Molecular Mechanisms and Potential Therapeutic Targets of Ischemia–Reperfusion Injury in Kidney Transplantation
Abstract
1. Introduction
2. Ischemia–Reperfusion Injury and Its Molecular Mechanisms
2.1. Mitochondrial Oxidative Stress, Immune, and Inflammatory Responses in IRI
2.2. Programmed Cell Death
2.3. Inflammatory and Immune Responses
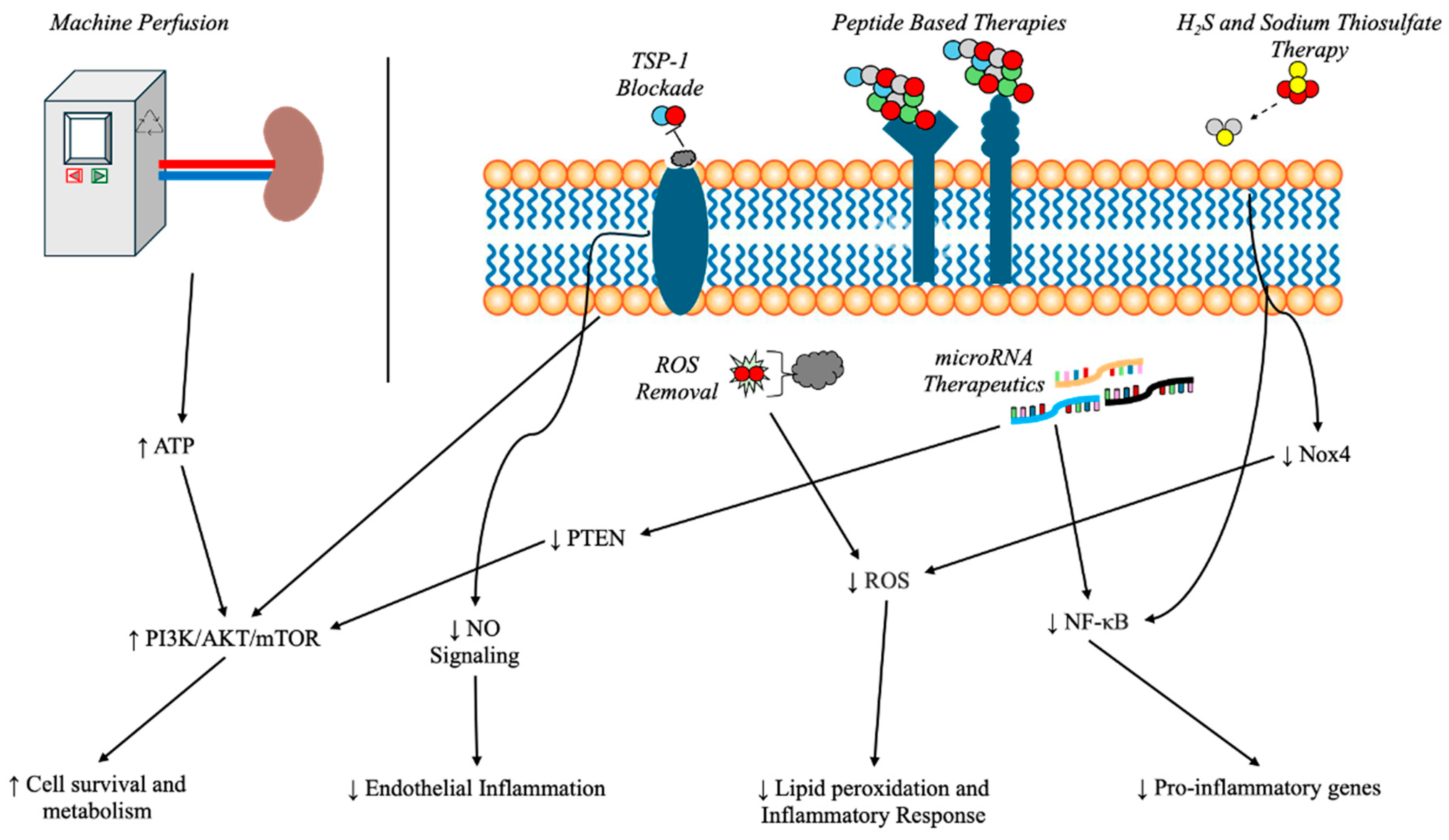
3. Potential Therapeutic Targets and Surgical Considerations
3.1. Cold Storage vs. Machine Perfusion
3.2. Ischemic Conditioning
3.3. MicroRNA as Therapeutic Targets
3.4. Peptide-Based Therapies
3.5. Thrombospondin-1 Blockade Using Nanoparticles
3.6. Reactive Oxygen Species (ROS) Removal via Nanoparticles
3.7. Hydrogen Sulfide
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ESRD | End-stage renal disease |
| DCD | Donor after circulatory death |
| DBD | Donor after brain death |
| IRI | Ischemia–reperfusion injury |
| ROS | Reactive oxygen species |
| NOS | Nitric oxide synthase |
| Drp1 | Dynamin-related protein 1 |
| mtDNA | Mitochondrial DNA |
| mtROS | Mitochondrial reactive oxygen species |
| TFAM | Mitochondrial transcription factor A |
| ATP | Adenosine triphosphate |
| PAF | Platelet-activating factor |
| PAF-LPL | Platelet-activating factor phospholipids |
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| FasL | Fas ligand |
| SCS | Static cold storage |
| MP | Machine perfusion |
| DGF | Delayed graft function |
| eGFR | Estimated glomerular filtration rate |
| miRNA | MicroRNA |
| TSP-1 | Thrombospondin-1 |
| H2S | Hydrogen sulfide |
| STS | Sodium thiosulfate |
| ATN | Acute tubular necrosis |
References
- NKF Patient Education Team. Kidney Failure. Available online: https://www.kidney.org/kidney-topics/patient-education-library-brochures (accessed on 8 April 2025).
