Abstract
Cerebral ischemia-reperfusion injury (CIRI) is a complex pathological process triggered by transient obstruction of blood flow and subsequent reperfusion, ultimately leading to intracellular disturbances such as oxidative stress, inflammatory responses, and programmed cell death. Among the various types of cell death, pyroptosis (an inflammatory kind of regulated cell death) has received increasing attention due to its involvement in key neurovascular unit cells, including endothelial cells, neurons, microglia, and astrocytes. Intriguingly, accumulating evidence demonstrates that non-coding RNAs (ncRNAs), including long non-coding RNAs, microRNAs, and circular RNAs, can modulate multiple stages of pyroptosis in CIRI. This review synthesizes recent findings on the ncRNAs-regulated pyroptosis in CIRI. We highlight the molecular underpinnings of pyroptotic activation following ischemic injury and discuss how ncRNAs shape these mechanisms. By elucidating the interactions between ncRNAs and pyroptosis-related pathways, we intend to present innovative viewpoints for early diagnosis and the development of potential therapeutic strategies to mitigate CIRI.
1. Introduction
Stroke is one of the most severe cerebrovascular diseases in the elderly, characterized by elevated rates of disability and death [1]. Clinically, stroke is classified into ischemic and hemorrhagic types. According to surveys, ischemic stroke accounted for 86.8% of all strokes in 2020 [2]. Ischemic stroke can lead to irreversible neuronal functional damage in brain tissue [3]. Subsequently, reperfusion can lead to secondary damage in ischemic brain tissue, known as CIRI [4]. CIRI is believed to be the primary mechanism of pathological injury in stroke. Moreover, various pathological processes, including inflammatory response [5], oxidative stress [6], autophagy [7], ferroptosis [8], mitochondrial dysfunction [9], angiogenesis [10], and others are closely related to CIRI, with the inflammatory response showing predominance.
Pyroptosis is a form of cell death that promotes inflammation. It is characterized by the formation of pores in the plasma membrane due to the action of gasdermin(GSDM) family proteins. Activation of cysteine aspartate-specific proteases (caspases) triggers their interaction with the GSDM protein family to create channels in the plasma membrane, leading to the discharge of pro-inflammatory substances, including interleukin-1β (IL-1β) and interleukin-18 (IL-18) [11]. Pyroptosis has emerged as a new area of research in CIRI [12], with abnormal pyroptosis reported in endothelial cells [13], neurons [14], microglia [15], and astrocytes in CIRI [16].
Many upstream signals cause pyroptosis, and ncRNAs have recently been found to be closely related to pyroptosis [17]. ncRNAs belong to the RNA class that do not encode proteins but play an essential role in regulating the expression of coding genes and their corresponding functions. Based on their length and shape, ncRNAs are classified into different categories. ncRNAs are primarily categorized into three subtypes based on structural features: (1) long non-coding RNAs (lncRNAs) (>200 nucleotides), (2) microRNAs (miRNAs) (20–22 nucleotides), and (3) circular RNAs (circRNAs), distinguished by their covalently closed circular topology. miRNAs can bind to complementary sequences in target mRNAs, leading to their degradation via the RNA-induced silencing complex (RISC) [18]. In contrast, lncRNAs and circRNAs regulate gene expression through various mechanisms, such as folding into specific structures to facilitate interactions with DNA, RNA, and proteins [19] or acting as miRNA sponges to prevent the degradation of target RNAs [20]. Studies have shown that ncRNAs are closely associated with and play a pivotal role in the occurrence and progression of CIRI [21].
In this review, we used “ncRNA”, “pyroptosis”, and “cerebral ischemia-reperfusion injury” as the core keywords and included their synonyms or related terms. We retrieved relevant articles between 1992 and 2024 from databases such as Web of Science and PubMed, which laid the foundation for this review. This article summarizes the research progress on ncRNAs-mediated pyroptosis in CIRI and provides new insights into the occurrence, development, diagnosis, and treatment.
2. The Role of Pyroptosis in CIRI
2.1. The Concept of Pyroptosis
In the early 1990s, researchers discovered a novel type of cell death in Shigella flexneri-infected macrophages [22]. In 2001, Brad Cookson and Molly Brennan named this newly discovered pro-inflammatory programmed cell death “pyroptosis” [23], defining it as a cell-death model associated with the release of pro-inflammatory factors [24]. From this point, pyroptosis was formally differentiated from apoptosis and other forms of cellular death mechanisms. Further research has shown that pyroptosis affects not only macrophages but also other cell types, including neurons, microglia, and endothelial cells. Moreover, pyroptosis has been linked to ischemia-reperfusion injury in vital organs such as the brain, heart, and liver [25,26,27].
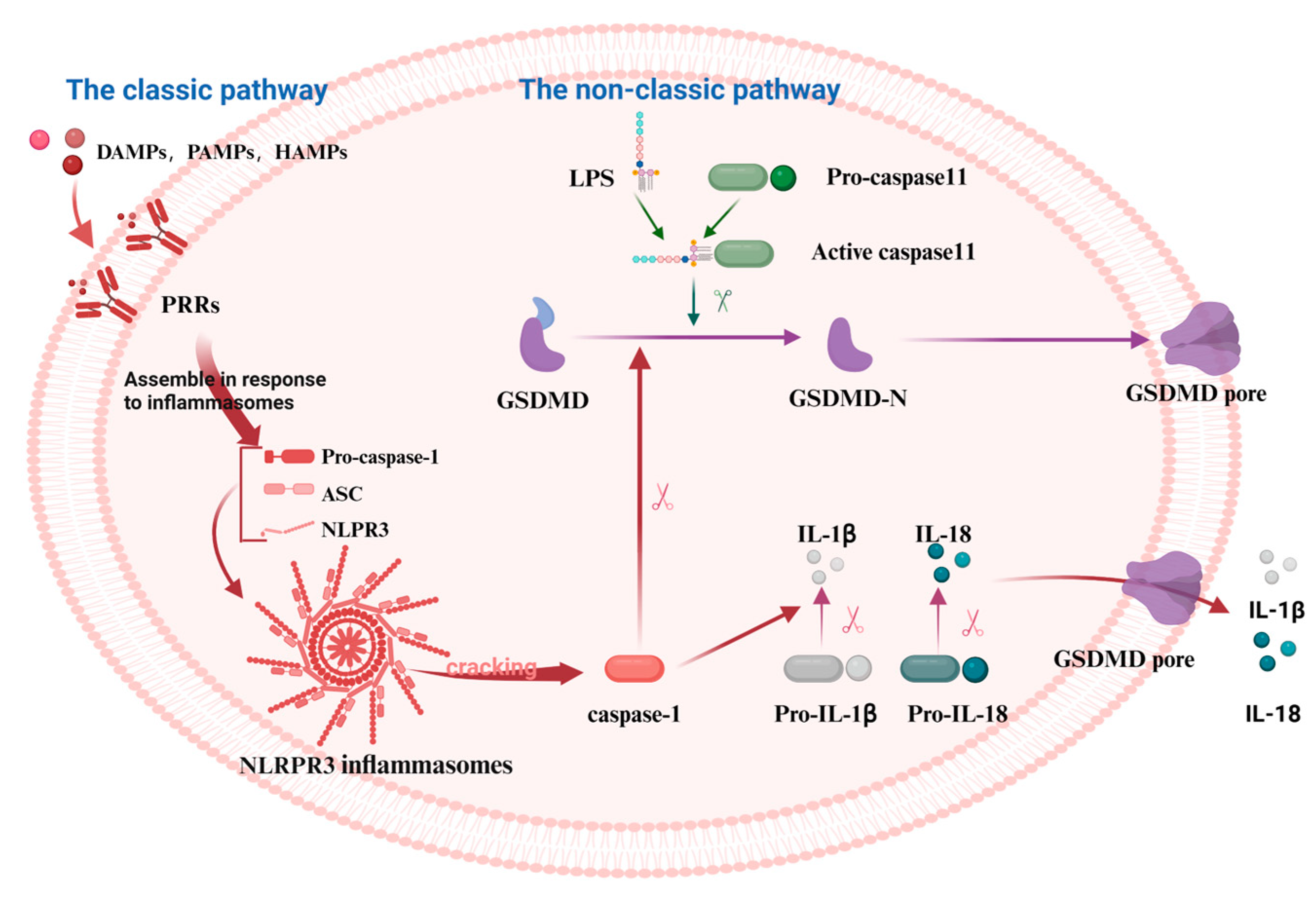
Pyroptosis is a programmed cell death (PCD) mode mediated by the GSDM protein family. It can be classified into the classic pathway, the non-classic pathway, and the alternative pathways [28]. NOD-like receptor thermal protein domain-associated protein 3 (NLRP3), a protein involved in immune responses, gets activated by various signals. These include damage-associated molecular patterns (DAMPs), pathogen-associated molecular patterns (PAMPs), and homeostasis-altering molecular processes (HAMPs). This activation in the classic pathway leads to the conversion of pro-caspase-1 into its active form, caspase-1. Furthermore, caspase-1 cleaves GSDMD, pro-IL-1β, and pro-IL-18 into their mature forms: the GSDMD-N-terminal fragments (GSDMD-NT), IL-1β, and IL-18 [29]. IL-1β and IL-18 stimulate the generation of additional inflammatory molecules, including cytokines, chemokines, and adhesion factors. Conversely, the NT is lipophilic and can move to the membrane film structure, where it gradually polymerizes to form pores, causing osmotic imbalance and rapid cell expansion, resulting in membrane rupture and leading to the leakage of cell contents [30]. However, no inflammasomes and cytoplasmic pattern recognition receptors (PRRs) are involved in the non-classic pathway, instead, cell death is mediated by the activation of other subunits in the caspase family (Figure 1). Rapid oligomerization of murine caspase-11 and human caspase-4/5 was found to lead to the cleavage of GSDMD, which forms membrane pores when stimulated by molecules such as LPS [31,32]. In addition to GSDMD, the cleavage of other members of the gasdermin family (GSDMA, GSDMB, GSDMC, and GSDME) results in the release of pro-inflammatory mediators such as IL-1β and subsequent cell lysis [33]. Recent studies have shown that caspase-3 can cleave GSDME to generate the GSDME-NT, causing pyroptosis [33,34]. Similarly, caspase-8 can regulate pyroptosis by cleaving GSDME and GSDMD proteins [35,36]. Under hypoxia, phosphorylated STAT3 (p-STAT3) binds to programmed death-ligand 1 (PD-L1), promoting PD-L1 nuclear translocation. This interaction activates GSDMC and caspase-8, thereby triggering the non-classic pyroptosis pathway [37].
Figure 1.
The mechanism of the classical and the non-classical pyroptosis pathway. In the classical pathway, PRRs recognize DAMPs, PAMPs, and HAMPs, activating the assembly of inflammasomes and pro-caspase-1. This leads to the cleavage of pro-caspase-1 into active caspase-1, which subsequently cleaves GSDMD, producing active GSDMD-NT that form cell membrane pores, resulting in pyroptosis. In the non-classical pathway, caspase-11 directly recognizes the lipopolysaccharide (LPS) of the pathogen, thereby activating the pyroptosis process via the non-inflammasome pathway. Caspase-11 binds to LPS and directly cleaves GSDMD, producing GSDMD-NT that form cell membrane pores, leading to pyroptosis. The figure was created using BioRender.
