.; Jansen Steur, E.N.H.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef] [PubMed]
- Gesi, M.; Soldani, P.; Giorgi, F.S.; Santinami, A.; Bonaccorsi, I.; Fornai, F. The role of the locus coeruleus in the development of Parkinson’s disease. Neurosci. Biobehav. Rev. 2000, 24, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef] [PubMed]
- Jardanhazi-Kurutz, D.; Kummer, M.P.; Terwel, D.; Vogel, K.; Dyrks, T.; Thiele, A.; Heneka, M.T. Induced LC degeneration in APP/PS1 transgenic mice accelerates early cerebral amyloidosis and cognitive deficits. Neurochem. Int. 2010, 57, 375–382. [Google Scholar] [CrossRef]
- Heneka, M.T.; Ramanathan, M.; Jacobs, A.H.; Dumitrescu-Ozimek, L.; Bilkei-Gorzo, A.; Debeir, T.; Sastre, M.; Galldiks, N.; Zimmer, A.; Hoehn, M.; et al. Locus Ceruleus degeneration promotes Alzheimer pathogenesis in amyloid precursor protein 23 transgenic mice. J. Neurosci. 2006, 26, 1343–1354. [Google Scholar] [CrossRef]
- Kalinin, S.; Feinstein, D.L.; Xu, H.; Huesa, G.; Pelligrino, D.A.; Galea, E. Degeneration of noradrenergic fibres from the locus coeruleus causes tight-junction disorganisation in the rat brain. Eur. J. Neurosci. 2006, 24, 3393–3400. [Google Scholar] [CrossRef]
- Morales, M.; Margolis, E.B. Ventral tegmental area: Cellular heterogeneity, connectivity and behaviour. Nat. Rev. Neurosci. 2017, 18, 73–85. [Google Scholar] [CrossRef]
- Becker-Krail, D.D.; Walker, W.H.; Nelson, R.J. The Ventral Tegmental Area and Nucleus Accumbens as Circadian Oscillators: Implications for Drug Abuse and Substance Use Disorders. Front. Physiol. 2022, 13, 886704. [Google Scholar] [CrossRef]
- Bariselli, S.; Glangetas, C.; Tzanoulinou, S.; Bellone, C. Ventral tegmental area subcircuits process rewarding and aversive experiences. J. Neurochem. 2016, 139, 1071–1080. [Google Scholar] [CrossRef]
- Oliva, I.; Wanat, M.J. Ventral tegmental area afferents and drug-dependent behaviors. Front. Psychiatry 2016, 7, 181416. [Google Scholar] [CrossRef] [PubMed]
- D’Amelio, M.; Puglisi-Allegra, S.; Mercuri, N. The role of dopaminergic midbrain in Alzheimer’s disease: Translating basic science into clinical practice. Pharmacol. Res. 2018, 130, 414–419. [Google Scholar] [CrossRef] [PubMed]
- Latif, S.; Jahangeer, M.; Maknoon Razia, D.; Ashiq, M.; Ghaffar, A.; Akram, M.; El Allam, A.; Bouyahya, A.; Garipova, L.; Ali Shariati, M.; et al. Dopamine in Parkinson’s disease. Clin. Chim. Acta 2021, 522, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Smiley, J.F.; Mesulam, M.-M. Cholinergic neurons of the nucleus basalis of Meynert receive cholinergic, catecholaminergic and GABAergic synapses: An electron microscopic investigation in the monkey. Neuroscience 1999, 88, 241–255. [Google Scholar] [CrossRef]
- Bartus, R.T.; Dean, R.L., 3rd; Beer, B.; Lippa, A.S. The cholinergic hypothesis of geriatric memory dysfunction. Science 1982, 217, 408–414. [Google Scholar] [CrossRef]
- Solari, N.; Hangya, B. Cholinergic modulation of spatial learning, memory and navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef]
- Abudukeyoumu, N.; Hernandez-Flores, T.; Garcia-Munoz, M.; Arbuthnott, G.W. Cholinergic modulation of striatal microcircuits. Eur. J. Neurosci. 2018, 49, 604–622. [Google Scholar] [CrossRef]
- Grothe, M.J.; Schuster, C.; Bauer, F.; Heinsen, H.; Prudlo, J.; Teipel, S.J. Atrophy of the cholinergic basal forebrain in dementia with Lewy bodies and Alzheimer’s disease dementia. J. Neurol. 2014, 261, 1939–1948. [Google Scholar] [CrossRef] [PubMed]
- Carsana, E.V.; Audano, M.; Breviario, S.; Pedretti, S.; Aureli, M.; Lunghi, G.; Mitro, N. Metabolic Profile Variations along the Differentiation of Human-Induced Pluripotent Stem Cells to Dopaminergic Neurons. Biomedicines 2022, 10, 2069. [Google Scholar] [CrossRef]
- Kim, J.; Jeon, J.; Song, B.; Lee, N.; Ko, S.; Cha, Y.; Leblanc, P.; Seo, H.; Kim, K.S. Spotting-based differentiation of functional dopaminergic progenitors from human pluripotent stem cells. Nat. Protoc. 2022, 17, 890–909. [Google Scholar] [CrossRef]
- Novosadova, E.; Dolotov, O.; Inozemtseva, L.; Novosadova, L.; Antonov, S.; Shimchenko, D.; Bezuglov, V.; Vetchinova, A.; Tarantul, V.; Grivennikov, I.; et al. Influence of N-Arachidonoyl Dopamine and N-Docosahex-aenoyl Dopamine on the Expression of Neurotrophic Factors in Neuronal Differentiated Cultures of Human Induced Pluripotent Stem Cells under Conditions of Oxidative Stress. Antioxidants 2022, 11, 142. [Google Scholar] [CrossRef] [PubMed]
- Jansch, C.; Ziegler, G.C.; Forero, A.; Gredy, S.; Wäldchen, S.; Vitale, M.R.; Svirin, E.; Zöller, J.E.M.; Waider, J.; Günther, K.; et al. Serotonin-specific neurons differentiated from human iPSCs form distinct subtypes with synaptic protein assembly. J. Neural Transm. 2021, 128, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Luo, S.; Qian, W.; Zhang, L.; Chen, C.; Xu, M.; Wang, G.; Wang, Z.; Wang, J.; Wang, W. Developmental deficits and early signs of neurodegeneration revealed by PD patient derived dopamine neurons. Stem Cell Res. 2020, 49, 102027. [Google Scholar] [CrossRef]
