The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Methodology and Subjects
2.2. Sample Collection
2.3. Sample Analysis
2.4. Ethical Principles
2.5. Statistical Analysis
3. Results
3.1. Study Group Characteristics
3.2. Anti-Inflammatory Proteins Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Breastfeeding and COVID-19—Scientific Brief, 23 June 2020. Available online: https://www.who.int/news-room/commentaries/detail/breastfeeding-and-covid-19 (accessed on 1 April 2024).
- Bacalu, D.A.; Lazea, C.; Mirel, S.; Stan, O.-P.; Lotrean, L.M. Breastfeeding in the First Year of Life: The Situation in Romania in the European Context. Sustainability 2024, 16, 636. [Google Scholar] [CrossRef]
- Van de Perre, P. Transfer of antibody via mother's milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Cianga, P.; Medesan, C.; Richardson, J.A.; Ghetie, V.; Ward, E.S. Identification and function of neonatal Fc receptor in mammary gland of lactating mice. Eur J Immunol. 1999, 29, 2515–2523. [Google Scholar] [CrossRef]
- Brandtzaeg, P. The mucosal immune system and its integration with the mammary glands. J Pediat. 2010, 156, S8–S15. [Google Scholar] [CrossRef]
- Agarwal, S.; Karmaus, W.; Davis, S.; Gangur, V. Immune markers in breast milk and fetal and maternal body fluids: A systematic review of perinatal concentrations. J. Hum. Lact. 2011, 27, 171–186. [Google Scholar] [CrossRef]
- Castellote, C.; Casillas, R.; Ramírez-Santana, C.; Pérez-Cano, F.J.; Castell, M.; Moretones, M.G.; López-Sabater, M.C.; Franch, A. Premature delivery influences the immunological composition of colostrum and transitional and mature human milk. J. Nutr. 2011, 141, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Trofin, F.; Nastase, E.V.; Iancu, L.S.; Constantinescu, D.; Cianga, C.M.; Lunca, C.; Ursu, R.G.; Cianga, P.; Dorneanu, O.S. Anti-RBD IgA and IgG Response and Transmission in Breast Milk of Anti-SARS-CoV-2 Vaccinated Mothers. Pathogens 2022, 11, 286. [Google Scholar] [CrossRef]
- Zhu, J.; Dingess, K.A. The Functional Power of the Human Milk Proteome. Nutrients 2019, 11, 1834. [Google Scholar] [CrossRef]
- Jakuszko, K.; Kościelska-Kasprzak, K.; Żabińska, M.; Bartoszek, D.; Poznański, P.; Rukasz, D.; Kłak, R.; Królak-Olejnik, B.; Krajewska, M. Immune Response to Vaccination against COVID-19 in Breastfeeding Health Workers. Vaccines 2021, 9, 663. [Google Scholar] [CrossRef]
- Corona, L.; Lussu, A.; Bosco, A.; Pintus, R.; Cesare Marincola, F.; Fanos, V.; Dessì, A. Human Milk Oligosaccharides: A Comprehensive Review towards Metabolomics. Children 2021, 8, 804. [Google Scholar] [CrossRef]
- Lechosa-Muñiz, C.; Paz-Zulueta, M.; Mendez-Legaza, J.M.; Irure-Ventura, J.; Cuesta González, R.; Calvo Montes, J.; López-Hoyos, M.; Llorca, J.; Cabero-Pérez, M.J. Induction of SARS-CoV-2-Specific IgG and IgA in Serum and Milk with Different SARS-CoV-2 Vaccines in Breastfeeding Women: A Cross-Sectional Study in Northern Spain. Int. J. Environ. Res. Public Health 2021, 18, 8831. [Google Scholar] [CrossRef] [PubMed]