2.2. The Role of Pyroptosis in CIRI
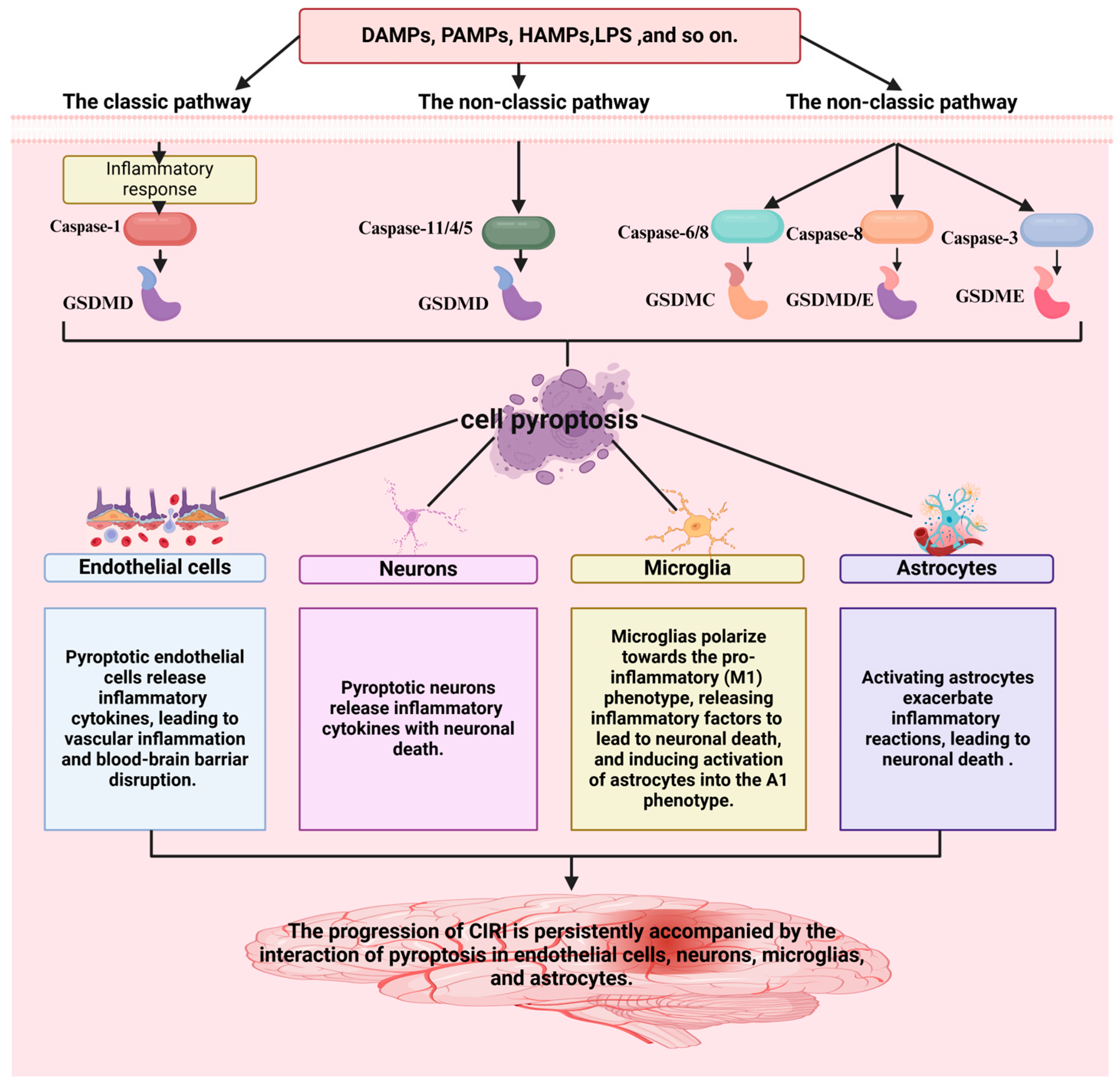
Neuroinflammation is generally considered an important secondary injury after stroke [5]. To date, inflammasomes NLRP1, NLRP3, NLRP6, NLRC4, and AIM have been associated with ischemic stroke [38,39,40]. According to statistics, the NLRP3 inflammasome is currently the most studied, and caspase-1 is activated in the sterile inflammatory response [41]. Findings demonstrate that pharmacological inhibition or genetic ablation of the NLRP3 inflammasome can enhance neurological performance [16]. The NLRP3 inflammasome is intimately linked to pyroptosis, a pro-inflammatory form of regulated cell death. In CIRI, neurovascular unit cells, including endothelial cells [42], neurons [43], microglia [44], and astrocytes [45], all undergo NLRP3-mediated pyroptosis, exacerbating neuroinflammation and tissue damage.
Pyroptotic endothelial cells release inflammatory cytokines, leading to vascular inflammation and blood-brain barrier disruption in CIRI [46]. A previous study investigating oxygen-glucose deprivation/reoxygenation (OGD/R) in endothelial cells and middle cerebral artery blockage/restoration (MCAO/R) mice revealed that NLRP3 is closely associated with pyroptosis. Endothelial cell pyroptosis is a crucial pathophysiological mechanism of ischemic stroke. Sodium Danshensu (SDSS) was found to inhibit the triggering of the NLRP3 inflammasome and reduce chloride efflux by binding to chloride intracellular channel 4 (CLIC4), thereby inhibiting caspase-1 cleavage and GSDMD-N shearing, suppresses the production and release of IL-1β and IL-18, reducing vascular endothelial cell pyroptosis, and alleviating CIRI [13]. Therefore, inhibiting the pyroptosis of vascular endothelial cells represents a potential therapeutic option for CIRI [47].
Pyroptotic neurons release inflammatory cytokines, leading to neuronal death in CIRI [48,49]. In MCAO mouse neurons and OGD/R neurons, BRCA1/BRCA2 Containing Complex Subunit 3 (BRCC3) was highly expressed, which activated the NLRP6 inflammasome, leading to pyroptosis [49]. Experiments showed that siRNA BRCC3 significantly downregulated the protein levels of NLRP6, cleavated caspase-1 and IL-1β, reduced neutrophil infiltration, and improved neurological dysfunction in MCAO mice [48].
Microglia polarize towards the pro-inflammatory (M1) phenotype, releasing inflammatory factors that lead to neuronal death and induce the activation of astrocytes into the A1 phenotype in CIRI. Microglia are crucial intrinsic immune cells that participate in regulating the body’s immune response and brain function [50]. Microglia can be divided into two types: the pro-inflammatory microglia (M1) and the anti-inflammatory microglia (M2) phenotype [51]. The M1 phenotype produces substances such as tumor necrosis factor-alpha (TNF-α) and inducible nitric oxide synthase (iNOS), which have toxic effects on nerves, exacerbating the inflammatory response and leading to further brain damage. In contrast, the M2 microglia exerts anti-inflammatory effects [52]. In a rat MCAO/R model, the M1 phenotype was activated by recognition of PAMPs or DAMPs, which enhanced NLRP3 and pro-IL-1β synthesis, promoting the maturation of GSDMD-NT and IL-1β, and leading to microglial pyroptosis. Consequently, inflammatory factors were released, leading to neuronal death and inducing the activation of astrocytes into the A1 phenotype. Therefore, inhibition of the NLRP3 inflammasome can alleviate pyroptosis and play a protective role in neurons [53,54,55,56].
Activated astrocytes exacerbate inflammatory reactions, leading to neuronal death in CIRI [57]. Scientific studies increasingly show a causal link between CIRI pathogenesis and astrocytic pyroptosis [58]. For example, pioglitazone can inhibit pyroptosis of astrocytes in brain damage [59], by regulating the expression of the NLRP3 inflammasome. Hispidulin inhibits astrocyte pyroptosis, alleviates neurological symptoms, reduces inflammatory responses, and improves cerebral edema [60]. Moreover, Lipocalin-2mediated astrocytic pyroptosis causes neuronal damage [16].
The above findings demonstrate that the progression of CIRI is persistently accompanied by the interaction of pyroptosis in endothelial cells, neurons, microglia, and astrocytes (Figure 2). Hence, inhibiting the pyroptosis of key cells (endothelial cells [42], neurons [43], microglia [44], and astrocytes [45]) in the neurovascular unit represents a potential therapeutic option for CIRI.
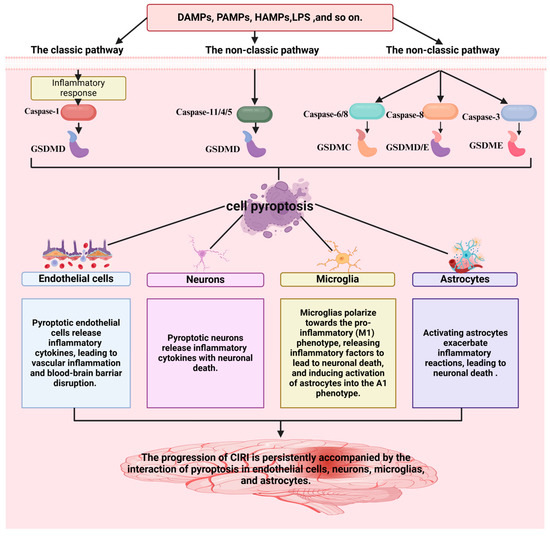
Figure 2.
A strong association is observed between pyroptosis and the progression of CIRI. The figure was created using BioRender.
3. The Role of ncRNAs in CIRI
3.1. ncRNAs
RNA was once thought to be primarily a messenger, carrying instructions encoded by DNA so that other molecules, such as ribosomes, could use this code to produce proteins. However, in recent studies, researchers have discovered the existence of various types of RNA, the most important of which is ncRNA (mainly including lncRNA, miRNA, and circRNA), which does not participate in protein production. With the progress of research, the discovery of tens of thousands of ncRNAs has completely transformed this field and changed scientists’ views on physiology and disease development. ncRNA accounts for over 90% of the RNA in the human genome, yet most of it remains unexplored [61,62].
Specifically, miRNAs are involved in the post-transcriptional control of gene expression by binding to non-coding sequences located at the 3′-untranslated region (3′-UTR) of mRNA [63]. Precursor RNAs (pre-miRNAs) are transcribed and processed in the nucleus by RNA polymerase II, Drosha, and DiGeorge Critical Region 8(DGCR8) and then are exported from the nucleus via exportin-5 [64]. The pre-miRNA is further processed by Dicer and TAR RNA-binding proteins (TRBPs) to generate mature miRNAs that bind to Argonaute proteins to form RISC. RISC binds to mRNA through complementary miRNA response elements (MREs) that suppresses translation or induces the degradation of the target mRNA [65]. However, the pairing between miRNAs and target mRNAs may not be specific, allowing miRNAs to modulate a wide range of mRNA targets. Furthermore, miRNAs are involved in all cellular activities, including biological and disease-related processes such as cell growth, division, differentiation, and apoptosis. Additionally, lncRNAs are a class of transcriptional regulators transcribed by RNA polymerase II, which are involved in alternative splicing and protein post-translational modification of mRNA. They competitively bind to miRNAs to regulate various miRNAs [66,67,68]. CircRNAs consist of introns, exons, or both and lack polyadenylated tails. The biogenetic pathways of circRNAs are varied, including but not limited to classical splicing and reverse splicing [69]. CircRNAs primarily act as miRNA sponges, protein bait antagonists, RNA splicing regulators, parental gene transcription regulators, and even translation templates to control the physiological and pathological functions of cells [70,71]. Some circRNAs have been found to function as molecular sponges to control the mRNA binding of proteins. Hence, circRNAs are likely more stable and suitable as biomarkers than lncRNAs [20].