- Song, B.; Cha, Y.; Ko, S.; Jeon, J.; Lee, N.; Seo, H.; Park, K.J.; Lee, I.H.; Lopes, C.; Feitosa, M.; et al. Human autologous iPSC-derived dopaminergic progenitors restore motor function in Parkinson’s disease models. J. Clin. Investig. 2020, 130, 904–920. [Google Scholar] [CrossRef]
- Mahajani, S.; Raina, A.; Fokken, C.; Kügler, S.; Bähr, M. Homogenous generation of dopaminergic neurons from multiple hiPSC lines by transient expression of transcription factors. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Bono, F.; Savoia, P.; Guglielmi, A.; Gennarelli, M.; Piovani, G.; Sigala, S.; Leo, D.; Espinoza, S.; Gainetdinov, R.R.; Devoto, P.; et al. Role of Dopamine D2/D3 Receptors in Development, Plasticity, and Neuroprotection in Human iPSC-Derived Midbrain Dopaminergic Neurons. Mol. Neurobiol. 2018, 55, 1054–1067. [Google Scholar] [CrossRef]
- Jung-Klawitter, S.; Blau, N.; Sebe, A.; Ebersold, J.; Göhring, G.; Opladen, T. Generation of an iPSC line from a patient with tyrosine hydroxylase (TH) deficiency: TH-1 iPS.C. Stem Cell Res. 2016, 17, 580–583. [Google Scholar] [CrossRef][Green Version]
- Sheng, Y.; Filichia, E.; Shick, E.; Preston, K.L.; Phillips, K.A.; Cooperman, L.; Lin, Z.; Tesar, P.; Hoffer, B.; Luo, Y. Using iPSC-derived human DA neurons from opioid-dependent subjects to study dopamine dynamics. Brain Behav. 2016, 6, e00491. [Google Scholar] [CrossRef]
- Lu, J.; Zhong, X.; Liu, H.; Hao, L.; Huang, C.T.L.; Sherafat, M.A.; Jones, J.; Ayala, M.; Li, L.; Zhang, S.C. Generation of serotonin neurons from human pluripotent stem cells. Nat. Biotechnol. 2016, 34, 89–94. [Google Scholar] [CrossRef]
- Vadodaria, K.C.; Mertens, J.; Paquola, A.; Bardy, C.; Li, X.; Jappelli, R.; Fung, L.; Marchetto, M.C.; Hamm, M.; Gorris, M.; et al. Generation of functional human serotonergic neurons from fibroblasts. Mol. Psychiatry 2016, 21, 49–61. [Google Scholar] [CrossRef]
- Konagaya, S.; Iwata, H. Microencapsulation of dopamine neurons derived from human induced pluripotent stem cells. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Woodard, C.M.; Campos, B.A.; Kuo, S.H.; Nirenberg, M.J.; Nestor, M.W.; Zimmer, M.; Mosharov, E.V.; Sulzer, D.; Zhou, H.; Paull, D.; et al. IPSC-derived dopamine neurons reveal differences between monozygotic twins discordant for parkinson’s disease. Cell Rep. 2014, 9, 1173–1182. [Google Scholar] [CrossRef]
- Sagal, J.; Zhan, X.; Xu, J.; Tilghman, J.; Karuppagounder, S.S.; Chen, L.; Dawson, V.L.; Dawson, T.M.; Laterra, J.; Ying, M. Proneural Transcription Factor Atoh1 Drives Highly Efficient Differentiation of Human Pluripotent Stem Cells Into Dopaminergic Neurons. Stem Cells Transl. Med. 2014, 3, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Hartfield, E.M.; Yamasaki-Mann, M.; Ribeiro Fernandes, H.J.; Vowles, J.; James, W.S.; Cowley, S.A.; Wade-Martins, R. Physiological characterisation of human iPS-derived dopaminergic neurons. PLoS ONE 2014, 9, e87388. [Google Scholar] [CrossRef]
- Theka, I.; Caiazzo, M.; Dvoretskova, E.; Leo, D.; Ungaro, F.; Curreli, S.; Managò, F.; Dell’Anno, M.T.; Pezzoli, G.; Gainetdinov, R.R.; et al. Rapid Generation of Functional Dopaminergic Neurons From Human Induced Pluripotent Stem Cells Through a Single-Step Procedure Using Cell Lineage Transcription Factors. Stem Cells Transl. Med. 2013, 2, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Danés, A.; Richaud-Patin, Y.; Carballo-Carbajal, I.; Jiménez-Delgado, S.; Caig, C.; Mora, S.; Di Guglielmo, C.; Ezquerra, M.; Patel, B.; Giralt, A.; et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol. Med. 2012, 4, 380–395. [Google Scholar] [CrossRef]
- Caiazzo, M.; Dell’Anno, M.T.; Dvoretskova, E.; Lazarevic, D.; Taverna, S.; Leo, D.; Sotnikova, T.D.; Menegon, A.; Roncaglia, P.; Colciago, G.; et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature 2011, 476, 224–227. [Google Scholar] [CrossRef]
- Nguyen, H.N.; Byers, B.; Cord, B.; Shcheglovitov, A.; Byrne, J.; Gujar, P.; Kee, K.; Schüle, B.; Dolmetsch, R.E.; Langston, W.; et al. LRRK2 mutant iPSC-derived da neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011, 8, 267–280. [Google Scholar] [CrossRef]
- Cooper, O.; Hargus, G.; Deleidi, M.; Blak, A.; Osborn, T.; Marlow, E.; Lee, K.; Levy, A.; Perez-Torres, E.; Yow, A.; et al. Differentiation of human ES and Parkinson’s disease iPS cells into ventral midbrain dopaminergic neurons requires a high activity form of SHH, FGF8a and specific regionalization by retinoic acid. Mol. Cell. Neurosci. 2010, 45, 258–266. [Google Scholar]
- Swistowski, A.; Peng, J.; Liu, Q.; Mali, P.; Rao, M.S.; Cheng, L.; Zeng, X. Efficient generation of functional dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells 2010, 28, 1893–1904. [Google Scholar] [CrossRef]