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Dor, A.; Pauly, M.V.; Eichleay, M.A.; Held, P.J. End-stage renal disease and economic incentives: The International Study of Health Care Organization and Financing (ISHCOF). Int. J. Health Care Financ. Econ. 2007, 7, 73–111. [Google Scholar] [CrossRef] [PubMed]
- United States Renal Data System. 2022 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Available online: https://usrds-adr.niddk.nih.gov/2022 (accessed on 8 April 2025).
- Ortiz, A.; Covic, A.; Fliser, D.; Fouque, D.; Goldsmith, D.; Kanbay, M.; Mallamaci, F.; Massy, Z.A.; Rossignol, P.; Vanholder, R.; et al. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 2014, 383, 1831–1843. [Google Scholar] [CrossRef] [PubMed]
- Hemmelgarn, B.R.; James, M.T.; Manns, B.J.; O’Hare, A.M.; Muntner, P.; Ravani, P.; Quinn, R.R.; Turin, T.C.; Tan, Z.; Tonelli, M.; et al. Rates of Treated and Untreated Kidney Failure in Older vs Younger Adults. JAMA 2012, 307, 2507–2515. [Google Scholar] [CrossRef]
- Berns, J. Patient Education: Dialysis or Kidney Transplantation—Which Is Right for Me? (Beyond the Basics). Available online: https://renalassociateswmi.com/wp-content/uploads/2020/01/Dialysis-or-Kidney-Transplantation.pdf (accessed on 8 April 2025).
- Garcia-Garcia, G.; Briseño-Rentería, G.; Luquín-Arellan, V.H.; Gao, Z.; Gill, J.; Tonelli, M. Survival among Patients with Kidney Failure in Jalisco, Mexico. J. Am. Soc. Nephrol. 2007, 18, 1922–1927. [Google Scholar] [CrossRef]
- Kidney Disease Statistics for the United States. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). Available online: https://www.niddk.nih.gov/health-information/kidney-disease (accessed on 8 April 2025).
