Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tribulus terrestris Fruits Collection
2.2. Preparation of TT Extracts
2.3. Identification of Methicillin-Resistant S. epidermidis (MRSE)
2.4. Antimicrobial Susceptibility Testing of MRSE
2.5. Well Diffusion Assay for Anti-MRSE Activity of Methanolic Fruit Extract
2.6. TT Fractionation Using Gradient HPLC
2.7. Well Diffusion Assay for Anti-MRSE Activity of HPLC Fractions
2.8. Identification of Bioactive Compounds
2.9. Prediction of Lead Hits
2.10. Preparation of PBP2a for Docking Assessment
2.11. Statistical Analysis
3. Results
3.1. Confirmation of MRSE
3.2. HPLC Fractions of TT Methanol Extract Against MRSE
3.3. Identification of Bioactive Compounds by GC-MS Analysis
3.4. Druggable Characteristics of Bioactive Compounds
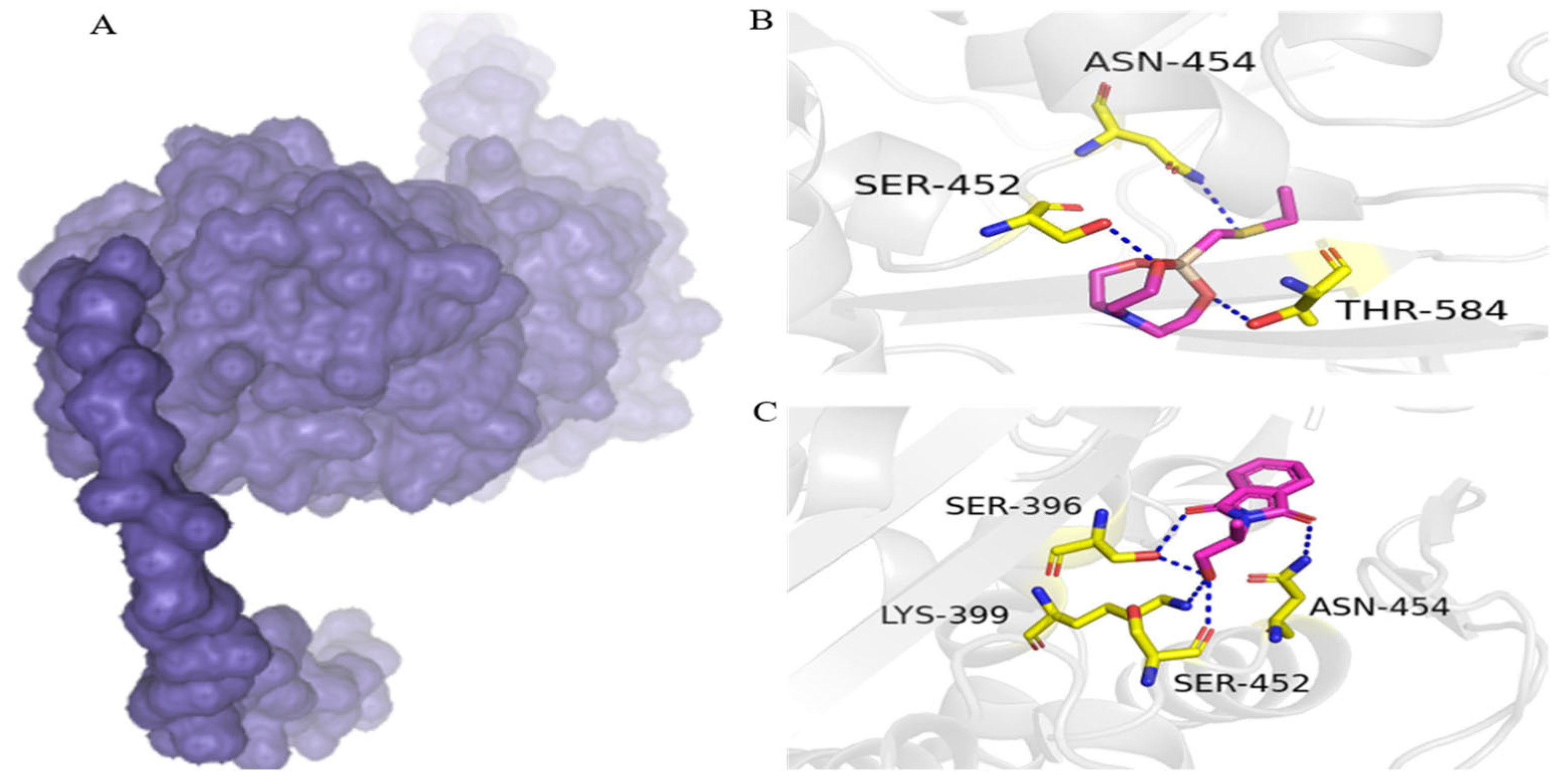
3.5. Putative PBP2a Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doughari, J.H.; El-mahmood, A.M.; Tyoyina, I. Antimicrobial activity of leaf extracts of Senna obstusifolia. Afr. J. Pharm. Pharmacol. 2008, 2, 344–346. [Google Scholar]
- Kivanç, M.; Kunduhoğlu, B. Antimıcrobial activity of fresh plant juice on the growth of bacteria and yeast. J. Qafqaz Univ. 1997, 1, 27–35. [Google Scholar]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu India. BMC Complement. Altern. Med. 2006, 6, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Dey, P.; Lamb, C. Phytoalexins: Enzymology and molecular biology. Adv. Enzymol. 1983, 55, 1–69. [Google Scholar]
- Gauthaman, K.; Adaikan, P.G.; Prasad, R.N. Aphrodisiac properties of Tribulus terrestris extract (Protodioscin) in normal and castrated rats. Life Sci. 2002, 71, 1385–1396. [Google Scholar] [CrossRef]
- Chhatre, S.; Nesari, T.; Somani, G.; Kanchan, D.; Sathaye, S. Phytopharmacological overview of Tribulus terrestris. Pharmacogn. Rev. 2014, 8, 45–51. [Google Scholar] [CrossRef]
- Al-Asmari, A.K.; Al-Elaiwi, A.M.; Athar, M.T.; Tariq, M.; Al Eid, A.; Al-Asmary, S.M. A review of hepatoprotective plants used in Saudi traditional medicine. Evid.-Based Complement. Altern. Med. 2014, 2014, 890842. [Google Scholar] [CrossRef]