- Soldner, F.; Hockemeyer, D.; Beard, C.; Gao, Q.; Bell, G.W.; Cook, E.G.; Hargus, G.; Blak, A.; Cooper, O.; Mitalipova, M.; et al. Parkinson’s Disease Patient-Derived Induced Pluripotent Stem Cells Free of Viral Reprogramming Factors. Cell 2009, 136, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Sagar, R.; Zivko, C.; Xydia, A.; Weisman, D.C.; Lyketsos, C.G.; Mahairaki, V. Generation and Characterization of a Human-Derived and Induced Pluripotent Stem Cell (iPSC) Line from an Alzheimer’s Disease Patient with Neuropsychiatric Symptoms. Biomedicines 2023, 11, 3313. [Google Scholar] [CrossRef] [PubMed]
- Sheta, R.; Teixeira, M.; Idi, W.; Oueslati, A. Optimized protocol for the generation of functional human induced-pluripotent-stem-cell-derived dopaminergic neurons. STAR Protoc. 2023, 4, 102486. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.K.; Nakamura, S.; Nakai, K.; Sato, T.; Shiga, T.; Abe, Y.; Hoashi, Y.; Inoue, T.; Akamatsu, W.; Baba, K. Increased excitability of human iPSC-derived neurons in HTR2A variant-related sleep bruxism. Stem Cell Res. 2022, 59, 102658. [Google Scholar] [CrossRef]
- Doi, D.; Magotani, H.; Kikuchi, T.; Ikeda, M.; Hiramatsu, S.; Yoshida, K.; Amano, N.; Nomura, M.; Umekage, M.; Morizane, A.; et al. Pre-clinical study of induced pluripotent stem cell-derived dopaminergic progenitor cells for Parkinson’s disease. Nat. Commun. 2020, 11, 3369. [Google Scholar] [CrossRef]
- Hoashi, Y.; Okamoto, S.; Abe, Y.; Matsumoto, T.; Tanaka, J.; Yoshida, Y.; Imaizumi, K.; Mishima, K.; Akamatsu, W.; Okano, H.; et al. Generation of neural cells using iPSCs from sleep bruxism patients with 5-HT2A polymorphism. J. Prosthodont. Res. 2017, 61, 242–250. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Mouse Embryonic and Adult Fibroblast Cultures by Defined Factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Komatsu, M.; Konagaya, S.; Egawa, E.Y.; Iwata, H. Maturation of human iPS cell-derived dopamine neuron precursors in alginate-Ca2+ hydrogel. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1669–1675. [Google Scholar] [CrossRef]
- Li, H.; Jiang, H.; Li, H.; Li, L.; Yan, Z.; Feng, J. Generation of human A9 dopaminergic pacemakers from induced pluripotent stem cells. Mol. Psychiatry 2022, 27, 4407–4418. [Google Scholar] [CrossRef]
- Stanslowsky, N.; Haase, A.; Martin, U.; Naujock, M.; Leffler, A.; Dengler, R.; Wegner, F. Functional differentiation of midbrain neurons from human cord blood-derived induced pluripotent stem cells. Stem Cell Res. Ther. 2014, 5, 35. [Google Scholar] [CrossRef]
- Xi, J.; Liu, Y.; Liu, H.; Chen, H.; Emborg, M.E.; Zhang, S.C. Specification of midbrain dopamine neurons from primate pluripotent stem cells. Stem Cells 2012, 30, 1655–1663. [Google Scholar] [CrossRef]
- Revilla, A.; González, C.; Iriondo, A.; Fernández, B.; Prieto, C.; Marín, C.; Liste, I. Current advances in the generation of human iPS cells: Implications in cell-based regenerative medicine. J. Tissue Eng. Regen. Med. 2016, 10, 893–907. [Google Scholar] [CrossRef] [PubMed]
- Bayart, E.; Cohen-Haguenauer, O. Technological Overview of iPS Induction from Human Adult Somatic Cells. Curr. Gene Ther. 2013, 13, 73–92. [Google Scholar] [CrossRef] [PubMed]
- Katolikova, N.V.; Khudiakov, A.A.; Shafranskaya, D.D.; Prjibelski, A.D.; Masharskiy, A.E.; Mor, M.S.; Golovkin, A.S.; Zaytseva, A.K.; Neganova, I.E.; Efimova, E.V.; et al. Modulation of Notch Signaling at Early Stages of Differentiation of Human Induced Pluripotent Stem Cells to Dopaminergic Neurons. Int. J. Mol. Sci. 2023, 24, 1429. [Google Scholar] [CrossRef] [PubMed]
- Cardo, L.F.; Monzón-Sandoval, J.; Li, Z.; Webber, C.; Li, M. Single-Cell Transcriptomics and In Vitro Lineage Tracing Reveals Differential Susceptibility of Human iPSC-Derived Midbrain Dopaminergic Neurons in a Cellular Model of Parkinson’s Disease. Cells 2023, 12, 2860. [Google Scholar] [CrossRef] [PubMed]
- Rao, M.S.; Malik, N. Assessing iPSC reprogramming methods for their suitability in translational medicine. J. Cell Biochem. 2012, 113, 3061–3068. [Google Scholar] [CrossRef]
- Tao, Y.; Li, X.; Dong, Q.; Kong, L.; Petersen, A.J.; Yan, Y.; Xu, K.; Zima, S.; Li, Y.; Schmidt, D.K.; et al. Generation of locus coeruleus norepinephrine neurons from human pluripotent stem cells. Nat. Biotechnol. 2023, 1–13. [Google Scholar] [CrossRef]
- Diao, X.; Wang, F.; Becerra-Calixto, A.; Soto, C.; Mukherjee, A. Induced pluripotent stem cell-derived dopaminergic neurons from familial parkinson’s disease patients display α-synuclein pathology and abnormal mitochondrial morphology. Cells 2021, 10, 2402. [Google Scholar] [CrossRef]
- Valiulahi, P.; Vidyawan, V.; Puspita, L.; Oh, Y.; Juwono, V.B.; Sittipo, P.; Friedlander, G.; Yahalomi, D.; Sohn, J.W.; Lee, Y.K.; et al. Generation of caudal-type serotonin neurons and hindbrain-fate organoids from hPSCs. Stem Cell Rep. 2021, 16, 1938–1952. [Google Scholar] [CrossRef]