- Wiciński, M.; Sawicka, E.; Gębalski, J.; Kubiak, K.; Malinowski, B. Human Milk Oligosaccharides: Health Benefits, Potential Applications in Infant Formulas, and Pharmacology. Nutrients 2020, 12, 266. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhao, A.; Jiang, H.; Yan, J.; Zhong, W.; Xun, Y.; Zhang, Y. Patterns of Human Milk Oligosaccharides in Mature Milk Are Associated with Certain Gut Microbiota in Infants. Nutrients 2024, 16, 1287. [Google Scholar] [CrossRef]
- Bode, L.; Kuhn, L.; Kim, H.Y.; Hsiao, L.; Nissan, C.; Sinkala, M.; Kankasa, C.; Mwiya, M.; Thea, D.M.; Aldrovandi, G.M. Human milk oligosaccharide concentration and risk of postnatal transmission of HIV through breastfeeding. Am. J. Clin Nutr. 2012, 96, 831–839. [Google Scholar] [CrossRef]
- Ballard, O.; Morrow, A.L. Human milk composition: Nutrients and bioactive factors. Pediatr. Clin. N. Am. 2013, 60, 49–74. [Google Scholar] [CrossRef] [PubMed]
- Adamkin, D.H. Mother’s milk, feeding strategies, and lactoferrin to prevent necrotizing enterocolitis. JPEN J. Parenter Enter. Nutr. 2012, 36, 25S–29S. [Google Scholar] [CrossRef]
- Kusunoki, R.; Ishihara, S.; Aziz, M.; Oka, A.; Tada, Y.; Kinoshita, Y. Roles of milk fat globule-epidermal growth factor 8 in intestinal inflammation. Digestion 2012, 85, 103–107. [Google Scholar] [CrossRef]
- Savino, F.; Sorrenti, M.; Benetti, S.; Lupica, M.M.; Liguori, S.A.; Oggero, R. Resistin and leptin in breast milk and infants in early life. Early Hum. Dev. 2012, 88, 779–782. [Google Scholar] [CrossRef]
- Donalisio, M.; Cirrincione, S.; Rittà, M.; Lamberti, C.; Civra, A.; Francese, R.; Tonetto, P.; Sottemano, S.; Manfredi, M.; Lorenzato, A.; et al. Extracellular Vesicles in Human Preterm Colostrum Inhibit Infection by Human Cytomegalovirus In Vitro. Microorganisms 2020, 8, 1087. [Google Scholar] [CrossRef]
- Frank, N.M.; Lynch, K.F.; Uusitalo, U. The relationship between breastfeeding and reported respiratory and gastrointestinal infection rates in young children. BMC Pediatr. 2019, 19, 339. [Google Scholar] [CrossRef]
- Garofoli, F.; Civardi, E.; Pisoni, C.; Angelini, M.; Ghirardello, S. Anti-Inflammatory and Anti-Allergic Properties of Colostrum from Mothers of Full-Term and Preterm Babies: The Importance of Maternal Lactation in the First Days. Nutrients 2023, 15, 4249. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, A.; Jeleniewska, A.; Porębska, K.; Królikowska, K.; Rustecka, A.; Lipińska-Opałka, A.; Będzichowska, A.; Zdanowski, R.; Aleksandrowicz, K.; Kloc, M.; et al. Immunomodulatory Effect of Infectious Disease of a Breastfed Child on the Cellular Composition of Breast Milk. Nutrients 2023, 15, 3844. [Google Scholar] [CrossRef]
- Kim, S.Y.; Yi, D.Y. Components of human breast milk: From macronutrient to microbiome and microRNA. Clin Exp Pediatr. 2020, 63, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Quitadamo, P.A.; Comegna, L.; Cristalli, P. Anti-Infective, Anti-Inflammatory, and Immunomodulatory Properties of Breast Milk Factors for the Protection of Infants in the Pandemic From COVID-19. Front. Public Health 2021, 8, 589736. [Google Scholar] [CrossRef]