- Wu, T.S.; Shi, L.S.; Kuo, S.C. Alkaloids and other constituents from Tribulus terrestris. Phytochemistry 1999, 50, 1411–1415. [Google Scholar] [CrossRef]
- Shahid, M.; Riaz, M.; Talpur, M.M.; Pirzada, T. Phytopharmacology of Tribulus terrestris. J. Biol. Regul. Homeost. Agents 2016, 30, 785–788. [Google Scholar]
- Zhu, W.; Du, Y.; Meng, H.; Dong, Y.; Li, L. A review of traditional pharmacological uses, phytochemistry, and pharmacological activities of Tribulus terrestris. Chem. Cent. J. 2017, 11, 60. [Google Scholar] [CrossRef]
- Xu, Z.; Cave, R.; Chen, L.; Yangkyi, T.; Liu, Y.; Li, K.; Meng, G.; Niu, K.; Zhang, W.; Tang, N.; et al. Antibiotic resistance and molecular characteristics of methicillin-resistant Staphylococcus epidermidis re-covered from hospital personnel in China. J. Glob. Antimicrob. Resist. 2020, 22, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Coagulase-negative staphylococci as reservoirs of genes facilitating MRSA infection: Staphylococcal commensal species such as Staphylococcus epidermidis are being recognized as important sources of genes promoting MRSA colonization and virulence. Bioessays 2013, 35, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Diekema, D.J.; Pfaller, M.A.; Schmitz, F.J.; Smayevsky, J.; Bell, J.; Jones, R.N.; Beach, M. Survey of infections due to Staphylococcus species: Frequency of occurrence and antimicrobial susceptibility of isolates collected in the United States, Canada, Latin America, Europe, and the Western Pacific region for the SENTRY Antimicrobial Surveillance Program, 1997–1999. Clin. Infect. Dis. 2001, 32, S114–S132. [Google Scholar] [PubMed]
- Gaisford, W.C.; Reynolds, P.E. Methicillin resistance in Staphylococcus epidermidis Relationship between the additional penicillin-binding protein and an attachment transpeptidase. Eur. J. Biochem. 1989, 185, 211–218. [Google Scholar] [CrossRef]
- Mariana, G.P.; Hermínia de, L.; Alexander, T. An acquired and a native penicillin-binding protein cooperate in building the cell wall of drug-resistant staphylococci. Proc. Natl. Acad. Sci. USA 2001, 98, 10886–10891. [Google Scholar]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial bio-films. Int. J. Med. Microbiol. 2002, 292, 107–113. [Google Scholar] [CrossRef]