- Amimoto, N.; Nishimura, K.; Shimohama, S.; Takata, K. Generation of striatal neurons from human induced pluripotent stem cells by controlling extrinsic signals with small molecules. Stem Cell Res. 2021, 55, 102486. [Google Scholar] [CrossRef]
- Hiller, B.M.; Marmion, D.J.; Gross, R.M.; Thompson, C.A.; Chavez, C.A.; Brundin, P.; Wakeman, D.R.; McMahon, C.W.; Kordower, J.H. Mitomycin-C treatment during differentiation of induced pluripotent stem cell-derived dopamine neurons reduces proliferation without compromising survival or function in vivo. Stem Cells Transl. Med. 2021, 10, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Sanz Muñoz, S.; Engel, M.; Balez, R.; Do-Ha, D.; Castro Cabral-Da-Silva, M.; Hernández, D.; Berg, T.; Fifita, J.A.; Grima, N.; Yang, S.; et al. A simple differentiation protocol for generation of induced pluripotent stem cell-derived basal forebrain-like cholinergic neurons for alzheimer’s disease and frontotemporal dementia disease modeling. Cells 2020, 9, 2018. [Google Scholar] [CrossRef] [PubMed]
- Collo, G.; Cavalleri, L.; Chiamulera, C.; Merlo Pich, E. (2 R,6 R)-Hydroxynorketamine promotes dendrite outgrowth in human inducible pluripotent stem cell-derived neurons through AMPA receptor with timing and exposure compatible with ketamine infusion pharmacokinetics in humans. Neuroreport 2018, 29, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Fedele, S.; Collo, G.; Behr, K.; Bischofberger, J.; Müller, S.; Kunath, T.; Christensen, K.; Gündner, A.L.; Graf, M.; Jagasia, R.; et al. Expansion of human midbrain floor plate progenitors from induced pluripotent stem cells increases dopaminergic neuron differentiation potential. Sci. Rep. 2017, 7, 6036. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Morizane, A.; Doi, D.; Onoe, H.; Hayashi, T.; Kawasaki, T.; Saiki, H.; Miyamoto, S.; Takahashi, J. Survival of human induced pluripotent stem cell-derived midbrain dopaminergic neurons in the brain of a primate model of Parkinson’s disease. J. Park. Dis. 2011, 1, 395–412. [Google Scholar] [CrossRef]
- Kriks, S.; Shim, J.W.; Piao, J.; Ganat, Y.M.; Wakeman, D.R.; Xie, Z.; Carrillo-Reid, L.; Auyeung, G.; Antonacci, C.; Buch, A.; et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s disease. Nature 2011, 480, 547–551. [Google Scholar] [CrossRef]
- Inman, G.J.; Nicolás, F.J.; Nicolás, N.; Callahan, J.F.; Harling, J.D.; Gaster, L.M.; Reith, A.D.; Laping, N.J.; Hill, C.S. SB-431542 Is a Potent and Specific Inhibitor of Transforming Growth Factor-Superfamily Type I Activin Receptor-Like Kinase (ALK) Receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 2002, 62, 65–74. [Google Scholar] [CrossRef]
- Boergermann, J.H.; Kopf, J.; Yu, P.B.; Knaus, P. Dorsomorphin and LDN-193189 inhibit BMP-mediated Smad, p38 and Akt signalling in C2C12 cells. Int. J. Biochem. Cell Biol. 2010, 42, 1802–1807. [Google Scholar] [CrossRef]
- Rhee, Y.-H.; Ko, J.-Y.; Chang, M.-Y.; Yi, S.-H.; Kim, D.; Kim, C.-H.; Shim, J.W.; Jo, A.Y.; Kim, B.W.; Lee, H.; et al. Protein-based human iPS cells efficiently generate functional dopamine neurons and can treat a rat model of Parkinson disease. J. Clin. Investig. 2011, 121, 2326–2335. [Google Scholar] [CrossRef]
- Okada, Y.; Shimazaki, T.; Sobue, G.; Okano, H. Retinoic-acid-concentration-dependent acquisition of neural cell identity during in vitro differentiation of mouse embryonic stem cells. Dev. Biol. 2004, 275, 124–142. [Google Scholar] [CrossRef]
- Kittappa, R.; Chang, W.W.; Awatramani, R.B.; McKay, R.D.G. The foxa2 gene controls the birth and spontaneous degeneration of dopamine neurons in old age. PLoS Biol. 2007, 5, 2875–2884. [Google Scholar] [CrossRef] [PubMed]
- Doucet-Beaupré, H.; Ang, S.L.; Lévesque, M. Cell fate determination, neuronal maintenance and disease state: The emerging role of transcription factors Lmx1a and Lmx1b. FEBS Lett. 2015, 589, 3727–3738. [Google Scholar] [CrossRef] [PubMed]
- Moreno, E.L.; Hachi, S.; Hemmer, K.; Trietsch, S.J.; Baumuratov, A.S.; Hankemeier, T.; Vulto, P.; Schwamborn, J.C.; Fleming, R.M. Differentiation of neuroepithelial stem cells into functional dopaminergic neurons in 3D microfluidic cell culture. Lab. Chip. 2015, 15, 2419–2428. [Google Scholar] [CrossRef] [PubMed]
- Sibuea, S.; Ho, J.K.; Pouton, C.W.; Haynes, J.M. TGFβ3, dibutyryl cAMP and a notch inhibitor modulate phenotype late in stem cell-derived dopaminergic neuron maturation. Front. Cell Dev. Biol. 2023, 11, 1111705. [Google Scholar] [CrossRef]
- Flanders, K.C.; Ren, R.F.Â.; Lippa, C.F. Transforming growth factor-βs in neurodegenerative disease. Prog. Neurobiol. 1998, 54, 71–85. [Google Scholar] [CrossRef]
- Schober, A.; Peterziel, H.; von Bartheld, C.S.; Simon, H.; Krieglstein, K.; Unsicker, K. GDNF applied to the MPTP-lesioned nigrostriatal system requires TGF-β for its neuroprotective action. Neurobiol. Dis. 2007, 25, 378–391. [Google Scholar] [CrossRef]