- Schold, J.D.; Buccini, L.D.; Goldfarb, D.A.; Flechner, S.M.; Poggio, E.D.; Sehgal, A.R. Association between Kidney Transplant Center Performance and the Survival Benefit of Transplantation Versus Dialysis. Clin. J. Am. Soc. Nephrol. 2014, 9, 1773–1780. [Google Scholar] [CrossRef]
- Tonelli, M.; Wiebe, N.; Knoll, G.; Bello, A.; Browne, S.; Jadhav, D.; Klarenbach, S.; Gill, J. Systematic Review: Kidney Transplantation Compared With Dialysis in Clinically Relevant Outcomes. Am. J. Transplant. 2011, 11, 2093–2109. [Google Scholar] [CrossRef]
- Poggio, E.D.; Augustine, J.J.; Arrigain, S.; Brennan, D.C.; Schold, J.D. Long-term kidney transplant graft survival—Making progress when most needed. Am. J. Transplant. 2021, 21, 2824–2832. [Google Scholar] [CrossRef]
- Summers, D.M.; Watson, C.J.E.; Pettigrew, G.J.; Johnson, R.J.; Collett, D.; Neuberger, J.M.; Bradley, J.A. Kidney donation after circulatory death (DCD): State of the art. Kidney Int. 2015, 88, 241–249. [Google Scholar] [CrossRef]
- Peters-Sengers, H.; Houtzager, J.H.E.; Idu, M.M.; Heemskerk, M.B.A.; van Heurn, E.L.W.; van der Heide, J.J.H.; Kers, J.; Berger, S.P.; van Gulik, T.M.; Bemelman, F.J. Impact of Cold Ischemia Time on Outcomes of Deceased Donor Kidney Transplantation: An Analysis of a National Registry. Transplant. Direct 2019, 5, e448. [Google Scholar] [CrossRef]
- Lum, E.L.; Homkrailas, P.; Abdalla, B.; Danovitch, G.M.; Bunnapradist, S. Cold Ischemia Time, Kidney Donor Profile Index, and Kidney Transplant Outcomes: A Cohort Study. Kidney Med. 2023, 5, 100570. [Google Scholar] [CrossRef] [PubMed]
- Cavaillé-Coll, M.; Bala, S.; Velidedeoglu, E.; Hernandez, A.; Archdeacon, P.; Gonzalez, G.; Neuland, C.; Meyer, J.; Albrecht, R. Summary of FDA Workshop on Ischemia Reperfusion Injury in Kidney Transplantation. Am. J. Transplant. 2013, 13, 1134–1148. [Google Scholar] [CrossRef] [PubMed]
- Saat, T.C.; van den Akker, E.K.; IJzermans, J.N.M.; Dor, F.J.M.F.; de Bruin, R.W.F. Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: Lost in translation? J. Transl. Med. 2016, 14, 20. [Google Scholar] [CrossRef]
- Granata, S.; Votrico, V.; Spadaccino, F.; Catalano, V.; Netti, G.S.; Ranieri, E.; Stallone, G.; Zaza, G. Oxidative Stress and Ischemia/Reperfusion Injury in Kidney Transplantation: Focus on Ferroptosis, Mitophagy and New Antioxidants. Antioxidants 2022, 11, 769. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2008, 417, 1–13. [Google Scholar] [CrossRef]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Li, N.; Wang, H.; Jiang, C.; Zhang, M. Renal ischemia/reperfusion-induced mitophagy protects against renal dysfunction via Drp1-dependent-pathway. Exp. Cell Res. 2018, 369, 27–33. [Google Scholar] [CrossRef]
- Shi, L.; Zha, H.; Huang, H.; Xia, Y.; Li, H.; Huang, J.; Yue, R.; Li, C.; Zhu, J.; Song, Z. miR-199a-5p aggravates renal ischemia-reperfusion and transplant injury by targeting AKAP1 to disrupt mitochondrial dynamics. Am. J. Physiol.-Ren. Physiol. 2024, 327, F910–F929. [Google Scholar] [CrossRef]