- Liu, L.G.; Zhu, Y.L.; Hu, L.F.; Cheng, J.; Ye, Y.; Li, J.B. Comparative study of the mutant prevention concentrations of vancomycin alone and in combination with levofloxacin, rifampicin and fosfomycin against methicillin-resistant Staphylococcus epidermidis. J. Antibiot. 2013, 66, 709–712. [Google Scholar] [CrossRef]
- Baldi, A. Computational Approaches for Drug Design and Discovery: An Overview. Syst. Rev. Pharm. 2010, 1, 99. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Bouamrane, S.; Khaldan, A.; Hajji, H.; El-mernissi, R.; Maghat, H.; Ajana, M.A.; Sbai, A.; Bouachrine, M.; Lakhlifi, T. 3D-QSAR, molecular docking, molecular dynamic simulation, and ADMET study of bioactive compounds against candida albicans. Mor. J. Chem. 2022, 10, 523–541. [Google Scholar]
- Pinzi, L.; Rastelli, G. Molecular Docking: Shifting Paradigms in Drug Discovery. Int. J. Mol. Sci. 2019, 20, 4331. [Google Scholar] [CrossRef] [PubMed]
- Lahyaoui, M.; El-Idrissi, H.; Saffaj, T.; Ihssane, B.; Saffaj, N.; Mamouni, R.; Rodi, Y.K. QSAR modeling, molecular docking and molecular dynamic simulation of phosphorus-substituted quinoline derivatives as topoisomerase I inhibitors. Arab. J. Chem. 2023, 16, 104783. [Google Scholar] [CrossRef]
- Priyanka, B.; Emanuel, K.; Mathias, D.; Robert, P. ProTox 3.0: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2024, 52, 513–520. [Google Scholar]
- Wendakoon, C.; Calderon, P.; Gagnon, D. Evaluation of selected medicinal plants extracted in different ethanol concentrations for antibacterial activity against human pathogens. J. Med. Act. Plants 2012, 1, 60–68. [Google Scholar]
- Alipour, M.; Khanmohammadi, O. Antibacterial activity of plant extracts against oral and skin pathogens. Afr. J. Microbiol. Res. 2011, 5, 2909–2911. [Google Scholar]
- Ahmad, S.; Rahman, H.; Mumtaz, S.; Qasim, M.; Rahman, Z.U.; Alsuwat, M.A.; Halawani, I.F.; Alzahrani, F.M.; Ali, S. mecA and fdh: Markers of pathogenicity and commensalism in Staphylococcus epidermidis of pediatric origin from Pakistan. Diagn. Microbiol. Infect. Dis. 2024, 108, 116109. [Google Scholar] [CrossRef]