3.2. The Role of ncRNAs in CIRI
ncRNAs do not encode proteins but are involved in various physiological and pathological processes, including cell pyroptosis [17], proliferation [72], and apoptosis [73]. Dysregulated ncRNA levels have been linked to a variety of diseases, including tumors, diabetes, and various types of ischemia-reperfusion injury [74,75]. ncRNAs, including miRNAs such as miR-155 [76], miR-377 [77], and miR-1202 [78], lncRNAs such as SNHG12 [79], MALAT1 [80], and ANRIL [81], and circRNAs such as circ-camk4 [82], circ0072309 [83], and circCCDC9 [84], are closely associated with CIRI. These can serve as biomarkers and therapeutic targets for neuroinflammation in ischemic stroke [21,85,86,87].
4. The Role of ncRNAs-Mediated Pyroptosis in CIRI
ncRNAs may contribute to CIRI by regulating the transcription or translation of pyroptosis-related genes. The role of ncRNAs-mediated pyroptosis in CIRI has garnered growing interest among researchers.
4.1. The Role of lncRNAs-Mediated Pyroptosis in CIRI
Recently, an increasing amount of research has documented the impact of lncRNA-regulated pyroptosis in CIRI.
X-inactive-specific transcript (XIST) is associated with abnormal inflammation regulators and acts as an sponge of miR-96-5p in CIRI [88]. In MCAO rats and OGD/R microvascular endothelial cells, XIST expression was downregulated, while NLRP3, Caspase-1, and GSDMD were upregulated, leading to increased IL-1β secretion. Protocatechol or MCC950 (pyroptosis inhibitor) improved cell damage and enhanced XIST expression. However, in the XIST silencing group, protocatechol did not alleviate pyroptosis in microvascular endothelial cells, thereby alleviating CIRI [47].
Opa-interacting protein 5 antisense transcript 1 (OIP5-AS1) is a highly conserved gene and plays an essential role in various diseases, including nervous system disorders, tumors, and inflammation [89]. A previous study has shown that overexpression of OIP5-AS1 in MCAO/R rats downregulates the expression levels of miR-186-5p, attenuates oxidative stress, and reduces the inflammatory response [90]. OIP5-AS1 was significantly downregulated in OGD/R neurons, MCAO/R mice, and ischemic stroke patients. Overexpression of OIP5-AS1 promoted thioredoxin-interacting protein (TXNIP) ubiquitination and degradation via the E3 protein ubiquitin ligase (E3) Itch, suppressed NLRP3 expression in brain tissue, inhibited neuronal pyroptosis, and alleviated CIRI [91].
Recent studies have documented that lncRNA Gm44206 has been closely linked to CIRI. OGD/R microglia showed enhanced the expression of Gm44206. In addition, NLRP3, Caspase-1, GSDMD, and apoptosis-associated speck-like proteins containing C-terminal caspase recruitment domains (ASC) were involved in microglial pyroptosis. Silencing lncRNA Gm44206 could reduce pro-inflammatory cytokine release, including IL-1 β, IL-6, IL-18, and TNF-α, through the classical pathway, thereby alleviating OGD/R microglial pyroptosis, thereby alleviating CIRI [15].
Some studies have shown that maternally expressed gene 3 (MEG3) binds competitively to miRNAs and participates in apoptosis, endoplasmic reticulum stress, oxidative stress, inflammation, epithelial-mesenchymal transition (EMT), and other processes [92]. LncRNA MEG3 exerts a range of pathological effects on neurons, astrocytes, microglia, and endothelial cells, showing potential for clinical application in the prevention and treatment of encephalopathy [93,94]. Knockdown of MEG3 inhibited caspase-1 signaling, decreased the levels of absent in melanoma 2 (AIM2), ASC, cleaved-caspase-1, and GSDMD-NT, and inhibited pyroptosis and inflammation in both MCAO rats and OGD/R-treated neurons, thereby alleviating CIRI [95].
KCNQ1 opposite strand/antisense transcript 1 (KCNQ1OT1) has been shown to play a key role in hypoxia through inflammation and oxidative stress [96]. In CIRI, KCNQ1OT1 acts as a sponge for miR-153-3p [97], miR-140-3P [98], miR-30b [99], and miR-9 [100]. KCNQ1OT1 exhibited robust expression in murine and in vitro neuronal cell models. Specifically, knockdown of KCNQ1OT1 or overexpression of miR-153-3p attenuated OGD/R-induced neuronal injury and regulated Foxo3 expression to inhibit pyroptosis, thereby alleviating CIRI [97].
Many studies have shown that lncRNA Taurine Up-regulated Gene 1 (TUG1) plays a key role in the I/R-related inflammatory response [101]. TUG1 downregulates miR-9a-5p and upregulates KLF5 expression, leading to cardiomyocyte apoptosis following myocardial ischemia and reperfusion [102]. The expression of TUG1 was upregulated in OGD/R-treated astrocytes, which in turn regulated miR-145 and aquaporin 4 (AQP4) to induce neuronal death [103]. Knockdown of TUG1 increased the level of miR-200a-3p, and decreased the levels of NLRP3, caspase-1, GSDMD-NT, IL-18,and IL-1β, thereby exacerbating CIRI [104].
Low levels of lncRNA RGD1564534 were found in MCAO rats and OGD/R cells. RGD1564534 could sponge miR-101a-3p to increase Dusp1 levels and upregulate its expression in neurons, thereby inhibiting the activation of the NLRP3 inflammasome. Its protective effect on neurons was related to promoting autophagy and inhibiting pyroptosis, thereby alleviating CIRI [105].
Recent studies have found that nuclear overexpressed transcript 1 (NEATl) is strongly associated with brain-related diseases. NEATl sponges miR-22-3p [106], miR-874-3p [107], and miR-214 [108], thereby exacerbating CIRI. In MCAO rats and OGD/R-treated neurons, NEAT1 was found to sponge miR-22-3p and decrease the expression of NLRP3 and cleaved caspase-1 to suppress pyroptosis, thereby alleviating CIRI [106].
Moreover, lncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was found to exacerbate cerebral infarction through the mouse double minute 2 (MDM2)-p53 pathway [109]. MALAT1 was highly expressed in a diabetic cerebral ischemia model, and MALAT1 knockdown of effectively inhibited inflammation and pyroptosis in BV2 cells. In addition, MALAT1 interacted with STAT1 and can enhance the transcriptional activity of NLRP3. Knockdown of STAT1 significantly inhibited MALAT1 transcription. Thus, the interaction between MALAT1 and STAT1 promoted diabetic cerebral ischemia-induced microglial pyroptosis by activating NLRP3 transcription, thereby alleviating CIRI [110].
LOC102555978 is a newly discovered CIRI-related lncRNA. LOC102555978 promoted NLRP3-mediated pyroptosis of microglia in MCAO/R rats and OGD/R microglia. Astragaloside IV inhibits microglia pyroptosis by downregulating LOC102555978, thereby alleviating CIRI [111].
Some studies have found that lncRNA FENDRR plays an important role in gastric cancer, osteosarcoma, bile duct cancer, and other diseases [112]. FENDRR can promote apoptosis in intracranial microvascular endothelial cells [113]. The results revealed increased expression of FENDRR, as well as upregulation of the NLRC4 inflammasome and pyroptosis in diabetic rats following CIRI. Similarly, in the OGD/R model, the expression of NLRC4 and related inflammatory factors decreased after FENDRR knockdown, thereby alleviating CIRI [114].
4.2. The Role of miRNAs-Mediated Pyroptosis in CIRI
Recently, miR-139 has been associated with inflammatory regulation in various diseases. Elevated miR-139 expression regulates the c-Jun/NLRP3 inflammasome pathway, thereby reducing CIRI [115]. In MCAO/R mice and OGD/R neurons, miR-139-5p was downregulated, the antioxidant pathway of Nrf2 was inhibited after the expression of the forkhead boxO1 (FoxO1) and Keap1 were up-regulated, and pyroptosis was mediated by NLRP3 inflammasome. Ginsenoside Rd inhibits the transcriptional regulation of Keap1 by FoxO1 by upregulating miR-139-5p, thereby activating the Nrf2 pathway and reducing pyroptosis mediated by the ROS/TXNIP/NLRP3 inflammasome pathway, thereby alleviating CIRI [116].
miR-155-5p is a newly discovered inflammatory regulator involved in a series of inflammatory diseases [117]. Previous studies have shown that miR-155-5p in CIRI can improve neuroinflammation by targeting dual-specificity phosphatase 14 (DUSP14) [118]. However, Que et al. established an elderly rat model and reported that the overexpression of DUSP14 could inhibit the NLRP3 inflammasome, reduce the inflammatory response, and improve cognitive function in elderly rats [119]. miR-155-5p, TXNIP, and NLRP3 were significantly upregulated in CIRI. In addition, knockdown of miR-155-5p inhibits inflammation and pyroptosis and plays a protective role in CIRI [120].
miR-96-5p plays a crucial role in cancer [121]. miR-96-5p lowered hypoxia-induced apoptosis and cardiac fibrosis in cardiomyocytes [122]. A previous study revealed that the expression of miR-96-5p is significantly downregulated in animal models of cerebral ischemia. miR-96-5p mimics significantly inhibited the expression of caspase-1 and improved pyroptosis in OGD/R treated N2a cells, while the miR-96-5p inhibitor caused the opposite results, thereby alleviating CIRI [123].
miR-29 is first discovered in human HeLa cells in 2001 and plays a critical role in cancer [124]. miR-29a-3p is associated with lung fibrosis [125] and cardiomyocyte apoptosis [126]. Astrocyte-derived extracellular vesicles (EVs) improved miR-29a expression in MCAO/R rat brains and OGD/R treatment N9 microglia. miR-29a from astrocyte-derived EVs alleviated CIRI by reducing tumor protein p53-Inducible Nuclear Protein 1 (TP53INP1) expression and the NF-κB/NLRP3 pathway to inhibit cell pyroptosis [127].
miR-135a-5p plays a pivotal role in numerous illnesses and is associated with autophagy, proliferation, apoptosis, etc. [128]. miR-135a-5P has been shown to prevent CIRI development [129]. Overexpression of miR-135a-5p was observed in EVs of M2 microglia, which delivered miR-135a-5p to neurons to inhibit TXNIP expression, thereby inhibiting NLRP3 inflammasome activation and reducing neuronal pyroptosis and cerebral ischemia [130].
miR-223 has been shown to be a biomarker for various human metabolic diseases and is involved in inflammation, autoimmune disorders, and other diseases. In addition, miR-223 protects against brain damage caused by glutamate excitotoxicity [131]. miR-223 contains a binding site in the 3′ UTR of NLRP3 mRNA, enabling its regulation [132]. Electroacupuncture was found to inhibit the increase of miR-223 expression in brain tissue after ischemia-reperfusion injury, while the expression of NLRP3, caspase-1, IL-1ß, and IL-18 decreased. However, antagomir-223 could partially inhibit the protective effect of electroacupuncture against cerebral ischemia [133].
miR-124 plays a vital neuroprotective role, and injection of miR-124 reduces neuronal damage induced by hypoxia and glucose deprivation in the early stages of ischemic stroke [134]. In addition, Ponomarev et al. showed that the higher levels of miR-124 prevented allergic meningitis by blocking microglial activation. The protective effect of miR-124 may be intricately linked to inflammation [135]. miRNA-124 agonists inhibited the expression and activation of STAT3 in a targeted manner, which also decreased the extent of pyroptosis. However, miR-124 antagonist reversed miR-124 agonist-mediated effects [136].
miR-423-5p is implicated in several diseases but has rarely been reported in CIRI. Luo et al. demonstrated that the levels of elevated miRNA-124 reduced I/R inflammation and pyroptosis in the brain through the NLRP3/caspase-1 pathway. Additionally, the knockdown of miR-423-5p improved venous nerve parameters such as cerebral infarct area, nerve score, cerebral edema, and neuronal injury, while inhibiting NLRP3 inflammasomes, pyroptosis, and oxidative stress [137].