- Perry, H.M.; Huang, L.; Wilson, R.J.; Bajwa, A.; Sesaki, H.; Yan, Z.; Rosin, D.L.; Kashatus, D.F.; Okusa, M.D. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 194–206. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yu, Y.; Zhu, S.; Hu, Y.; Ling, X.; Xu, L.; Zhang, H.; Guo, K. The emerging role of regulated cell death in ischemia and reperfusion-induced acute kidney injury: Current evidence and future perspectives. Cell Death Discov. 2024, 10, 216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Sun, T.; Yu, F.; Liu, W.; Gao, J.; Chen, J.; Zheng, H.; Liu, J.; Miao, C.; Guo, H.; et al. PAFAH2 suppresses synchronized ferroptosis to ameliorate acute kidney injury. Nat. Chem. Biol. 2024, 20, 835–846. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Peng, X.; Zhou, X.; Li, M.; Chen, G.; Shi, W.; Yu, H.; Zhang, C.; Li, Y.; Feng, Z.; et al. Small extracellular vesicles delivering lncRNA WAC-AS1 aggravate renal allograft ischemia–reperfusion injury by inducing ferroptosis propagation. Cell Death Differ. 2023, 30, 2167–2186. [Google Scholar] [CrossRef]
- Wang, X.; Kim, C.S.; Adams, B.C.; Wilkinson, R.; Hill, M.M.; Shah, A.K.; Mohamed, A.; Dutt, M.; Ng, M.S.Y.; Ungerer, J.P.J.; et al. Human proximal tubular epithelial cell-derived small extracellular vesicles mediate synchronized tubular ferroptosis in hypoxic kidney injury. Redox Biol. 2024, 70, 103042. [Google Scholar] [CrossRef]
- Linkermann, A.; Hackl, M.J.; Kunzendorf, U.; Walczak, H.; Krautwald, S.; Jevnikar, A.M. Necroptosis in Immunity and Ischemia-Reperfusion Injury. Am. J. Transplant. 2013, 13, 2797–2804. [Google Scholar] [CrossRef]
- Linkermann, A.; Bräsen, J.H.; Himmerkus, N.; Liu, S.; Huber, T.B.; Kunzendorf, U.; Krautwald, S. Rip1 (Receptor-interacting protein kinase 1) mediates necroptosis and contributes to renal ischemia/reperfusion injury. Kidney Int. 2012, 81, 751–761. [Google Scholar] [CrossRef]
- Jang, H.R.; Rabb, H. The innate immune response in ischemic acute kidney injury. Clin. Immunol. 2009, 130, 41–50. [Google Scholar] [CrossRef]
- Fan, H.; Liu, J.; Sun, J.; Feng, G.; Li, J. Advances in the study of B cells in renal ischemia-reperfusion injury. Front. Immunol. 2023, 14, 1216094. [Google Scholar] [CrossRef]
- Ysebaert, D.K.; De Greef, K.E.; De Beuf, A.; Van Rompay, A.R.; Vercauteren, S.; Persy, V.P.; De brOE, M.E. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004, 66, 491–496. [Google Scholar] [CrossRef]
- Santarsiero, D.; Aiello, S. The Complement System in Kidney Transplantation. Cells 2023, 12, 791. [Google Scholar] [CrossRef] [PubMed]
- Ko, G.J.; Jang, H.R.; Huang, Y.; Womer, K.L.; Liu, M.; Higbee, E.; Xiao, Z.; Yagita, H.; Racusen, L.; Hamad, A.R.A.; et al. Blocking Fas Ligand on Leukocytes Attenuates Kidney Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2011, 22, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Oliveira, V.; Amano, M.T.; Correa-Costa, M.; Castoldi, A.; Felizardo, R.J.F.; de Almeida, D.C.; Bassi, E.J.; Moraes-Vieira, P.M.; Hiyane, M.I.; Rodas, A.C.D.; et al. Gut Bacteria Products Prevent AKI Induced by Ischemia-Reperfusion. J. Am. Soc. Nephrol. 2015, 26, 1877–1888. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shi, J.; Xia, T.C.; Xu, R.; He, X.; Xia, Y. Preservation Solutions for Kidney Transplantation: History, Advances and Mechanisms. Cell Transpl. 2019, 28, 1472–1489. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; van Kasterop-Kutz, M.; van der Heide, J.J.H.; Squifflet, J.P.; van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef]
- Savoye, E.; Macher, M.A.; Videcoq, M.; Gatault, P.; Hazzan, M.; Abboud, I.; Thierry, A.; Bertrand, D.; Drouin, S.; Sayegh, J.; et al. Evaluation of outcomes in renal transplantation with hypothermic machine perfusion for the preservation of kidneys from expanded criteria donors. Clin. Transpl. 2019, 33, e13536. [Google Scholar] [CrossRef]