- Bauer, A.W.; Perry, D.M.; Kirby, W.M.M. Single disc antibiotic sensitivity testing of Staphylococci. Arch. Intern. Med. 1959, 104, 208–216. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standard Institute (CLSI). Performance Standards for Anti-Microbial Susceptibility Testing, 30th ed.; M100; Clinical and Laboratory Standard Institute: Wayne, PA, USA, 2020. [Google Scholar]
- Azarm, A.; Ayoobi, F.; Zare-Bidaki, M.; Taheri, M.; Zarandi, E.R. Antibacterial and antibiofilm activities of Tribulus terrestris methanolic extract against Streptococcus mutans, Streptococcus sobrinus, and Lactobacillus acidophilus: An in vitro study. Dent. Res. J. 2024, 21, 57. [Google Scholar] [CrossRef]
- Khan, I.; Rahman, H.; Abd El-Salam, N.M.; Tawab, A.; Hussain, A.; Khan, T.A.; Khan, U.A.; Qasim, M.; Adnan, M.; Azizullah, A. Punica granatum peel extracts: HPLC fractionation and LC MS analysis to quest compounds having activity against multidrug resistant bacteria. BMC Complement. Altern. Med. 2017, 17, 247. [Google Scholar] [CrossRef]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Orabi, M.A.A.; Abdel-Wahab, B.A.; Walbi, I.A.; Habeeb, M.S.; Khateeb, M.M.; Shettar, A.K.; Hoskeri, J.H. Tribulus terrestris Cytotoxicity against Breast Cancer MCF-7 and Lung Cancer A549 Cell Lines Is Mediated via Activation of Apoptosis, Caspase-3, DNA Degradation, and Suppressing Bcl-2 Activity. Separations 2022, 9, 383. [Google Scholar] [CrossRef]
- Amani, A.T.; Tahany, A.T.; Zahraa, A.E.; Al, N.; ZIinah, A.K. Phytochemical Investigation and GC-MS analysis of Tribulus terrestris L. cultivated in Iraq. OBAT J. Ris. Ilmu Farm. Dan. Kesehat. 2024, 2, 22–29. [Google Scholar] [CrossRef]
- Stefanescu, R.; Tero-Vescan, A.; Negroiu, A.; Aurică, E.; Vari, C.-E. A Comprehensive Review of the Phytochemical, Pharmacological, and Toxicological Properties of Tribulus terrestris L. Biomolecules 2020, 10, 752. [Google Scholar] [CrossRef] [PubMed]
- Rhetso, T.; Shubharani, R.; Roopa, M.S.; Sivaram, V. Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Futur. J. Pharm. Sci. 2020, 6, 102. [Google Scholar] [CrossRef]