4.3. The Role of circRNAs-Mediated Pyroptosis in CIRI
Compared with other ncRNAs, the circular structure of circRNAs provides higher stability for miRNA binding and plays a vital role in diagnosis. However, the mechanism of circRNA-mediated pyroptosis in CIRI remains poorly understood. CircRIMS is recognized as a proto-oncogenic circular RNA in gastric cancer and esophageal squamous cell carcinoma [138]. Li W et al. found that the levels of circRIMS were elevated in both the MCAO/R model and OGD/R model and that circRIMS contributed to CIRI progression through the regulation of the miR-96-5p/JAK/STAT1 axis [139]. Overexpression of miR-96-5p can inhibit pyroptosis and alleviate CIRI in mice [123].
CircCCDC6 is a recently discovered circRNA associated with CIRI. MCAO/R and OGD/R-treated SH-SY5Y cells showed increased levels of circCCDC6. Silencing circCCDC6 reduced neuronal pyroptosis and inflammation in MCAO/R rats. CircCCDC6 modulates miR-128-3p to trigger TXNIP/NLRP3, promoting pyroptosis and inflammation in response to OGD/R [140].
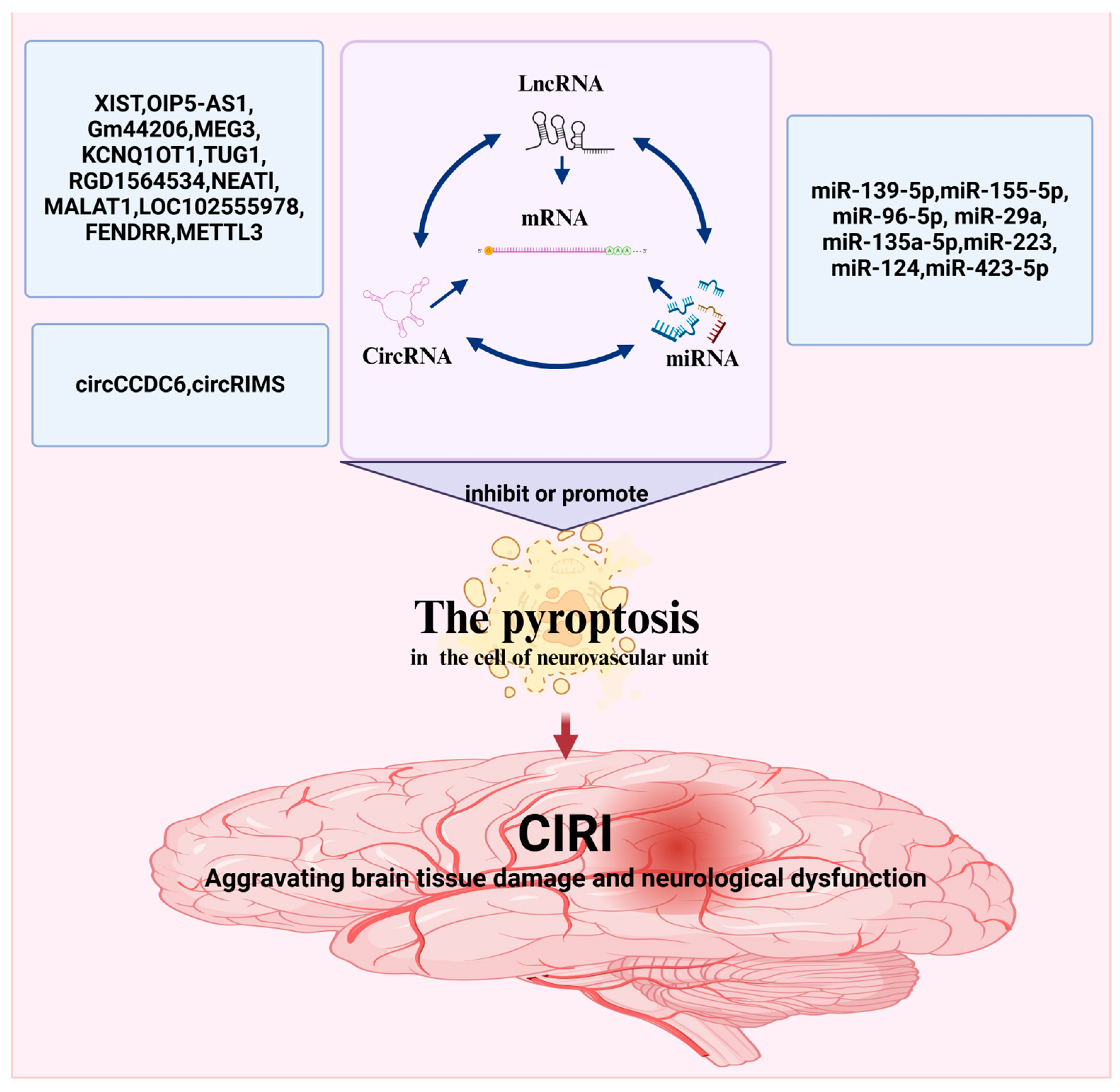
ncRNAs are widely present in various human organs. ncRNAs are released into the blood according to the degree of pyroptosis. As shown above, altering ncRNA expression levels in CIRI can promote pyroptosis (Figure 3 and Table 1).
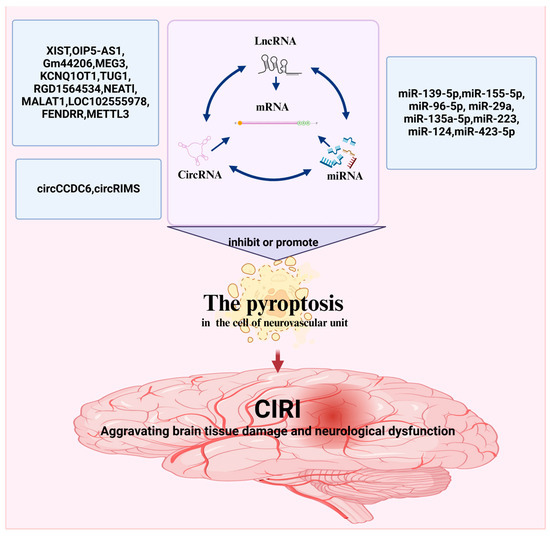
Figure 3.
ncRNAs-mediated regulation of pyroptosis in CIRI. The figure was created using BioRender.

Table 1.
The role of ncRNAs-mediated pyroptosis in CIRI.
5. Conclusions and Prospect
This narrative review, rather than a systematic review, did not follow a formal systematic review or scoping review protocol. We found that ncRNAs affected the pathological process of CIRI by influencing the pyroptosis of and cerebral microvascular endothelial cells, neurons, microglia and astrocytes, and other cells [136,141]. Pyroptosis-related ncRNAs form a complex regulatory network, providing new insights into the development and progression of CIRI and offering potential applications for early screening, diagnosis, and treatment. Extensive research has been conducted on pyroptosis-related lncRNAs and miRNAs, but the role of pyroptosis-associated circRNAs in CIRI remains largely unknown. Further research may uncover additional ncRNAs associated with pyroptosis, contributing to a better understanding of how CIRI works.
Currently, research is still in its early stages. Future studies should further explore the regulatory mechanisms of ncRNAs in CIRI, particularly the interactions between ncRNAs and pyroptosis, as well as functional differences in various types of cell. In terms of clinical applications, although ncRNAs has great potential as a therapeutic target, the development of its stability and delivery systems remains a pressing issue. Future research should focus on developing more effective ncRNAs delivery systems to enhance stability and targeting capabilities within the body. Additionally, combining ncRNAs-regulated pyroptosis strategies with other therapeutic approaches, such as pharmacological interventions and acupuncture, may yield better therapeutic outcomes. In summary, the role of ncRNAs in regulating pyroptosis provides new ideas and directions for treating CIRI, but further research and validation are necessary for clinical application.
Author Contributions
Writing—original draft preparation, R.X. and Q.P.; writing—review and editing, R.X., Q.P., W.C., X.C. and G.W.; visualization, R.X.; supervision, X.C. and G.W.; project administration, R.X. and Q.P.; funding acquisition, X.C. and G.W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (82474115, 81874375), the Project of the Natural Science Foundation of Hunan Province (2024JJ9443, 2025JJ90275), Key funded project of the Hunan Provincial Health Commission (A202303066904), the Project of the Hunan Province Education Department (22A0266), Funding for First-class Discipline of Basic Medicine at the Hunan University of Chinese Medicine (2023), and Funding for Hunan Provincial First-class Discipline of Integrated Traditional Chinese and Western Medicine (2023).