- Luo, S.X.; Timbang, L.; Kim, J.I.; Shang, Y.; Sandoval, K.; Tang, A.A.; Whistler, J.L.; Ding, J.B.; Huang, E.J. TGF-β Signaling in Dopaminergic Neurons Regulates Dendritic Growth, Excitatory-Inhibitory Synaptic Balance, and Reversal Learning. Cell Rep. 2016, 17, 3233–3245. [Google Scholar] [CrossRef]
- Mena, M.A.; Casarejos, M.J.; Bonin, A.; Ramos, J.A.; De Yébenes, G. Effects of Dibutyryl Cyclic AMP and Retinoic Acid on the Differentiation of Dopamine Neurons: Prevention of Cell Death by Dibutyryl Cyclic AMP. J. Neurochem. 1995, 65, 2612–2620. [Google Scholar] [CrossRef]
- Wulansari, N.; Kim, E.H.; Sulistio, Y.A.; Rhee, Y.H.; Song, J.J.; Lee, S.H. Vitamin C-Induced Epigenetic Modifications in Donor NSCs Establish Midbrain Marker Expressions Critical for Cell-Based Therapy in Parkinson’s Disease. Stem Cell Rep. 2017, 9, 1192–1206. [Google Scholar] [CrossRef][Green Version]
- Pattyn, A.; Goridis, C.; Brunet, J.F. Specification of the central noradrenergic phenotype by the homeobox gene Phox2b. Mol. Cell Neurosci. 2000, 15, 235–243. [Google Scholar] [CrossRef]
- Morin, X.; Cremer, H.; Hirsch, M.-R. Defects in Sensory and Autonomic Ganglia and Absence of Locus Coeruleus in Mice Deficient for the Homeobox Gene Phox2a. Neuron 1997, 18, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Vera, E.; Bosco, N.; Studer, L. Generating Late-Onset Human iPSC-Based Disease Models by Inducing Neuronal Age-Related Phenotypes through Telomerase Manipulation. Cell Rep. 2016, 17, 1184–1192. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Jiang, H.; Hu, Z.; Fan, K.; Wang, J.; Janoschka, S.; Wang, X.; Ge, S.; Feng, J. Parkin Mutations Reduce the Complexity of Neuronal Processes in iPSC-Derived Human Neurons. Stem Cells 2015, 33, 68–78. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beevers, J.E.; Lai, M.C.; Collins, E.; Booth, H.D.E.; Zambon, F.; Parkkinen, L.; Vowles, J.; Cowley, S.A.; Wade-Martins, R.; Caffrey, T.M. MAPT Genetic Variation and Neuronal Maturity Alter Isoform Expression Affecting Axonal Transport in iPSC-Derived Dopamine Neurons. Stem Cell Rep. 2017, 9, 587–599. [Google Scholar] [CrossRef]
- Zambon, F.; Cherubini, M.; Fernandes, H.J.R.; Lang, C.; Ryan, B.J.; Volpato, V.; Bengoa-Vergniory, N.; Vingill, S.; Attar, M.; Booth, H.D.E.; et al. Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum. Mol. Genet. 2019, 28, 2001–2013. [Google Scholar] [CrossRef]
- Fernandes, H.J.R.; Hartfield, E.M.; Christian, H.C.; Emmanoulidou, E.; Zheng, Y.; Booth, H.; Bogetofte, H.; Lang, C.; Ryan, B.J.; Sardi, S.P.; et al. ER Stress and Autophagic Perturbations Lead to Elevated Extracellular α-Synuclein in GBA-N370S Parkinson’s iPSC-Derived Dopamine Neurons. Stem Cell Rep. 2016, 6, 342–356. [Google Scholar] [CrossRef]
- Kumar, M.; Acevedo-Cintrón, J.; Jhaldiyal, A.; Wang, H.; Andrabi, S.A.; Eacker, S.; Karuppagounder, S.S.; Brahmachari, S.; Chen, R.; Kim, H.; et al. Defects in Mitochondrial Biogenesis Drive Mitochondrial Alterations in PARKIN-Deficient Human Dopamine Neurons. Stem Cell Rep. 2020, 15, 629–645. [Google Scholar] [CrossRef]
- Mazzulli, J.R.; Zunke, F.; Tsunemi, T.; Toker, N.J.; Jeon, S.; Burbulla, L.F.; Patnaik, S.; Sidransky, E.; Marugan, J.J.; Sue, C.M.; et al. Activation of β-Glucocerebrosidase Reduces Pathological α-Synuclein and Restores Lysosomal Function in Parkinson’s Patient Midbrain Neurons. J. Neurosci. 2016, 36, 7693–7706. [Google Scholar] [CrossRef]
- Lang, C.; Campbell, K.R.; Ryan, B.J.; Carling, P.; Attar, M.; Vowles, J.; Perestenko, O.V.; Bowden, R.; Baig, F.; Kasten, M.; et al. Single-Cell Sequencing of iPSC-Dopamine Neurons Reconstructs Disease Progression and Identifies HDAC4 as a Regulator of Parkinson Cell Phenotypes. Cell Stem Cell 2019, 24, 93–106.e6. [Google Scholar] [CrossRef] [PubMed]
- Cukier, H.N.; Kim, H.; Griswold, A.J.; Codreanu, S.G.; Prince, L.M.; Sherrod, S.D.; McLean, J.A.; Dykxhoorn, D.M.; Ess, K.C.; Hedera, P.; et al. Genomic, transcriptomic, and metabolomic profiles of hiPSC-derived dopamine neurons from clinically discordant brothers with identical PRKN deletions. Npj Park. Dis. 2022, 8, 1–17. [Google Scholar] [CrossRef]
- López de Maturana, R.; Lang, V.; Zubiarrain, A.; Sousa, A.; Vázquez, N.; Gorostidi, A.; Águila, J.; López de Munain, A.; Rodríguez, M.; Sánchez-Pernaute, R. Mutations in LRRK2 impair NF-ΚB pathway in iPSC-derived neurons. J. Neuroinflam. 2016, 13, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.I.; Bogetofte, H.; Ritter, L.; Agergaard, J.B.; Hammerich, D.; Kabiljagic, A.A.; Wlodarczyk, A.; Lopez, S.G.; Sørensen, M.D.; Jørgensen, M.L.; et al. Microglia-Secreted Factors Enhance Dopaminergic Differentiation of Tissue- and iPSC-Derived Human Neural Stem Cells. Stem Cell Rep. 2021, 16, 281–294. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Yin, X.; Jhaldiyal, A.; Khan, M.R.; Martin, I.; Xie, Z.; Perez-Rosello, T.; Kumar, M.; Abalde-Atristain, L.; Xu, J.; et al. Defects in mRNA Translation in LRRK2-Mutant hiPSC-Derived Dopaminergic Neurons Lead to Dysregulated Calcium Homeostasis. Cell Stem Cell 2020, 27, 633–645.e7. [Google Scholar] [CrossRef] [PubMed]