- Batista, C.R.A.; Godin, A.M.; Melo, I.S.F.; Coura, G.M.E.; Matsui, T.C.; Dutra, M.B.; Brito, A.M.S.; Canhestro, W.G.; Alves, R.J.; Araujo, D.P.; et al. The phthalimide analogues N-3-hydroxypropylphthalimide and N-carboxymethyl-3-nitrophthalimide exhibit activity in experimental models of inflammatory and neuropathic pain. Pharmacol. Rep. 2019, 71, 1177–1183. [Google Scholar] [CrossRef]
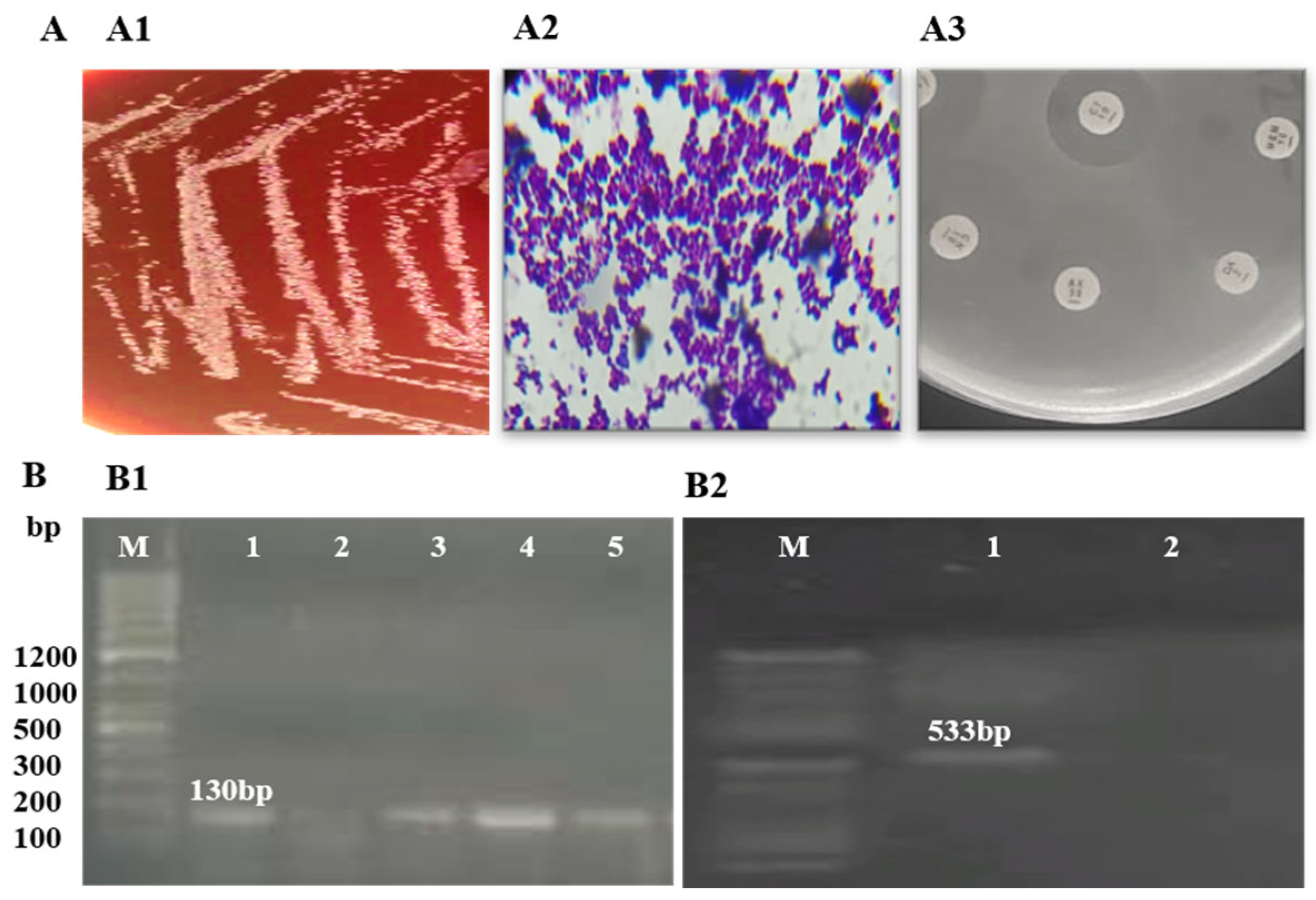

| Isolates | PCR Detection of MRSE | Antibiotic Susceptibility Profile of MRSE | HPLC Fractions | Antibiotic Control | Solvent Control | ||||
|---|---|---|---|---|---|---|---|---|---|
| rdr | mecA | Resistance | Sensitive | Zone of Inhibition (mm) | |||||
| F01 | F02 | F03 | Augmentin | DMSO | |||||
| MRSE01 | Detected | Detected | M, P, AUG, E, CIP | VA, AZM, LIN, TE, CN, CLR | 12 ± 0.32 | 15 ± 0.13 | 10 ± 0.20 | 0 | 0 |
| MRSE02 | Detected | Detected | M, P, AUG, CLR, E, CIP | VA, TE, LIN, CN, AZM | 10 ± 0.20 | 13 ± 0.21 | 9 ± 0.21 | 0 | 0 |
| MRSE03 | Detected | Detected | M, P, E, AUG, CN, AZM | VA, CLR, CN, LIN, TE, CIP | 11 ± 0.10 | 16 ± 0.14 | 8 ± 0.20 | 0 | 0 |
| S.No. | Compound | Molecular Formula | Ret Time | CAS No. | m/z | 2D/3D Structure |
|---|---|---|---|---|---|---|
| 1. | 13-Hexyloxacyclotridec-10-en-2-one | C18H32O2 | 18.163 | 127062-51-5 | 32.00 |  |
| 2. | Bicyclo[5.3.1]undecan-11-one | C11H18O | 18.163 | 013348-11-3 | 41.10 | 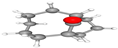 |
| 3. | 13-Oxabicyclo[10.1.0]tridecane | C12H22O | 18.163 | 000286-99-7 | 7.10 | 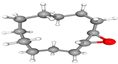 |
| 4. | 10,13-Octadecadienoic acid, methyl ester | C19H34O2 | 18.432 | 056554-62-2 | 55.10 |  |
| 5. | 1-Ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane | C9H19NO3SSi | 18.885 | 063331-02-2 | 175.10 |  |
| 6. | N-[[2-p-Tolylsulfonyl]ethyl]phthalimide | C17H15NO4S | 18.885 | 069384-65-2 | 314.30 |  |
| 7. | Phthalimide, N-(1-hydroxy-2-propyl)- | C11H11NO3 | 18.885 | 1000164-06-1 | 41.10 |  |
| 8. | 9-Eicosene, (E)- | C20H40 | 20.136 | 074685-29-3 | 116.00 |  |
| 9. | Cyclopropaneoctanal, 2-octyl- | C19H36O | 21.174 | 056196-06-6 | 69.10 |  |
| 10. | Isopropyl linoleate | C21H38O2 | 21.503 | 022882-95-7 | 55.10 |  |
| 11. | Butyl 9,12-octadecadienoate | C22H40O2 | 21.503 | 1000336-54-1 | 79.10 |  |
| 12. | 9-Octadecenal, (Z)- | C18H34O | 21.565 | 002423-10-1 | 69.10 |  |