Data Availability Statement
Data sharing is not applicable for this article, as no new data were developed or analyzed.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| KCNQ1OT1 | KCNQ1 opposite strand/antisense transcript 1 |
| IL-1β | interleukin-1β |
| IL-18 | interleukin-18 |
| GSDMD-NT | GSDMD-N-terminal fragments |
| PD-L1 | programmed death-ligand 1 |
| p-STAT3 | phosphorylated STAT3 |
| CLIC4 | chloride intracellular channel 4 |
| iNOS | inducible nitric oxide synthase |
| TNF-α | tumor necrosis factor-alpha |
| DGCR8 | DiGeorge Critical Region 8 |
| 3′-UTR | 3′-untranslated region |
| OIP5-AS1 | Opa-interacting protein 5 antisense transcript 1 |
| TXNIP | thioredoxin-interacting protein |
| MEG3 | maternally expressed gene 3 |
| EMT | epithelial-mesenchymal transition |
| AIM2 | absent in melanoma 2 |
| TUG1 | Taurine Up-regulated Gene 1 |
| MDM2 | mouse double minute 2 |
| FoxO1 | forkhead boxO1 |
| TP53INP1 | tumor protein p53-Inducible Nuclear Protein 1 |
| CIRI | Cerebral ischemia-reperfusion injury |
| ncRNAs | non-coding RNAs |
| caspase | cysteine aspartate-specific proteases |
| lncRNAs | long non-coding RNAs |
| miRNAs | microRNAs |
| circRNAs | circular RNAs |
| GSDM | gasdermin |
| NLRP3 | NOD-like receptor thermal protein domain-associated protein 3 |
| PRRs | pattern recognition receptors |
| OGD/R | oxygen-glucose deprivation/reoxygenation |
| MCAO/R | middle cerebral artery blockage/restoration |
| SDSS | Sodium Danshensu |
| BRCC3 | BRCA1/BRCA2-Containing Complex Subunit 3 |
| M1 | the pro-inflammatory microglia |
| M2 | the anti-inflammatory microglia |
| pre-miRNAs | Precursor microRNAs |
| TRBPs | TAR RNA-binding proteins |
| RISC | RNA-induced silencing complex |
| MREs | miRNA response elements |
| CSF | cerebrospinal fluid |
| XIST | X-inactive-specific transcript |
| ASC | apoptosis-associated speck-like proteins containing a C-terminal caspase recruitment domain |
| AQP4 | encoding aquaporin 4 |
| NEATl | nuclear abundance transcript 1 |
| MALAT1 | metastasis-associated lung adenocarcinoma transcript 1 |
| DUSP14 | Dual specificity ATPase 14 |
| EVs | extracellular vesicles |
References
- Bindal, P.; Kumar, V.; Kapil, L.; Singh, C.; Singh, A. Therapeutic management of ischemic stroke. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2024, 397, 2651–2679. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.-J.; Zhao, Z.; Yin, P.; Cao, L.; Zeng, J.; Chen, H.; Fan, D.; Fang, Q.; Gao, P.; Gu, Y.; et al. Estimated Burden of Stroke in China in 2020. JAMA Netw. Open 2023, 6, e231455. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Liu, G.; Wang, Z.; Li, Y.; Fang, W. Circular RNAs: Promising Treatment Targets and Biomarkers of Ischemic Stroke. Int. J. Mol. Sci. 2023, 25, 178. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Y.; Wu, X.; Chen, B.; Liu, S.; Huang, J.; Kong, L.; Wang, G.; Ye, Z. Research progress on the mechanism of curcumin in cerebral ischemia/reperfusion injury: A narrative review. Apoptosis 2023, 28, 1285–1303. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jia, H.; Si, M.; Li, X.; Ma, Z.; Zhu, Y.; Sun, W.; Zhu, F.; Luo, S. Loureirin B protects against cerebral ischemia/reperfusion injury through modulating M1/M2 microglial polarization via STAT6/NF-kappaB signaling pathway. Eur. J. Pharmacol. 2023, 953, 175860. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; He, W.; Wei, H.; Chang, C.; Yang, L.; Meng, J.; Long, T.; Xu, Q.; Zhang, C. Srs11-92, a ferrostatin-1 analog, improves oxidative stress and neuroinflammation via Nrf2 signal following cerebral ischemia/reperfusion injury. CNS Neurosci. Ther. 2023, 29, 1667–1677. [Google Scholar] [CrossRef]
- Mahemuti, Y.; Kadeer, K.; Su, R.; Abula, A.; Aili, Y.; Maimaiti, A.; Abulaiti, S.; Maimaitituerxun, M.; Miao, T.; Jiang, S.; et al. TSPO exacerbates acute cerebral ischemia/reperfusion injury by inducing autophagy dysfunction. Exp. Neurol. 2023, 369, 114542. [Google Scholar] [CrossRef]
- Zhang, J.; Cai, W.; Wei, X.; Shi, Y.; Zhang, K.; Hu, C.; Wan, J.; Luo, K.; Shen, W. Moxibustion ameliorates cerebral ischemia-reperfusion injury by regulating ferroptosis in rats. Clin. Exp. Pharmacol. Physiol. 2023, 50, 779–788. [Google Scholar] [CrossRef]
- Huang, J.; Chen, L.; Yao, Z.M.; Sun, X.R.; Tong, X.H.; Dong, S.Y. The role of mitochondrial dynamics in cerebral ischemia-reperfusion injury. Biomed. Pharmacother. 2023, 162, 114671. [Google Scholar] [CrossRef]
- Wei, J.; Xie, J.; He, J.; Li, D.; Wei, D.; Li, Y.; Li, X.; Fang, W.; Wei, G.; Lai, K. Active fraction of Polyrhachis vicina (Roger) alleviated cerebral ischemia/reperfusion injury by targeting SIRT3-mediated mitophagy and angiogenesis. Phytomedicine 2023, 121, 155104. [Google Scholar] [CrossRef]
- Duan, W.L.; Wang, X.J.; Ma, Y.P.; Sheng, Z.M.; Dong, H.; Zhang, L.Y.; Zhang, B.G.; He, M.T. Therapeutic strategies targeting the NLRP3-mediated inflammatory response and pyroptosis in cerebral ischemia/reperfusion injury (Review). Mol. Med. Rep. 2024, 29, 46. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Fan, L.L.; Ding, Y.L.; Wu, D.; Zheng, J.Y.; Cai, Y.F.; Huang, Y.; Qiao, L.J.; Zhang, S.J.; Zhan, J. Pre-electroacupuncture Ameliorates Cerebral Ischemia-reperfusion Injury by Inhibiting Microglial RhoA/pyrin/GSDMD Signaling Pathway. Neurochem. Res. 2024, 49, 3105–3117. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, Q.; Zhang, R.; Zhang, Y.; Zeng, W.; Yu, Q.; Zeng, M.; Gan, J.; Li, H.; Yang, L.; et al. Sodium Danshensu ameliorates cerebral ischemia/reperfusion injury by inhibiting CLIC4/NLRP3 inflammasome-mediated endothelial cell pyroptosis. Biofactors 2024, 50, 74–88. [Google Scholar] [CrossRef]
- Bai, W.; Huo, S.; Zhou, G.; Li, J.; Yang, Y.; Shao, J. Biliverdin modulates the Nrf2/A20/eEF1A2 axis to alleviate cerebral ischemia-reperfusion injury by inhibiting pyroptosis. Biomed. Pharmacother. 2023, 165, 115057. [Google Scholar] [CrossRef]
- Yang, L.; Gao, Y.; Huang, J.; Yang, H.; Zhao, P.; Li, C.; Yang, Z. LncRNA Gm44206 Promotes Microglial Pyroptosis Through NLRP3/Caspase-1/GSDMD Axis and Aggravate Cerebral Ischemia-Reperfusion Injury. DNA Cell Biol. 2023, 42, 554–562. [Google Scholar] [CrossRef]
- Li, J.; Xu, P.; Hong, Y.; Xie, Y.; Peng, M.; Sun, R.; Guo, H.; Zhang, X.; Zhu, W.; Wang, J.; et al. Lipocalin-2-mediated astrocyte pyroptosis promotes neuroinflammatory injury via NLRP3 inflammasome activation in cerebral ischemia/reperfusion injury. J. Neuroinflamm. 2023, 20, 148. [Google Scholar] [CrossRef]
- Feng, X.; Yang, X.; Zhong, Y.; Cheng, X. The role of ncRNAs-mediated pyroptosis in diabetes and its vascular complications. Cell Biochem. Funct. 2024, 42, e3968. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, X.; Cai, Z.; Zhou, J.; Cao, R.; Zhao, Y.; Chen, Z.; Wang, D.; Ruan, W.; Zhao, Q.; et al. A novel class of microRNA-recognition elements that function only within open reading frames. Nat. Struct. Mol. Biol. 2018, 25, 1019–1027. [Google Scholar] [CrossRef]
- Wang, N.; Yu, Y.; Xu, B.; Zhang, M.; Li, Q.; Miao, L. Pivotal prognostic and diagnostic role of the long non-coding RNA colon cancer-associated transcript 1 expression in human cancer (Review). Mol. Med. Rep. 2019, 19, 771–782. [Google Scholar] [CrossRef]
- Militello, G.; Weirick, T.; John, D.; Döring, C.; Dimmeler, S.; Uchida, S. Screening and validation of lncRNAs and circRNAs as miRNA sponges. Brief Bioinform. 2017, 18, 780–788. [Google Scholar] [CrossRef]
- Ye, J.; Shan, Y.; Zhou, X.; Tian, T.; Gao, W. Identification of Novel Circular RNA Targets in Key Penumbra Region of Rats After Cerebral Ischemia-Reperfusion Injury. J. Mol. Neurosci. 2023, 73, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Zychlinsky, A.; Prevost, M.C.; Sansonetti, P.J. Shigella flexneri induces apoptosis in infected macrophages. Nature 1992, 358, 167–169. [Google Scholar] [CrossRef] [PubMed]
- Cookson, B.T.; Brennan, M.A. Pro-inflammatory programmed cell death. Trends Microbiol. 2001, 9, 113–114. [Google Scholar] [CrossRef]
- Brennan, M.A.; Cookson, B.T. Salmonella induces macrophage death by caspase-1-dependent necrosis. Mol. Microbiol. 2000, 38, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Stoess, C.; Choi, Y.K.; Onyuru, J.; Friess, H.; Hoffman, H.M.; Hartmann, D.; Feldstein, A.E. Cell Death in Liver Disease and Liver Surgery. Biomedicines 2024, 12, 559. [Google Scholar] [CrossRef]
- Toldo, S.; Mauro, A.G.; Cutter, Z.; Abbate, A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1553–H1568. [Google Scholar] [CrossRef]
- Wang, L.; Ren, W.; Wu, Q.; Liu, T.; Wei, Y.; Ding, J.; Zhou, C.; Xu, H.; Yang, S. NLRP3 Inflammasome Activation: A Therapeutic Target for Cerebral Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2022, 15, 847440. [Google Scholar] [CrossRef]
- Yarovinsky, T.O.; Su, M.; Chen, C.; Xiang, Y.; Tang, W.H.; Hwa, J. Pyroptosis in cardiovascular diseases: Pumping gasdermin on the fire. Semin. Immunol. 2023, 69, 101809. [Google Scholar] [CrossRef]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Kayagaki, N.; Lee, B.L.; Stowe, I.B.; Kornfeld, O.S.; O’Rourke, K.; Mirrashidi, K.M.; Haley, B.; Watanabe, C.; Roose-Girma, M.; Modrusan, Z.; et al. IRF2 transcriptionally induces GSDMD expression for pyroptosis. Sci. Signal. 2019, 12, eaax4917. [Google Scholar] [CrossRef]