- Azkona, G.; López de Maturana, R.; Del Rio, P.; Sousa, A.; Vazquez, N.; Zubiarrain, A.; Jimenez-Blasco, D.; Bolaños, J.P.; Morales, B.; Auburger, G.; et al. LRRK2 expression is deregulated in fibroblasts and neurons from parkinson patients with mutations in PINK1. Mol. Neurobiol. 2018, 55, 506–516. [Google Scholar] [CrossRef]
- Kuzumaki, N.; Suda, Y.; Iwasawa, C.; Narita, M.; Sone, T.; Watanabe, M.; Maekawa, A.; Matsumoto, T.; Akamatsu, W.; Igarashi, K.; et al. Cell-specific overexpression of COMT in dopaminergic neurons of Parkinson’s disease. Brain 2019, 142, 1675–1689. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, O.; Poberezhniy, D.; Novosadova, E.; Gerasimova, T.; Novosadova, L.; Arsenyeva, E.; Stepanenko, E.; Shimchenko, D.; Volovikov, E.; Anufrieva, K.; et al. Overexpression of Parkin in the Neuronal Progenitor Cells from a Patient with Parkinson’s Disease Shifts the Transcriptome Towards the Normal State. Mol. Neurobiol. 2023, 60, 3522–3533. [Google Scholar] [CrossRef]
- Oni, E.N.; Halikere, A.; Li, G.; Toro-Ramos, A.J.; Swerdel, M.R.; Verpeut, J.L.; Moore, J.C.; Bello, N.T.; Bierut, L.J.; Goate, A.; et al. Increased nicotine response in iPSC-derived human neurons carrying the CHRNA5 N398 allele. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Collo, G.; Cavalleri, L.; Zoli, M.; Maskos, U.; Ratti, E.; Pich, E.M. Alphα6-containing nicotinic acetylcholine receptors mediate nicotine-induced structural plasticity in mouse and human iPSC-derived dopaminergic neurons. Front. Pharmacol. 2018, 9, 344362. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Savoia, P.; Barbon, A.; Bellucci, A.; Missale, C.; Fiorentini, C. Nicotine prevents alpha-synuclein accumulation in mouse and human iPSC-derived dopaminergic neurons through activation of the dopamine D3- acetylcholine nicotinic receptor heteromer. Neurobiol. Dis. 2019, 129, 1–12. [Google Scholar] [CrossRef]
- Mutti, V.; Bono, F.; Tomasoni, Z.; Bontempi, L.; Guglielmi, A.; Bolognin, S.; Schwamborn, J.C.; Missale, C.; Fiorentini, C. Structural Plasticity of Dopaminergic Neurons Requires the Activation of the D3R-nAChR Heteromer and the PI3K-ERK1/2/Akt-Induced Expression of c-Fos and p70S6K Signaling Pathway. Mol. Neurobiol. 2022, 59, 2129–2149. [Google Scholar] [CrossRef]
- Bono, F.; Mutti, V.; Devoto, P.; Bolognin, S.; Schwamborn, J.C.; Missale, C.; Fiorentini, C. Impaired dopamine D3 and nicotinic acetylcholine receptor membrane localization in iPSCs-derived dopaminergic neurons from two Parkinson’s disease patients carrying the LRRK2 G2019S mutation. Neurobiol. Aging 2021, 99, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Novak, G.; Kyriakis, D.; Grzyb, K.; Bernini, M.; Rodius, S.; Dittmar, G.; Finkbeiner, S.; Skupin, A. Single-cell transcriptomics of human iPSC differentiation dynamics reveal a core molecular network of Parkinson’s disease. Commun. Biol. 2022, 5, 1–19. [Google Scholar] [CrossRef]
- Ren, Y.; Jiang, H.; Pu, J.; Li, L.; Wu, J.; Yan, Y.; Zhao, G.; Guttuso, T.J.; Zhang, B.; Feng, J. Molecular Features of Parkinson’s Disease in Patient-Derived Midbrain Dopaminergic Neurons. Mov. Disord. 2022, 37, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Vadodaria, K.C.; Ji, Y.; Skime, M.; Paquola, A.C.; Nelson, T.; Hall-Flavin, D.; Heard, K.J.; Fredlender, C.; Deng, Y.; Elkins, J.; et al. Altered serotonergic circuitry in SSRI-resistant major depressive disorder patient-derived neurons. Mol. Psychiatry 2019, 24, 808–818. [Google Scholar] [CrossRef]
- Vadodaria, K.C.; Ji, Y.; Skime, M.; Paquola, A.; Nelson, T.; Hall-Flavin, D.; Fredlender, C.; Heard, K.J.; Deng, Y.; Le, A.T.; et al. Serotonin-induced hyperactivity in SSRI-resistant major depressive disorder patient-derived neurons. Mol. Psychiatry 2019, 24, 795–807. [Google Scholar] [CrossRef]
- Seifan, A.; Ganzer, C.A.; Ryon, K.; Lin, M.; Mahmudur, R.; Adolfo, H.; Shih, C.; Jacobs, A.R.; Greenwald, M.; Isaacson, R.S. Detecting Non-cognitive Features of Prodromal Neurodegenerative Diseases. Curr. Aging Sci. 2019, 11, 242–249. [Google Scholar] [CrossRef]
- Neely, M.D.; Davison, C.A.; Aschner, M.; Bowman, A.B. From the Cover: Manganese and Rotenone-Induced Oxidative Stress Signatures Differ in iPSC-Derived Human Dopamine Neurons. Toxicol. Sci. 2017, 159, 366–379. [Google Scholar] [CrossRef] [PubMed]
- Pamies, D.; Wiersma, D.; Katt, M.E.; Zhao, L.; Burtscher, J.; Harris, G.; Smirnova, L.; Searson, P.C.; Hartung, T.; Hogberg, H.T. Human IPSC 3D brain model as a tool to study chemical-induced dopaminergic neuronal toxicity. Neurobiol. Dis. 2022, 169, 105719. [Google Scholar] [CrossRef]
- Cavalleri, L.; Merlo Pich, E.; Millan, M.J.; Chiamulera, C.; Kunath, T.; Spano, P.F.; Collo, G. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol. Psychiatry 2017, 23, 812–823. [Google Scholar] [CrossRef]