- Jiao, B.; Liu, S.; Liu, H.; Cheng, D.; Cheng, Y.; Liu, Y. Hypothermic Machine Perfusion Reduces Delayed Graft Function and Improves One-Year Graft Survival of Kidneys from Expanded Criteria Donors: A Meta-Analysis. PLoS ONE 2013, 8, e81826. [Google Scholar] [CrossRef]
- Tingle, S.J.; Figueiredo, R.S.; Moir, J.A.; Goodfellow, M.; Talbot, D.; Wilson, C.H. Machine perfusion preservation versus static cold storage for deceased donor kidney transplantation. Cochrane Database Syst. Rev. 2019, 3, CD011671. [Google Scholar] [CrossRef]
- Chatauret, N.; Coudroy, R.; Delpech, P.O.; Vandebrouck, C.; Hosni, S.; Scepi, M.; Hauet, T. Mechanistic Analysis of Nonoxygenated Hypothermic Machine Perfusion’, s Protection on Warm Ischemic Kidney Uncovers Greater eNOS Phosphorylation and Vasodilation. Am. J. Transplant. 2014, 14, 2500–2514. [Google Scholar] [CrossRef]
- Strandberg, G.; Öberg, C.M.; Blom, A.M.; Slivca, O.; Berglund, D.; Segelmark, M.; Nilsson, B.; Biglarnia, A.R. Prompt Thrombo-Inflammatory Response to Ischemia-Reperfusion Injury and Kidney Transplant Outcomes. Kidney Int. Rep. 2023, 8, 2592–2602. [Google Scholar] [CrossRef]
- Nicholson, M.L.; Hosgood, S.A. Renal Transplantation After Ex Vivo Normothermic Perfusion: The First Clinical Study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Callaghan, C.J.; Wilson, C.H.; Smith, L.; Mullings, J.; Mehew, J.; Oniscu, G.C.; Phillips, B.L.; Bates, L.; Nicholson, M.L. Normothermic machine perfusion versus static cold storage in donation after circulatory death kidney transplantation: A randomized controlled trial. Nat. Med. 2023, 29, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhong, Z.; Li, M.; Xiong, Y.; Wang, Y.; Peng, G.; Ye, Q. Hypothermic machine perfusion increases A20 expression which protects renal cells against ischemia/reperfusion injury by suppressing inflammation, apoptosis and necroptosis. Int. J. Mol. Med. 2016, 38, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Wever, K.E.; Menting, T.P.; Rovers, M.; van der Vliet, J.A.; Rongen, G.A.; Masereeuw, R.; Ritskes-Hoitinga, M.; Hooijmans, C.R.; Warlé, M. Ischemic Preconditioning in the Animal Kidney, a Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e32296. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, R.; Xue, S.; Zhu, H.; Sun, X.; Sun, X. Protective effects of three remote ischemic conditioning procedures against renal ischemic/reperfusion injury in rat kidneys: A comparative study. Irish J. Med. Sci. 2015, 184, 647–653. [Google Scholar] [CrossRef]
- Wu, J.; Feng, X.; Huang, H.; Shou, Z.; Zhang, X.; Wang, R.; Chen, Y.; Chen, J. Remote ischemic conditioning enhanced the early recovery of renal function in recipients after kidney transplantation: A randomized controlled trial. J. Surg. Res. 2014, 188, 303–308. [Google Scholar] [CrossRef]
- Bedir, S.; Ma, Y.; Antonelli, J.; Cadeddu, J.A.; Gahan, J.C. Ineffectiveness of Remote Ischemic Renal Preconditioning in a Porcine Solitary-Kidney Model. J. Endourol. 2015, 29, 590–594. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, H.; Wang, X.; Zhou, Z.; Luo, A.; Tian, Y. Remote Ischemic Preconditioning Fails to Improve Early Renal Function of Patients Undergoing Living-Donor Renal Transplantation: A Randomized Controlled Trial. Transplantation 2013, 95, e4–e6. [Google Scholar] [CrossRef]