- Ma, X.; Li, Y.; Shen, W.; Oladejo, A.O.; Yang, J.; Jiang, W.; Imam, B.H.; Wu, X.; Ding, X.; Yang, Y.; et al. LPS Mediates Bovine Endometrial Epithelial Cell Pyroptosis Directly Through Both NLRP3 Classical and Non-Classical Inflammasome Pathways. Front. Immunol. 2021, 12, 676088. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhao, Y.; Wang, Y.; Gao, W.; Ding, J.; Li, P.; Hu, L.; Shao, F. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 2014, 514, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Pelegrín, P.; Shao, F. The gasdermins, a protein family executing cell death and inflammation. Nat. Rev. Immunol. 2020, 20, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.J.; Liu, X.; Xia, S.; Zhang, Z.; Zhang, Y.; Zhao, J.; Ruan, J.; Luo, X.; Lou, X.; Bai, Y.; et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat. Immunol. 2020, 21, 736–745. [Google Scholar] [CrossRef]
- Sarhan, J.; Liu, B.C.; Muendlein, H.I.; Li, P.; Nilson, R.; Tang, A.Y.; Rongvaux, A.; Bunnell, S.C.; Shao, F.; Green, D.R.; et al. Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc. Natl. Acad. Sci. USA 2018, 115, e10888–e10897. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Yang, T.; Xiao, J.; Xu, C.; Alippe, Y.; Sun, K.; Kanneganti, T.D.; Monahan, J.B.; Abu-Amer, Y.; Lieberman, J.; et al. NLRP3 inflammasome activation triggers gasdermin D-independent inflammation. Sci. Immunol. 2021, 6, eabj3859. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Zhao, R.; Xia, W.; Chang, C.W.; You, Y.; Hsu, J.M.; Nie, L.; Chen, Y.; Wang, Y.C.; Liu, C.; et al. PD-L1-mediated gasdermin C expression switches apoptosis to pyroptosis in cancer cells and facilitates tumour necrosis. Nat. Cell Biol. 2020, 22, 1264–1275. [Google Scholar] [CrossRef]
- Meng, C.; Zhang, J.; Zhang, L.; Wang, Y.; Li, Z.; Zhao, J. Effects of NLRP6 in Cerebral Ischemia/Reperfusion (I/R) Injury in Rats. J. Mol. Neurosci. 2019, 69, 411–418. [Google Scholar] [CrossRef]
- Voet, S.; Srinivasan, S.; Lamkanfi, M.; van Loo, G. Inflammasomes in neuroinflammatory and neurodegenerative diseases. EMBO Mol. Med. 2019, 11, e10248. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, N.; Zhang, L.; Meng, C.; Zhao, J.; Wu, J. NLRP6 expressed in astrocytes aggravates neurons injury after OGD/R through activating the inflammasome and inducing pyroptosis. Int. Immunopharmacol. 2020, 80, 106183. [Google Scholar] [CrossRef]
- Feng, Y.S.; Tan, Z.X.; Wang, M.M.; Xing, Y.; Dong, F.; Zhang, F. Inhibition of NLRP3 Inflammasome: A Prospective Target for the Treatment of Ischemic Stroke. Front. Cell Neurosci. 2020, 14, 155. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Jiang, W.; Wang, R.; Zhong, H.; He, H.; Gao, X.; Zhong, S.; Yu, F.; Guo, Q.; Zhang, L.; et al. Brain endothelial GSDMD activation mediates inflammatory BBB breakdown. Nature 2024, 629, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Liu, M.; Fan, Y.; Zhang, J.; Liu, L.; Li, Y.; Zhang, Q.; Xie, H.; Jiang, C.; Wu, J.; et al. Intermittent theta-burst stimulation improves motor function by inhibiting neuronal pyroptosis and regulating microglial polarization via TLR4/NFκB/NLRP3 signaling pathway in cerebral ischemic mice. J. Neuroinflamm. 2022, 19, 141. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Li, H.; Liu, Y.; Ou, W.; Zhang, X.; Chai, H.; Huang, X.; Yang, W.; Wang, Q. Activating cGAS-STING axis contributes to neuroinflammation in CVST mouse model and induces inflammasome activation and microglia pyroptosis. J. Neuroinflamm. 2022, 19, 137. [Google Scholar] [CrossRef]
- Zhong, Y.; Wang, S.; Yin, Y.; Yu, J.; Liu, Y.; Gao, H. Dexmedetomidine suppresses hippocampal astrocyte pyroptosis in cerebral hypoxic-ischemic neonatal rats by upregulating microRNA-148a-3p to inactivate the STAT/JMJD3 axis. Int. Immunopharmacol. 2023, 121, 110440. [Google Scholar] [CrossRef]
- Liang, Y.; Song, P.; Chen, W.; Xie, X.; Luo, R.; Su, J.; Zhu, Y.; Xu, J.; Liu, R.; Zhu, P.; et al. Inhibition of Caspase-1 Ameliorates Ischemia-Associated Blood-Brain Barrier Dysfunction and Integrity by Suppressing Pyroptosis Activation. Front. Cell Neurosci. 2020, 14, 540669. [Google Scholar] [CrossRef]
- Guo, Y.; Yang, J.H.; He, Y.; Zhou, H.F.; Wang, Y.; Ding, Z.S.; Jin, B.; Wan, H.T. Protocatechuic aldehyde prevents ischemic injury by attenuating brain microvascular endothelial cell pyroptosis via lncRNA Xist. Phytomedicine 2022, 94, 153849. [Google Scholar] [CrossRef]
- Huang, X.; Gan, H.; Tan, J.; Wang, T.; Zhao, J.; Zhao, Y. BRCC3 promotes activation of the NLRP6 inflammasome following cerebral ischemia/reperfusion (I/R) injury in rats. Neurosci. Lett. 2021, 756, 135954. [Google Scholar] [CrossRef]
- Huang, X.; Tan, J.; Ji, Y.; Luo, J.; Zhao, Y.; Zhao, J. BRCC3 mediates inflammation and pyroptosis in cerebral ischemia/reperfusion injury by activating the NLRP6 inflammasome. CNS Neurosci. Ther. 2024, 30, e14697. [Google Scholar] [CrossRef]
- Rivest, S. Regulation of innate immune responses in the brain. Nat. Rev. Immunol. 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Xu, S.; Lu, J.; Shao, A.; Zhang, J.H.; Zhang, J. Glial Cells: Role of the Immune Response in Ischemic Stroke. Front. Immunol. 2020, 11, 294. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.C.; Zhang, N.N.; Zhang, Y.N.; Chen, H.S. Kv1.3 channel blockade alleviates cerebral ischemia/reperfusion injury by reshaping M1/M2 phenotypes and compromising the activation of NLRP3 inflammasome in microglia. Exp. Neurol. 2020, 332, 113399. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.B.; Deng, X.; Wang, J.; Qi, Y.; Zhao, W.; Guan, S. HAX-1 interferes in assembly of NLRP3-ASC to block microglial pyroptosis in cerebral I/R injury. Cell Death Discov. 2024, 10, 264. [Google Scholar] [CrossRef]
- Long, J.; Sun, Y.; Liu, S.; Yang, S.; Chen, C.; Zhang, Z.; Chu, S.; Yang, Y.; Pei, G.; Lin, M.; et al. Targeting pyroptosis as a preventive and therapeutic approach for stroke. Cell Death Discov. 2023, 9, 155. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, F.; Xu, H.; Yang, H.; Shao, M.; Xu, S.; Lyu, F. TLR4 aggravates microglial pyroptosis by promoting DDX3X-mediated NLRP3 inflammasome activation via JAK2/STAT1 pathway after spinal cord injury. Clin. Transl. Med. 2022, 12, e894. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.X.; Zheng, C.X.; Zhou, X.Q.; Chen, C.; Qiu, S.W.; Liu, W.H.; Li, H. Neuroprotective effects of Jie-du-huo-xue decoction on microglia pyroptosis after cerebral ischemia and reperfusion—From the perspective of glial-vascular unit. J. Ethnopharmacol. 2024, 318, 116990. [Google Scholar] [CrossRef]
- Jung, B.K.; Park, Y.; Yoon, B.; Bae, J.S.; Han, S.W.; Heo, J.E.; Kim, D.E.; Ryu, K.Y. Reduced secretion of LCN2 (lipocalin 2) from reactive astrocytes through autophagic and proteasomal regulation alleviates inflammatory stress and neuronal damage. Autophagy 2023, 19, 2296–2317. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Sun, H.; Xu, C.; Sun, H.; Wei, W.; Song, J.; Jia, F.; Zhong, D.; Li, G. A2 reactive astrocyte-derived exosomes alleviate cerebral ischemia-reperfusion injury by delivering miR-628. J. Cell. Mol. Med. 2024, 28, e70004. [Google Scholar] [CrossRef]
- Xia, P.; Pan, Y.; Zhang, F.; Wang, N.; Wang, E.; Guo, Q.; Ye, Z. Pioglitazone Confers Neuroprotection Against Ischemia-Induced Pyroptosis due to its Inhibitory Effects on HMGB-1/RAGE and Rac1/ROS Pathway by Activating PPAR-ɤ. Cell. Physiol. Biochem. 2018, 45, 2351–2368. [Google Scholar] [CrossRef]
- An, P.; Xie, J.; Qiu, S.; Liu, Y.; Wang, J.; Xiu, X.; Li, L.; Tang, M. Hispidulin exhibits neuroprotective activities against cerebral ischemia reperfusion injury through suppressing NLRP3-mediated pyroptosis. Life Sci. 2019, 232, 116599. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cheng, L.; Zhan, H.; Li, H.; Li, X.; Huang, Y.; Li, Y. The Roles of Noncoding RNAs in Systemic Sclerosis. Front. Immunol. 2022, 13, 856036. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yujiao, W.; Fang, W.; Linhui, Y.; Ziqi, G.; Zhichen, W.; Zirui, W.; Shengwang, W. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol. Res. 2020, 53, 40. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Tran, N. miRNA interplay: Mechanisms and consequences in cancer. Dis. Models Mech. 2021, 14, dmm047662. [Google Scholar] [CrossRef]
- Alarcón, C.R.; Lee, H.; Goodarzi, H.; Halberg, N.; Tavazoie, S.F. N6-methyladenosine marks primary microRNAs for processing. Nature 2015, 519, 482–485. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, Q.; Yu, L.; Zhu, D.; Li, Y.; Xue, Z.; Hua, Z.; Luo, X.; Song, Z.; Lu, C.; et al. The Role of miRNA in Tumor Immune Escape and miRNA-Based Therapeutic Strategies. Front. Immunol. 2021, 12, 807895. [Google Scholar] [CrossRef]
- Alvarez-Dominguez, J.R.; Lodish, H.F. Emerging mechanisms of long noncoding RNA function during normal and malignant hematopoiesis. Blood 2017, 130, 1965–1975. [Google Scholar] [CrossRef]
- Lin, C.; Yang, L. Long Noncoding RNA in Cancer: Wiring Signaling Circuitry. Trends Cell Biol. 2018, 28, 287–301. [Google Scholar] [CrossRef]