- Collo, G.; Cavalleri, L.; Merlo Pich, E. Structural Plasticity Induced by Ketamine in Human Dopaminergic Neurons as Mechanism Relevant for Treatment-Resistant Depression. Chronic Stress 2019, 3, 2470547019842545. [Google Scholar] [CrossRef]
- Laperle, A.H.; Sances, S.; Yucer, N.; Dardov, V.J.; Garcia, V.J.; Ho, R.; Fulton, A.N.; Jones, M.R.; Roxas, K.M.; Avalos, P.; et al. iPSC modeling of young-onset Parkinson’s disease reveals a molecular signature of disease and novel therapeutic candidates. Nat. Med. 2020, 26, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Orlando, R.; Ginerete, R.P.; Cavalleri, L.; Aliperti, V.; Imbriglio, T.; Battaglia, G.; Zuena, A.R.; Nicoletti, F.; Merlo Pich, E.; Collo, G. Synergic action of L-acetylcarnitine and L-methylfolate in Mouse Models of Stress-Related Disorders and Human iPSC-Derived Dopaminergic Neurons. Front. Pharmacol. 2022, 13, 913210. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, H.J.R.; Patikas, N.; Foskolou, S.; Field, S.F.; Park, J.E.; Byrne, M.L.; Bassett, A.R.; Metzakopian, E. Single-Cell Transcriptomics of Parkinson’s Disease Human In Vitro Models Reveals Dopamine Neuron-Specific Stress Responses. Cell Rep. 2020, 33, 108263. [Google Scholar] [CrossRef]
- Inoue, S.; Nishimura, K.; Gima, S.; Nakano, M.; Takata, K. CRISPR-Cas9-Edited SNCA Knockout Human Induced Pluripotent Stem Cell-Derived Dopaminergic Neurons and Their Vulnerability to Neurotoxicity. Biol. Pharm. Bull. 2023, 46, 517–522. [Google Scholar] [CrossRef]
- Suda, Y.; Kuzumaki, N.; Sone, T.; Narita, M.; Tanaka, K.; Hamada, Y.; Iwasawa, C.; Shibasaki, M.; Maekawa, A.; Matsuo, M.; et al. Down-regulation of ghrelin receptors on dopaminergic neurons in the substantia nigra contributes to Parkinson’s disease-like motor dysfunction. Mol. Brain 2018, 11, 1–9. [Google Scholar] [CrossRef]
- Korecka, J.A.; Talbot, S.; Osborn, T.M.; de Leeuw, S.M.; Levy, S.A.; Ferrari, E.J.; Moskites, A.; Atkinson, E.; Jodelka, F.M.; Hinrich, A.J.; et al. Neurite Collapse and Altered ER Ca2+ Control in Human Parkinson Disease Patient iPSC-Derived Neurons with LRRK2 G2019S Mutation. Stem Cell Rep. 2019, 12, 29–41. [Google Scholar] [CrossRef]
- Lewitt, P.A.; Library, W.O. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics. Mov. Disord. 2015, 30, 64–72. [Google Scholar] [CrossRef]
- Moriarty, N.; Kauhausen, J.A.; Pavan, C.; Hunt, C.P.J.; de Luzy, I.R.; Penna, V.; Ermine, C.M.; Thompson, L.H.; Parish, C.L. Understanding the Influence of Target Acquisition on Survival, Integration, and Phenotypic Maturation of Dopamine Neurons within Stem Cell-Derived Neural Grafts in a Parkinson’s Disease Model. J. Neurosci. 2022, 42, 4995–5006. [Google Scholar] [CrossRef] [PubMed]
- Fu, C.L.; Dong, B.C.; Jiang, X.; Li, D.; Yao, J. A cell therapy approach based on iPSC-derived midbrain organoids for the restoration of motor function in a Parkinson’s disease mouse model. Heliyon 2024, 10, e24234. [Google Scholar] [CrossRef]
- Zygogianni, O.; Kouroupi, G.; Taoufik, E.; Matsas, R. Engraftable Induced Pluripotent Stem Cell -Derived Neural Precursors for Brain Repair. Methods Mol. Biol. 2020, 2155, 23–39. [Google Scholar]
- Hills, R.; Mossman, J.A.; Bratt-Leal, A.M.; Tran, H.; Williams, R.M.; Stouffer, D.G.; Sokolova, I.V.; Sanna, P.P.; Loring, J.F.; Lelos, M.J. Neurite Outgrowth and Gene Expression Profile Correlate with Efficacy of Human Induced Pluripotent Stem Cell-Derived Dopamine Neuron Grafts. Stem Cells Dev. 2023, 32, 387–397. [Google Scholar] [CrossRef] [PubMed]
- de Luzy, I.R.; Pavan, C.; Moriarty, N.; Hunt, C.P.J.; Vandenhoven, Z.; Khanna, A.; Niclis, J.C.; Gantner, C.W.; Thompson, L.H.; Parish, C.L. Identifying the optimal developmental age of human pluripotent stem cell-derived midbrain dopaminergic progenitors for transplantation in a rodent model of Parkinson’s disease. Exp. Neurol. 2022, 358, 114219. [Google Scholar] [CrossRef] [PubMed]
- Shrigley, S.; Nilsson, F.; Mattsson, B.; Fiorenzano, A.; Mudannayake, J.; Bruzelius, A.; Ottosson, D.R.; Björklund, A.; Hoban, D.B.; Parmar, M. Grafts Derived from an α-Synuclein Triplication Patient Mediate Functional Recovery but Develop Disease-Associated Pathology in the 6-OHDA Model of Parkinson’s Disease. J. Park. Dis. 2021, 11, 515–528. [Google Scholar] [CrossRef]
- Hiller, B.M.; Marmion, D.J.; Thompson, C.A.; Elliott, N.A.; Federoff, H.; Brundin, P.; Mattis, V.B.; McMahon, C.W.; Kordower, J.H. Optimizing maturity and dose of iPSC-derived dopamine progenitor cell therapy for Parkinson’s disease. Npj Regen. Med. 2022, 7, 1–15. [Google Scholar] [CrossRef]
- Schweitzer, J.S.; Song, B.; Herrington, T.M.; Park, T.-Y.; Lee, N.; Ko, S.; Jeon, J.; Cha, Y.; Kim, K.; Li, Q.; et al. Personalized iPSC-Derived Dopamine Progenitor Cells for Parkinson’s Disease. N. Engl. J. Med. 2020, 382, 1926–1932. [Google Scholar] [CrossRef]