| 13. | Oxacyclododecan-2-one | C11H20O2 | 21.792 | 001725-03-7 | 129.00 |  |
| 14. | Oleoyl chloride | C18H33ClO | 21.792 | 000112-77-6 | 57.10 |  |
| 15. | Phthalic acid, di(2-propylpentyl) ester | C24H38O4 | 22.068 | 1000377-93-5 | 41.10 | 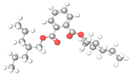 |
| 16. | 2,3-Dihydroxypropyl elaidate | C21H40O4 | 23.132 | 002716-53-2 | 69.10 | 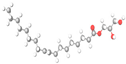 |
| 17. | Pyridine-3-carboxamide, oxime, N-(2-trifluoromethylphenyl)- | C13H10F3N3O | 23.337 | 288246-53-7 | 69.10 | 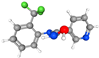 |
| Description | Characteristics of Druggable Compounds | |||||||
|---|---|---|---|---|---|---|---|---|
| 13-Hexyloxacyclotridec-10-en-2-one | Bicyclo[5.3.1]undecan-11-one | 13-Oxabicyclo[10.1.0]tridecane | 1-Ethylsulfanylmethyl-2,8,9-trioxa-5-aza-1-sila-bicyclo[3.3.3]undecane | N-[[2-p-Tolylsulfonyl]ethyl]phthalimide | Phthalimide, N-(1-Hydroxy-2-propyl)- | Oxacyclododecan-2-one | Pyridine-3-carboxamide, oxime, N-(2-trifluoromethylphenyl)- | |
| 1. Physicochemical Profile | ||||||||
| Chemistry | C18H32O2 | C11H18O | C12H22O | C9H19NO3SSi | C17H15NO4S | C11H11NO3 | C11H20O2 | C13H10F3N3O |
| M.W (g/mol) | 280.45 | 166.26 | 182.30 | 249.40 | 329.37 | 205.21 | 184.28 | 281.23 g/mol |
| Heavy atoms (n) | 20 | 12 | 13 | 15 | 23 | 15 | 13 | 20 |
| Aromatic heavy atoms (n) | 0 | 0 | 0 | 0 | 12 | 6 | 0 | 12 |
| Csp3 (fraction) | 0.83 | 0.91 | 1.00 | 1.00 | 0.18 | 0.27 | 0.91 | 0.08 |
| Rotatable bonds (n) | 5 | 0 | 0 | 3 | 4 | 2 | 0 | 4 |
| Num. H-bond acceptors(n) | 2 | 1 | 1 | 4 | 4 | 3 | 2 | 6 |
| Hydrogen donors (n) | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 2 |
| Refractivity (molar) | 87.34 | 50.96 | 56.65 | 66.67 | 89.11 | 57.50 | 54.16 | 66.47 |
| TPSA (Å2) | 26.30 | 17.07 | 12.53 | 56.23 | 79.90 | 57.61 | 26.30 | 57.51 |
| 2. Lipophilicity | ||||||||
| iLOGP | 4.03 | 2.26 | 2.85 | 2.83 | 2.28 | 1.75 | 2.62 | 1.99 |
| XLOGP3 | 6.67 | 3.12 | 4.70 | 0.90 | 2.20 | 1.06 | 3.60 | 2.36 |
| WLOGP | 5.56 | 2.94 | 3.67 | 0.22 | 2.76 | 0.28 | 3.05 | 4.31 |
| MLOGP | 4.17 | 2.59 | 3.02 | −0.51 | 2.33 | 0.90 | 2.48 | 2.77 |
| SILICOS-IT | 4.69 | 2.98 | 3.45 | 0.15 | 2.54 | 1.28 | 3.01 | 2.77 |