- Slaby, O.; Laga, R.; Sedlacek, O. Therapeutic targeting of non-coding RNAs in cancer. Biochem. J. 2017, 474, 4219–4251. [Google Scholar] [CrossRef]
- Chen, L.L.; Yang, L. Regulation of circRNA biogenesis. RNA Biol. 2015, 12, 381–388. [Google Scholar] [CrossRef]
- Yu, C.Y.; Kuo, H.C. The emerging roles and functions of circular RNAs and their generation. J. Biomed. Sci. 2019, 26, 29. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ran, L.; Zhao, H.; Yin, P.; Li, W.; Lin, J.; Mao, H.; Cai, D.; Ma, Q.; Pan, X.; et al. Circular RNA circ-TNPO3 suppresses metastasis of GC by acting as a protein decoy for IGF2BP3 to regulate the expression of MYC and SNAIL. Mol. Ther.-Nucleic Acids 2021, 26, 649–664. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Ji, H.; Jeong, S.D.; Pandey, P.R.; Gorospe, M.; Kim, H.H. LINC00162 regulates cell proliferation and apoptosis by sponging PAQR4-targeting miR-485-5p. J. Cell. Physiol. 2022, 237, 2943–2960. [Google Scholar] [CrossRef] [PubMed]
- Heydarnezhad Asl, M.; Pasban Khelejani, F.; Bahojb Mahdavi, S.Z.; Emrahi, L.; Jebelli, A.; Mokhtarzadeh, A. The various regulatory functions of long noncoding RNAs in apoptosis, cell cycle, and cellular senescence. J. Cell. Biochem. 2022, 123, 995–1024. [Google Scholar] [CrossRef]
- Li, X.; Peng, X.; Zhou, X.; Li, M.; Chen, G.; Shi, W.; Yu, H.; Zhang, C.; Li, Y.; Feng, Z.; et al. Small extracellular vesicles delivering lncRNA WAC-AS1 aggravate renal allograft ischemia–reperfusion injury by inducing ferroptosis propagation. Cell Death Differ. 2023, 30, 2167–2186. [Google Scholar] [CrossRef]
- Yang, K.; Zeng, L.; Ge, A.; Wang, S.; Zeng, J.; Yuan, X.; Mei, Z.; Wang, G.; Ge, J. A systematic review of the research progress of non-coding RNA in neuroinflammation and immune regulation in cerebral infarction/ischemia-reperfusion injury. Front. Immunol. 2022, 13, 930171. [Google Scholar] [CrossRef]
- Zingale, V.D.; Gugliandolo, A.; Mazzon, E. MiR-155: An Important Regulator of Neuroinflammation. Int. J. Mol. Sci. 2021, 23, 90. [Google Scholar] [CrossRef]
- Fan, Y.; Ding, S.; Sun, Y.; Zhao, B.; Pan, Y.; Wan, J. MiR-377 Regulates Inflammation and Angiogenesis in Rats After Cerebral Ischemic Injury. J. Cell Biochem. 2018, 119, 327–337. [Google Scholar] [CrossRef]
- Song, S.; Pan, Y.; Li, H.; Zhen, H. MiR-1202 Exerts Neuroprotective Effects on OGD/R Induced Inflammation in HM Cell by Negatively Regulating Rab1a Involved in TLR4/NF-κB Signaling Pathway. Neurochem. Res. 2020, 45, 1120–1129. [Google Scholar] [CrossRef]
- Yin, W.L.; Yin, W.G.; Huang, B.S.; Wu, L.X. LncRNA SNHG12 inhibits miR-199a to upregulate SIRT1 to attenuate cerebral ischemia/reperfusion injury through activating AMPK signaling pathway. Neurosci. Lett. 2019, 690, 188–195. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Q.; Su, D.; Xie, Y. Long Non-coding RNAMALAT1 Knockdown Alleviates Cerebral Ischemia/Reperfusion Injury of Rats Through Regulating the miR-375/PDE4D Axis. Front. Neurol. 2020, 11, 578765. [Google Scholar] [CrossRef]
- Deng, L.; Jiang, J.; Chen, S.; Lin, X.; Zuo, T.; Hu, Q.; Wu, Y.; Fan, X.; Dong, Z. Long Non-coding RNA ANRIL Downregulation Alleviates Neuroinflammation in an Ischemia Stroke Model via Modulation of the miR-671-5p/NF-κB Pathway. Neurochem. Res. 2022, 47, 2002–2015. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, Y.R.; Li, F.; Liu, X.L.; Zhang, H.; Zhu, Z.Z.; Huang, H.; Xu, X.H. Circ-camk4 involved in cerebral ischemia/reperfusion induced neuronal injury. Sci. Rep. 2020, 10, 7012. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, J.; Li, J.; Xu, L.; Lian, W. The decreased circular RNA hsa_circ_0072309 promotes cell apoptosis of ischemic stroke by sponging miR-100. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4420–4429. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xu, H.; Zhang, W.; Chen, Z.; Li, W.; Ke, W. Circular RNA circCCDC9 alleviates ischaemic stroke ischaemia/reperfusion injury via the Notch pathway. J. Cell Mol. Med. 2020, 24, 14152–14159. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Ho, E.S.; Mai, H.; Zang, J.; Liu, Y.; Li, Y.; Yang, B.; Ding, Y.; Tsang, C.K.; Xu, A. Identification of Blood Circular RNAs as Potential Biomarkers for Acute Ischemic Stroke. Front. Neurosci. 2020, 14, 81. [Google Scholar] [CrossRef] [PubMed]
- Ostolaza, A.; Blanco-Luquin, I.; Urdánoz-Casado, A.; Rubio, I.; Labarga, A.; Zandio, B.; Roldán, M.; Martínez-Cascales, J.; Mayor, S.; Herrera, M.; et al. Circular RNA expression profile in blood according to ischemic stroke etiology. Cell Biosci. 2020, 10, 34. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Han, B.; Meng, K.; Han, Y.; Ding, Y. Elucidating the Molecular Mechanism of Ischemic Stroke Using Integrated Analysis of miRNA, mRNA, and lncRNA Expression Profiles. Front. Integr. Neurosci. 2021, 15, 638114. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J.K.; Ma, J. Regulation of the long noncoding RNA XIST on the inflammatory polarization of microglia in cerebral infarction. Exp. Ther. Med. 2021, 22, 924. [Google Scholar] [CrossRef]
- Wooten, S.; Smith, K.N. Long non-coding RNA OIP5-AS1 (Cyrano): A context-specific regulator of normal and disease processes. Clin. Transl. Med. 2022, 12, e706. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, W.; Chen, M.; Sun, Q.; Chen, H.; Li, Y. Up-regulating lncRNA OIP5-AS1 protects neuron injury against cerebral hypoxia-ischemia induced inflammation and oxidative stress in microglia/macrophage through activating CTRP3 via sponging miR-186-5p. Int. Immunopharmacol. 2021, 92, 107339. [Google Scholar] [CrossRef]
- Li, Z.; Pang, Y.; Hou, L.; Xing, X.; Yu, F.; Gao, M.; Wang, J.; Li, X.; Zhang, L.; Xiao, Y. Exosomal OIP5-AS1 attenuates cerebral ischemia-reperfusion injury by negatively regulating TXNIP protein stability and inhibiting neuronal pyroptosis. Int. Immunopharmacol. 2024, 127, 111310. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Gao, J.; Sun, D.; Jiao, Q.; Ma, J.; Cui, W.; Lou, Y.; Xu, F.; Li, S.; Li, H. LncRNA MEG3: Potential stock for precision treatment of cardiovascular diseases. Front. Pharmacol. 2022, 13, 1045501. [Google Scholar] [CrossRef] [PubMed]
- Balusu, S.; Horré, K.; Thrupp, N.; Craessaerts, K.; Snellinx, A.; Serneels, L.; T’Syen, D.; Chrysidou, I.; Arranz, A.M.; Sierksma, A.; et al. MEG3 activates necroptosis in human neuron xenografts modeling Alzheimer’s disease. Science 2023, 381, 1176–1182. [Google Scholar] [CrossRef]
- Yao, L.; Peng, P.; Ding, T.; Yi, J.; Liang, J. m(6)A-Induced lncRNA MEG3 Promotes Cerebral Ischemia-Reperfusion Injury Via Modulating Oxidative Stress and Mitochondrial Dysfunction by hnRNPA1/Sirt2 Axis. Mol. Neurobiol. 2024, 61, 6893–6908. [Google Scholar] [CrossRef]
- Liang, J.; Wang, Q.; Li, J.Q.; Guo, T.; Yu, D. Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion injury through increasing pyroptosis by targeting miR-485/AIM2 axis. Exp. Neurol. 2020, 325, 113139. [Google Scholar] [CrossRef]
- Zhu, B.; Cheng, X.; Jiang, Y.; Cheng, M.; Chen, L.; Bao, J.; Tang, X. Silencing of KCNQ1OT1 Decreases Oxidative Stress and Pyroptosis of Renal Tubular Epithelial Cells. Diabetes Metab. Syndr. Obes. 2020, 13, 365–375. [Google Scholar] [CrossRef]
- Wang, H.J.; Tang, X.L.; Huang, G.; Li, Y.B.; Pan, R.H.; Zhan, J.; Wu, Y.K.; Liang, J.F.; Bai, X.X.; Cai, J. Long Non-Coding KCNQ1OT1 Promotes Oxygen-Glucose-Deprivation/Reoxygenation-Induced Neurons Injury Through Regulating MIR-153-3p/FOXO3 Axis. J. Stroke Cerebrovasc. Dis. 2020, 29, 105126. [Google Scholar] [CrossRef]
- Yi, M.; Li, Y.; Wang, D.; Zhang, Q.; Yang, L.; Yang, C. KCNQ1OT1 Exacerbates Ischemia-Reperfusion Injury Through Targeted Inhibition of miR-140-3P. Inflammation 2020, 43, 1832–1845. [Google Scholar] [CrossRef]
- Li, Y.; Yi, M.; Wang, D.; Zhang, Q.; Yang, L.; Yang, C. Correction to: LncRNA KCNQ1OT1 Regulates Endoplasmic Reticulum Stress to Affect Cerebral Ischemia-Reperfusion Injury Through Targeting miR-30b/GRP78. Inflammation 2022, 45, 935–936. [Google Scholar] [CrossRef]
- Ren, Y.; Gao, X.P.; Liang, H.; Zhang, H.; Hu, C.Y. LncRNA KCNQ1OT1 contributes to oxygen-glucose-deprivation/reoxygenation-induced injury via sponging miR-9 in cultured neurons to regulate MMP8. Exp. Mol. Pathol. 2020, 112, 104356. [Google Scholar] [CrossRef]
- Xu, Y.; Niu, Y.; Li, H.; Pan, G. Downregulation of lncRNA TUG1 attenuates inflammation and apoptosis of renal tubular epithelial cell induced by ischemia-reperfusion by sponging miR-449b-5p via targeting HMGB1 and MMP2. Inflammation 2020, 43, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Yu, J.; Liu, H.B.; Yan, X.Q.; Hu, J.; Yu, Y.; Guo, J.; Yuan, Y.; Du, Z.M. The long non-coding RNA TUG1-miR-9a-5p axis contributes to ischemic injuries by promoting cardiomyocyte apoptosis via targeting KLF5. Cell Death Dis. 2019, 10, 908. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Chen, W.; Zhao, X.; Pei, A.; Chen, M.; Yu, Y.; Zheng, Y.; Zhu, S. Long noncoding RNA TUG1 contributes to cerebral ischaemia/reperfusion injury by sponging mir-145 to up-regulate AQP4 expression. J. Cell Mol. Med. 2020, 24, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Chen, W.P.; Yin, X.P.; Tu, J.L.; Hu, N.; Li, Z.Y. LncRNA TUG1 Demethylated by TET2 Promotes NLRP3 Expression, Contributes to Cerebral Ischemia/Reperfusion Inflammatory Injury. ASN Neuro 2021, 13, 17590914211003247. [Google Scholar] [CrossRef]