- Grow, D.A.; McCarrey, J.R.; Navara, C.S. Advantages of nonhuman primates as preclinical models for evaluating stem cell-based therapies for Parkinson’s disease. Stem Cell Res. 2016, 17, 352–366. [Google Scholar] [CrossRef] [PubMed]
- Weinshenker, D. Long road to ruin: Noradrenergic dysfunction in neurodegenerative disease. Trends Neurosci. 2018, 41, 211–223. [Google Scholar] [CrossRef]
- Kelly, S.C.; Nelson, P.T.; Counts, S.E. Pontine Arteriolosclerosis and Locus Coeruleus Oxidative Stress Differentiate Resilience from Mild Cognitive Impairment in a Clinical Pathologic Cohort. J. Neuropathol. Exp. Neurol. 2021, 80, 325–335. [Google Scholar] [CrossRef]
- Kang, S.S.; Ahn, E.H.; Liu, X.; Bryson, M.; Miller, G.W.; Weinshenker, D.; Ye, K. ApoE4 inhibition of VMAT2 in the locus coeruleus exacerbates Tau pathology in Alzheimer’s disease. Acta Neuropathol. 2021, 142, 139–158. [Google Scholar] [CrossRef]
- Kang, S.S.; Meng, L.; Zhang, X.; Wu, Z.; Mancieri, A.; Xie, B.; Liu, X.; Weinshenker, D.; Peng, J.; Zhang, Z.; et al. Tau modification by the norepinephrine metabolite DOPEGAL stimulates its pathology and propagation. Nat. Struct. Mol. Biol. 2022, 29, 292–305. [Google Scholar] [CrossRef]
- Kang, S.S.; Liu, X.; Ahn, E.H.; Xiang, J.; Manfredsson, F.P.; Yang, X.; Luo, H.R.; Liles, L.C.; Weinshenker, D.; Ye, K. Norepinephrine metabolite DOPEGAL activates AEP and pathological Tau aggregation in locus coeruleus. J. Clin. Investig. 2020, 130, 422–437. [Google Scholar] [CrossRef] [PubMed]
- Capucciati, A.; Zucca, F.A.; Monzani, E.; Zecca, L.; Casella, L.; Hofer, T. Interaction of Neuromelanin with Xenobiotics and Consequences for Neurodegeneration; Promising Experimental Models. Antioxidants 2021, 10, 824. [Google Scholar] [CrossRef] [PubMed]
- Zucca, F.A.; Bellei, C.; Giannelli, S.; Terreni, M.R.; Gallorini, M.; Rizzio, E.; Pezzoli, G.; Albertini, A.; Zecca, L. Neuromelanin and iron in human locus coeruleus and substantia nigra during aging: Consequences for neuronal vulnerability. J. Neural Transm. 2006, 113, 757–767. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.J.; Castaño, A.; Venero, J.L.; Cano, J.; Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef]
- Song, S.; Wang, Q.; Jiang, L.; Oyarzabal, E.; Riddick, N.V.; Wilson, B.; Moy, S.S.; Shih, Y.I.; Hong, J.S. Noradrenergic dysfunction accelerates LPS-elicited inflammation-related ascending sequential neurodegeneration and deficits in non-motor/motor functions. Brain Behav. Immun. 2019, 81, 374–387. [Google Scholar] [CrossRef]
| Anatomical Definition | Histochemical Classification (Neurotransmitter) | CNS Targets | Physiological Effects |
|---|---|---|---|
| Dorsal Raphe Nucleus (DRN) | B8–B9 Serotonin (5HT) | Brainstem, hypothalamus, amygdala, hippocampus, cortex | Wake-promoting nucleus; pain and vegetative function regulation; mood and behavior modulation. |
| Locus Coeruleus (LC) | A6 Noradrenaline (NA) | Virtually the whole CNS | Wake-promoting nucleus; attention focusing and shifting; learning and memory promotion. |
| Substantia Nigra pars compacta (SNpc) | A9 Dopamine (DA) | Basal ganglia, thalamus, motor cortex | Movement regulation; planning and execution |
| Ventral Tegmental Area (VTA) | A10 Dopamine (DA) | Amygdala, hippocampus, nucleus accumbens, frontal cortex | Reward process and reinforced learning promotion; executive functions regulations. |
| Pedunculopontine Nucleus (PPN) and Lateral Tegmental Nucleus (LTN) | Ch5 and Ch6 Acetylcholine (Ach) | Other brainstem nuclei, hypothalamus | Wake-promoting nuclei |
| Nucleus Basalis of Meynert (NBM), Medial Septum Nucleus (MSN), horizontal and vertical limbs of the diagonal band of Broca (hDBB and vDBB) | Ch1, Ch2, Ch3 and Ch4 Acetylcholine (Ach) | Isocortex (NBM), hippocampus (MSN and vDBB), and olfactory cortex (hDBB) | Cortical activation and desynchronization; learning and memory promotion. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galgani, A.; Scotto, M.; Giorgi, F.S. The Neuroanatomy of Induced Pluripotent Stem Cells: In Vitro Models of Subcortical Nuclei in Neurodegenerative Disorders. Curr. Issues Mol. Biol. 2024, 46, 10180-10199. https://doi.org/10.3390/cimb46090607
Galgani A, Scotto M, Giorgi FS. The Neuroanatomy of Induced Pluripotent Stem Cells: In Vitro Models of Subcortical Nuclei in Neurodegenerative Disorders. Current Issues in Molecular Biology. 2024; 46(9):10180-10199. https://doi.org/10.3390/cimb46090607
Chicago/Turabian StyleGalgani, Alessandro, Marco Scotto, and Filippo S. Giorgi. 2024. "The Neuroanatomy of Induced Pluripotent Stem Cells: In Vitro Models of Subcortical Nuclei in Neurodegenerative Disorders" Current Issues in Molecular Biology 46, no. 9: 10180-10199. https://doi.org/10.3390/cimb46090607
APA StyleGalgani, A., Scotto, M., & Giorgi, F. S. (2024). The Neuroanatomy of Induced Pluripotent Stem Cells: In Vitro Models of Subcortical Nuclei in Neurodegenerative Disorders. Current Issues in Molecular Biology, 46(9), 10180-10199. https://doi.org/10.3390/cimb46090607