- Eldaif, S.M.; Deneve, J.A.; Wang, N.P.; Jiang, R.; Mosunjac, M.; Mutrie, C.J.; Guyton, R.A.; Zhao, Z.Q.; Vinten-Johansen, J. Attenuation of renal ischemia–reperfusion injury by postconditioning involves adenosine receptor and protein kinase C activation. Transpl. Int. 2010, 23, 217–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Li, Y.; Liao, D.; Hu, L.; Peng, K.; Liu, H.; Ji, F.; Shan, X. Remote ischemic conditioning may improve graft function following kidney transplantation: A systematic review and meta-analysis with trial sequential analysis. BMC Anesth. 2024, 24, 168. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Wang, X.; Zhang, C.; Zheng, M.; Ma, W.; Dai, Y. Effect of Remote Ischemic Conditioning on Organ Transplantation: A Meta-Analysis of Randomized Controlled Trials. Transpl. Proc. 2024, 56, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Park, H.Y.; Lim, C.H.; Kim, H.J.; Jang, Y.; Seong, H.; Kim, Y.H.; Shin, H.J. The effect of remote ischemic conditioning on mortality after kidney transplantation: The systematic review and meta-analysis of randomized controlled trials. Syst. Rev. 2024, 13, 201. [Google Scholar] [CrossRef] [PubMed]
- Douvris, A.; Burger, D.; Rodriguez, R.A.; Clark, E.G.; Viñas, J.; Lalu, M.M.; Shorr, R.; Burns, K.D. MicroRNA in Human Acute Kidney Injury: A Systematic Review Protocol. Can. J. Kidney Health Dis. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Kaltenmeier, C.; Wang, R.; Popp, B.; Geller, D.; Tohme, S.; Yazdani, H.O. Role of Immuno-Inflammatory Signals in Liver Ischemia-Reperfusion Injury. Cells 2022, 11, 2222. [Google Scholar] [CrossRef]
- Gale, D.P.; Gross, O.; Wang, F.; de la Rosa, R.J.E.; Hall, M.; Sayer, J.A.; Appel, G.; Hariri, A.; Liu, S.; Maski, M.; et al. A Randomized Controlled Clinical Trial Testing Effects of Lademirsen on Kidney Function Decline in Adults with Alport Syndrome. Clin. J. Am. Soc. Nephrol. 2024, 19, 995–1004. [Google Scholar] [CrossRef]
- Xiong, L.; Ding, S.; Yang, T. The protective function of miR-378 in the ischemia–reperfusion injury during renal transplantation and subsequent interstitial fibrosis of the renal allograft. Int. Urol. Nephrol. 2020, 52, 1791–1800. [Google Scholar] [CrossRef]
- Gueler, F.; Shushakova, N.; Mengel, M.; Hueper, K.; Chen, R.; Liu, X.; Park, J.K.; Haller, H.; Wensvoort, G.; Rong, S. A Novel Therapy to Attenuate Acute Kidney Injury and Ischemic Allograft Damage after Allogenic Kidney Transplantation in Mice. PLoS ONE 2015, 10, e0115709. [Google Scholar] [CrossRef]
- Kelm, N.Q.; Beare, J.E.; Weber, G.J.; LeBlanc, A.J. Thrombospondin-1 mediates Drp-1 signaling following ischemia reperfusion in the aging heart. FASEB Bioadv. 2020, 2, 304–314. [Google Scholar] [CrossRef]
- Hou, Y.; Xin, Y.; Liu, S.; Li, Y.; Meng, X.; Wang, J.; Xu, Z.; Sun, T.; Yang, Y.G. A biocompatible nanoparticle-based approach to inhibiting renal ischemia reperfusion injury in mice by blocking thrombospondin-1 activity. Am. J. Transplant. 2022, 22, 2246–2253. [Google Scholar] [CrossRef]
- Chen, W.; Li, D. Reactive Oxygen Species (ROS)-Responsive Nanomedicine for Solving Ischemia-Reperfusion Injury. Front. Chem. 2020, 8, 732. [Google Scholar] [CrossRef]
- Feng, S.; Ji, J.; Li, H.; Zhang, X. H2S alleviates renal ischemia and reperfusion injury by suppressing ERS-induced autophagy. Transpl. Immunol. 2024, 83, 102006. [Google Scholar] [CrossRef] [PubMed]