- Fan, W.; Qin, Y.; Tan, J.; Li, B.; Liu, Y.; Rong, J.; Shi, W.; Yu, B. RGD1564534 represses NLRP3 inflammasome activity in cerebral injury following ischemia-reperfusion by impairing miR-101a-3p-mediated Dusp1 inhibition. Exp. Neurol. 2023, 359, 114266. [Google Scholar] [CrossRef]
- Zhang, H.S.; Ouyang, B.; Ji, X.Y.; Liu, M.F. Gastrodin Alleviates Cerebral Ischaemia/Reperfusion Injury by Inhibiting Pyroptosis by Regulating the lncRNA NEAT1/miR-22-3p Axis. Neurochem. Res. 2021, 46, 1747–1758. [Google Scholar] [CrossRef]
- Liu, B.; Xu, T.; Meng, Y. IncRNA NEAT1 aggravates cerebral ischemia/reperfusion injury by sponging miR-874-3p. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Shen, S.; Ma, L.; Shao, F.; Jin, L.; Bian, Z. Long Non-Coding RNA (lncRNA) NEAT1 Aggravates Cerebral Ischemia-Reperfusion Injury by Suppressing the Inhibitory Effect of miR-214 on PTEN. Med. Sci. Monit. 2020, 26, e924781. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, H.; Li, Q.; Fu, J.; Huang, J.; Zhao, Y. MALAT1 Activates the P53 Signaling Pathway by Regulating MDM2 to Promote Ischemic Stroke. Cell Physiol. Biochem. 2018, 50, 2216–2228. [Google Scholar] [CrossRef]
- Zhao, N.; Hua, W.; Liu, Q.; Wang, Y.; Liu, Z.; Jin, S.; Wang, B.; Pang, Y.; Qi, J.; Song, Y. MALAT1 knockdown alleviates the pyroptosis of microglias in diabetic cerebral ischemia via regulating STAT1 mediated NLRP3 transcription. Mol. Med. 2023, 29, 44. [Google Scholar] [CrossRef]
- Gao, P.; Shi, H.; Jin, X.; Guo, S.; Zhou, X.; Gao, W. Mechanism of astragaloside IV regulating NLRP3 through LOC102555978 to attenuate cerebral ischemia reperfusion induced microglia pyroptosis. Int. Immunopharmacol. 2024, 131, 111862. [Google Scholar] [CrossRef]
- Qin, X.; Lu, M.; Zhou, Y.; Li, G.; Liu, Z. LncRNA FENDRR represses proliferation, migration and invasion through suppression of survivin in cholangiocarcinoma cells. Cell Cycle 2019, 18, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Zhou, B.; Sun, Z.; Huang, S.; Han, L.; Nie, H.; Chen, G.; Liu, S.; Zhang, Y.; Bao, N.; et al. LncRNA-FENDRR mediates VEGFA to promote the apoptosis of brain microvascular endothelial cells via regulating miR-126 in mice with hypertensive intracerebral hemorrhage. Microcirculation 2018, 25, e12499. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Q.; Zheng, Y.Y.; Zhou, H.J.; Zhang, X.X.; Wu, P.; Zhu, S.M. LncRNA-Fendrr protects against the ubiquitination and degradation of NLRC4 protein through HERC2 to regulate the pyroptosis of microglia. Mol. Med. 2021, 27, 39. [Google Scholar] [CrossRef]
- Wang, Q.S.; Luo, X.Y.; Fu, H.; Luo, Q.; Wang, M.Q.; Zou, D.Y. MiR-139 protects against oxygen-glucose deprivation/reoxygenation (OGD/R)-induced nerve injury through targeting c-Jun to inhibit NLRP3 inflammasome activation. J. Stroke Cerebrovasc. Dis. 2020, 29, 105037. [Google Scholar] [CrossRef]
- Yao, Y.; Hu, S.; Zhang, C.; Zhou, Q.; Wang, H.; Yang, Y.; Liu, C.; Ding, H. Ginsenoside Rd attenuates cerebral ischemia/reperfusion injury by exerting an anti-pyroptotic effect via the miR-139-5p/FoxO1/Keap1/Nrf2 axis. Int. Immunopharmacol. 2022, 105, 108582. [Google Scholar] [CrossRef]
- Yuan, X.W.; Yan, T.Q.; Tong, H. Effect of miR-515-5p on Proliferation and Drug Sensitivity of Retinoblastoma Cells. Cancer Manag. Res. 2020, 12, 12087–12098. [Google Scholar] [CrossRef]
- Shi, Y.; Li, K.; Xu, K.; Liu, Q.H. MiR-155-5p accelerates cerebral ischemia-reperfusion injury via targeting DUSP14 by regulating NF-κB and MAPKs signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1408–1419. [Google Scholar] [CrossRef]
- Que, Y.Y.; Zhu, T.; Zhang, F.X.; Peng, J. Neuroprotective effect of DUSP14 overexpression against isoflurane-induced inflammatory response, pyroptosis and cognitive impairment in aged rats through inhibiting the NLRP3 inflammasome. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7101–7113. [Google Scholar] [CrossRef]
- Shi, Y.; Li, Z.; Li, K.; Xu, K. miR-155-5p accelerates cerebral ischemia-reperfusion inflammation injury and cell pyroptosis via DUSP14/ TXNIP/NLRP3 pathway. Acta Biochim. Pol. 2022, 69, 787–793. [Google Scholar] [CrossRef]
- Dong, Q.; Long, X.; Cheng, J.; Wang, W.; Tian, Q.; Di, W. LncRNA GAS5 suppresses ovarian cancer progression by targeting the miR-96-5p/PTEN axis. Ann. Transl. Med. 2021, 9, 1770. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Duan, Y.; Li, S.; Wang, Q.; Zhen, W.; Zhang, W.; Zhang, Y.; Jiang, M.; Wang, C. miR-96-5p regulates myocardial infarction-induced cardiac fibrosis via Smad7/Smad3 pathway. Acta Biochim. Biophys. Sin. 2022, 54, 1874–1888. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.; Jin, L.; Wei, B.; Li, X.; Li, R.; Liu, W.; Guo, S.; Fan, H.; Duan, C. miR-96-5p alleviates cerebral ischemia-reperfusion injury in mice by inhibiting pyroptosis via downregulating caspase 1. Exp. Neurol. 2024, 374, 114676. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.Y.; Cao, S.Q. MiR-29a-3p: A potential biomarker and therapeutic target in colorectal cancer. Clin. Transl. Oncol. 2023, 25, 563–577. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hao, X.; Liu, J.; Zhang, Q.; Liang, Z.; Li, X.; Liu, H. miR-29a-3p Regulates Autophagy by Targeting Akt3-Mediated mTOR in SiO(2)-Induced Lung Fibrosis. Int. J. Mol. Sci. 2023, 24, 11440. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Wang, M. MiR-29a regulates cardiomyocyte apoptosis by targeting Bak1 in diabetic cardiomyopathy. J. Biochem. 2022, 171, 663–671. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Liu, Z.; Zhang, M.; Leng, Y. MircoRNA-29a in Astrocyte-derived Extracellular Vesicles Suppresses Brain Ischemia Reperfusion Injury via TP53INP1 and the NF-κB/NLRP3 Axis. Cell Mol. Neurobiol. 2022, 42, 1487–1500. [Google Scholar] [CrossRef]
- Luo, J.; Lang, J.; Xu, W.; Wang, L.; Zhao, Z.; Jia, J.; Lang, B. Electroacupuncture Alleviates Post-stroke Cognitive Impairment Through Inhibiting miR-135a-5p/mTOR/NLRP3 Axis-mediated Autophagy. Neuroscience 2024, 545, 185–195. [Google Scholar] [CrossRef]
- Chen, H.; Li, X. LncRNA ROR is involved in cerebral hypoxia/reoxygenation-induced injury in PC12 cells via regulating miR-135a-5p/ROCK1/2. Am. J. Transl. Res. 2019, 11, 6145–6158. [Google Scholar]
- Liu, Y.; Li, Y.P.; Xiao, L.M.; Chen, L.K.; Zheng, S.Y.; Zeng, E.M.; Xu, C.H. Extracellular vesicles derived from M2 microglia reduce ischemic brain injury through microRNA-135a-5p/TXNIP/NLRP3 axis. Lab. Investig. 2021, 101, 837–850. [Google Scholar] [CrossRef]
- Harraz, M.M.; Eacker, S.M.; Wang, X.; Dawson, T.M.; Dawson, V.L. MicroRNA-223 is neuroprotective by targeting glutamate receptors. Proc. Natl. Acad. Sci. USA 2012, 109, 18962–18967. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, L.; Xian, R.; Yuan, B. MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol. Immunol. 2015, 65, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Sha, R.; Zhang, B.; Han, X.; Peng, J.; Zheng, C.; Zhang, F.; Huang, X. Electroacupuncture Alleviates Ischemic Brain Injury by Inhibiting the miR-223/NLRP3 Pathway. Med. Sci. Monit. 2019, 25, 4723–4733. [Google Scholar] [CrossRef]
- Hamzei Taj, S.; Kho, W.; Riou, A.; Wiedermann, D.; Hoehn, M. MiRNA-124 induces neuroprotection and functional improvement after focal cerebral ischemia. Biomaterials 2016, 91, 151–165. [Google Scholar] [CrossRef]
- Ponomarev, E.D.; Veremeyko, T.; Barteneva, N.; Krichevsky, A.M.; Weiner, H.L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α-PU.1 pathway. Nat. Med. 2011, 17, 64–70. [Google Scholar] [CrossRef]
- Sun, H.; Li, J.J.; Feng, Z.R.; Liu, H.Y.; Meng, A.G. MicroRNA-124 regulates cell pyroptosis during cerebral ischemia-reperfusion injury by regulating STAT3. Exp. Ther. Med. 2020, 20, 227. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, N.; Chen, J.; Yu, G.; Zhao, J.; Yang, C.; Zhao, Y. Inhibition of miR-423-5p Exerts Neuroprotective Effects in an Experimental Rat Model of Cerebral Ischemia/Reperfusion Injury. Neuroscience 2022, 503, 95–106. [Google Scholar] [CrossRef]
- Wan, H.; Yuan, B.; Jiang, K.; Wei, J.; Feng, X.; Sun, B.; Wang, F. CircRNA CircRIMS is Overexpressed in Esophageal Squamous Cell Carcinoma and Downregulate miR-613 Through Methylation to Increase Cell Proliferation. Cancer Manag. Res. 2021, 13, 4587–4595. [Google Scholar] [CrossRef]
- Li, W.; Xie, L.; Wang, L.; Lin, F. CircRIMS promotes cerebral ischemia-reperfusion injury through increasing apoptosis and targeting the miR-96-5p/JAK/STAT1 axis. Brain Inj. 2023, 37, 1235–1244. [Google Scholar] [CrossRef]
- Wang, C.; Dong, M.; Zhang, X.; Wang, X.; Zhao, Y.; Cao, Y. Competitive binding of circCCDC6 to microRNA-128-3p activates TXNIP/NLRP3 pathway and promotes cerebral ischemia-reperfusion defects. Acta Biochim. Pol. 2023, 70, 807–815. [Google Scholar] [CrossRef]
- Cheng, X.; Ren, Z.; Jia, H.; Wang, G. METTL3 Mediates Microglial Activation and Blood-Brain Barrier Permeability in Cerebral Ischemic Stroke by Regulating NLRP3 Inflammasomes Through m6A Methylation Modification. Neurotox. Res. 2024, 42, 15. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).