| Log Po/w Consensus | 5.03 | 2.78 | 3.54 | 0.72 | 2.42 | 1.06 | 2.95 | 2.84 |
| 3. Water solubility | ||||||||
| ESOL | −5.45 | −2.84 | −3.93 | −1.76 | −3.39 | −1.94 | −3.25 | −3.25 |
| Solubility (mg/mL; mol/L) | 9.93 × 10−4; 3.54 × 10−6 | 2.42 × 10−1; 1.46 × 10−3 | 2.14 × 10−2; 1.17 × 10−4 | 4.38 × 10; 1.76 × 10−4 | 1.34 × 10−1; 4.07 × 10−4 | 2.33 × 10; 1.14 × 10−4 | 1.04 × 10−1; 5.62 × 10−4 | 1.58 × 10−1; 5.62 × 10−4 |
| Solubility class | Moderately soluble | Soluble | Soluble | Very soluble | Soluble | Very soluble | Soluble | Soluble |
| Log S (Ali) | −7.02 | −3.15 | −4.69 | −1.67 | −3.51 | −1.86 | −3.84 | −3.21 |
| Solubility (mg/mL; mol/L) | 2.65 × 10−5; 9.44 × 10−8 | 1.18 × 10−1; 7.12 × 10−4 | 3.71 × 10−3; 2.03 × 10−5 | 5.38 × 10; 2.16 × 10−2 | 1.01 × 10−1; 3.08 × 10−4 | 2.83 × 10; 1.38 × 10−2 | 2.67 × 10−2; 1.45 × 10−4 | 1.74 × 10−1; 6.20 × 10−4 |
| Solubility class | Poorly soluble | Soluble | Moderately soluble | Very soluble | Soluble | Very soluble | Soluble | Soluble |
| SILICOS-IT | −4.56 | −2.35 | −2.49 | −1.51 | −5.67 | −2.33 | −2.57 | −5.00 |
| Solubility (mg/mL; mol/L) | 7.72 × 10−3; 2.75 × 10−5 | 7.34 × 10−1; 4.42 × 10−3 | 5.86 × 10−1; 3.21 × 10−3 | 7.62 × 10; 3.05 × 10−2 | 6.96 × 10−4; 2.11 × 10−6 | 9.51 × 10−1; 4.63 × 10−3 | 4.94 × 10−1; 2.68 × 10−3 | 2.84 × 10−3; 1.01 × 10−5 |
| Solubility class | Moderately soluble | Soluble | Soluble | Soluble | Moderately soluble | Soluble | Soluble | Moderately soluble |
| 4. Pharmacokinetics | ||||||||
| Skin permeability (cm/s) | −3.28 | −5.10 | −4.08 | −7.18 | −6.75 | −6.80 | −4.87 | −6.34 |
| Blood brain barrier (permeability) | Yes | Yes | Yes | No | No | No | Yes | Yes |
| Gastrointestinal absorption | High | High | High | High | High | High | High | High |
| P-g proteins substrate | No | No | No | No | No | No | No | No |
| CYP2D6 inhibitor | No | No | No | No | No | No | No | No |
| CYP2C19 inhibitor | No | No | No | No | Yes | No | No | No |
| CYP3A4 inhibitor | No | No | No | No | No | No | No | No |
| CYP2C9 inhibitor | Yes | No | Yes | No | Yes | No | Yes | No |
| CYP1A2 inhibitor | Yes | No | No | No | No | No | No | No |
| 5. Druglikeness | ||||||||