- Dugbartey, G.J.; Juriasingani, S.; Richard-Mohamed, M.; Rasmussen, A.; Levine, M.; Liu, W.; Haig, A.; Whiteman, M.; Arp, J.; Luke, P.P.W.; et al. Static Cold Storage with Mitochondria-Targeted Hydrogen Sulfide Donor Improves Renal Graft Function in an Ex Vivo Porcine Model of Controlled Donation-after-Cardiac-Death Kidney Transplantation. Int. J. Mol. Sci. 2023, 24, 14017. [Google Scholar] [CrossRef] [PubMed]
- Scheid, S.; Goeller, M.; Baar, W.; Wollborn, J.; Buerkle, H.; Schlunck, G.; Lagrèze, W.; Goebel, U.; Ulbrich, F. Hydrogen Sulfide Reduces Ischemia and Reperfusion Injury in Neuronal Cells in a Dose- and Time-Dependent Manner. Int. J. Mol. Sci. 2021, 22, 10099. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Dugbartey, G.J.; Juriasingani, S.; Akbari, M.; Liu, W.; Haig, A.; McLeod, P.; Arp, J.; Sener, A. Sodium thiosulfate-supplemented UW solution protects renal grafts against prolonged cold ischemia-reperfusion injury in a murine model of syngeneic kidney transplantation. Biomed. Pharmacother. 2022, 145, 112435. [Google Scholar] [CrossRef]
- Abou Taka, M.; Dugbartey, G.J.; Richard-Mohamed, M.; McLeod, P.; Jiang, J.; Major, S.; Arp, J.; O’Neil, C.; Liu, W.; Gabril, M.; et al. Evaluating the Effects of Kidney Preservation at 10 °C with Hemopure and Sodium Thiosulfate in a Rat Model of Syngeneic Orthotopic Kidney Transplantation. Int. J. Mol. Sci. 2024, 25, 2210. [Google Scholar] [CrossRef]
- Ortas, S.; Dugbartey, G.; Richard-Mohamed, M.; Jiang, L.; Igbokwe, M.; Wang, J.; Sener, A. Supplementation of DCD Renal Graft Preservation Solutions with H2S Donor Molecule STS Against Ischemia-Reperfusion Injury in an Ex Vivo Porcine Model. Am. J. Transplant. 2025, 25, S103. [Google Scholar] [CrossRef]
| Type of Donors | Living | Circulatory Death | Brain Death |
|---|---|---|---|
| Graft survival (1 year average) | 97.8% | 79–94.3% | 84–92% |
| Graft survival (5 year average) | 86.5% | 58.8–85.9% | 73–84.5% |
| Incidence of delayed graft function | NA | 49–73% | 25–27% |
| Median ischemia time (in hours) | 1–2 | 4–46 | 3–45 |
| Median graft survival (in years) | 19.2 | 9.7 | NA |
| NA: (data) not available |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.J.; Sharma, G.K.; Parikh, R.; Jin, Z.; Darras, F.S.; Bergese, S.D. Molecular Mechanisms and Potential Therapeutic Targets of Ischemia–Reperfusion Injury in Kidney Transplantation. Curr. Issues Mol. Biol. 2025, 47, 282. https://doi.org/10.3390/cimb47040282
Huang AJ, Sharma GK, Parikh R, Jin Z, Darras FS, Bergese SD. Molecular Mechanisms and Potential Therapeutic Targets of Ischemia–Reperfusion Injury in Kidney Transplantation. Current Issues in Molecular Biology. 2025; 47(4):282. https://doi.org/10.3390/cimb47040282
Chicago/Turabian StyleHuang, Aaron J., Gaurav K. Sharma, Rohan Parikh, Zhaosheng Jin, Frank S. Darras, and Sergio D. Bergese. 2025. "Molecular Mechanisms and Potential Therapeutic Targets of Ischemia–Reperfusion Injury in Kidney Transplantation" Current Issues in Molecular Biology 47, no. 4: 282. https://doi.org/10.3390/cimb47040282
APA StyleHuang, A. J., Sharma, G. K., Parikh, R., Jin, Z., Darras, F. S., & Bergese, S. D. (2025). Molecular Mechanisms and Potential Therapeutic Targets of Ischemia–Reperfusion Injury in Kidney Transplantation. Current Issues in Molecular Biology, 47(4), 282. https://doi.org/10.3390/cimb47040282