| Lipinski (violation) | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ghose | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Veber | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Egan | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Muegge | 1 | 2 | 2 | 0 | 0 | 0 | 1 | 0 |
| Bioavailability (score) | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| 6. Medicinal Chemistry | ||||||||
| PAINS (alert) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Brenk (alert) | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 3 |
| Leadlikeness (violation) | 1 | 1 | 2 | 1 | 0 | 1 | 2 | 0 |
| Synthetic accessibility | 4.20 | 3.17 | 3.45 | 5.45 | 2.48 | 1.88 | 2.43 | 2.63 |
| 7. Toxicity | ||||||||
| LD50 predicted (mg/kg) | 34,900 | 500 | 5000 | 1800 | 1250 | 3500 | 5000 | 1500 |
| Toxicity class | 6 | 4 | 5 | 4 | 4 | 5 | 5 | 4 |
| Hepatotoxic | No | No | No | No | No | No | No | No |
| Nephrotoxic | No | No | No | No | No | No | No | No |
| Cardiotoxic | No | No | No | No | No | No | No | No |
| Neurotoxic | No | Yes | No | No | No | No | No | No |
| Carcinogenic | No | No | Yes | No | No | No | No | No |
| Cytotoxic | No | No | No | No | No | No | No | No |
| Mutagenic | No | No | No | No | No | No | No | No |
| Immunotoxic | No | No | No | No | No | No | No | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, K.J. Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis. Curr. Issues Mol. Biol. 2025, 47, 52. https://doi.org/10.3390/cimb47010052
Alzahrani KJ. Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis. Current Issues in Molecular Biology. 2025; 47(1):52. https://doi.org/10.3390/cimb47010052
Chicago/Turabian StyleAlzahrani, Khalid J. 2025. "Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis" Current Issues in Molecular Biology 47, no. 1: 52. https://doi.org/10.3390/cimb47010052
APA StyleAlzahrani, K. J. (2025). Tribulus terrestris Fruit Extract: Bioactive Compounds, ADMET Analysis, and Molecular Docking with Penicillin-Binding Protein 2a Transpeptidase of Methicillin-Resistant Staphylococcus epidermidis. Current Issues in Molecular Biology, 47(1), 52. https://doi.org/10.3390/cimb47010052