- Mazur, N.I.; Horsley, N.M.; Englund, J.A.; Nederend, M.; Magaret, A.; Kumar, A.; Jacobino, S.R.; Haan, C.A.M.d.; Khatry, S.K.; LeClerq, S.C.; et al. Breast Milk Prefusion F Immunoglobulin G as a Correlate of Protection Against Respiratory Syncytial Virus Acute Respiratory Illness. J. Infect. Dis. 2019, 219, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Wetzke, M.; Schwerk, N. Respiratory syncytial virus infections. Pneumologe 2019, 16, 232–241. [Google Scholar] [CrossRef]
- Hassiotou, F.; Hepworth, A.R.; Metzger, P.; Tat Lai, C.; Trengove, N.; Hartmann, P.E.; Filgueira, L. Maternal and infant infections stimulate a rapid leukocyte response in breastmilk. Clin. Transl. Immunol. 2020, 2, e3. [Google Scholar] [CrossRef]
- Riskin, A.; Almog, M.; Peri, R.; Halasz, K.; Srugo, I.; Kessel, A. Changes in immunomodulatory constituents of human milk in response to active infection in the nursing infant. Pediatr. Res. 2012, 71, 220–225. [Google Scholar] [CrossRef]
- Trofin, F.; Dorneanu, O.S.; Constantinescu, D.; Nastase, E.V.; Luncă, C.; Iancu, L.S.; Andrioaie, I.M.; Duhaniuc, A.; Cianga, C.M.; Pavel-Tanasa, M.; et al. Cytokines and Chemokines in Breastmilk of SARS-CoV-2 Infected or COVID-19 Vaccinated Mothers. Vaccines 2022, 10, 2001. [Google Scholar] [CrossRef]
- Chutipongtanate, S.; Kongsomros, S.; Cetinkaya, H.; Zhang, X.; Kuhnell, D.; Benefield, D.; Haffey, W.D.; Wyder, M.A.; Kwatra, G.; Conrey, S.C.; et al. Prenatal SARS-CoV-2 Infection Alters Human Milk-Derived Extracellular Vesicles. Cells 2025, 14, 284. [Google Scholar] [CrossRef]
- Groß, R.; Conzelmann, C.; Müller, J.A.; Stenger, S.; Steinhart, K.; Kirchhoff, F.; Münch, J. Detection of SARS-CoV-2 in human breastmilk. Lancet 2020, 395, 1757–1758. [Google Scholar] [CrossRef] [PubMed]
- Chambers, C.; Krogstad, P.; Bertrand, K.; Contreras, D.; Tobin, N.H.; Bode, L.; Aldrovandi, G. Evaluation for SARS-CoV-2 in Breast Milk From 18 Infected Women. JAMA 2020, 324, 1347–1348. [Google Scholar] [CrossRef] [PubMed]
- Almaghrabi, R.S.; Omrani, A.S. Middle East respiratory syndrome coronavirus (MERS-CoV) infection. Brit. J. Hosp. Med. 2017, 78, 23–26. [Google Scholar] [CrossRef][Green Version]
- Chen, H.; Guo, J.; Wang, C.; Luo, F.; Yu, X.; Zhang, W.; Li, J.; Zhao, D.; Xu, D.; Gong, Q.; et al. Clinical characteristics and intrauterine vertical transmission potential of COVID-19 infection in nine pregnant women: A retrospective review of medical records. Lancet 2020, 395, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Paquette, S.G.; Banner, D.; Huang, S.S.; Almansa, R.; Leon, A.; Xu, L.; Bartoszko, J.; Kelvin, D.J.; Kelvin, A.A. Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses. PLoS Pathog. 2015, 11, e1005173. [Google Scholar] [CrossRef]
- McCall, S.A.; Lichy, J.H.; Bijwaard, K.E.; Aguilera, N.S.; Chu, W.S.; Taubenberger, J.K. Epstein-Barr virus detection in ductal carcinoma of the breast. J. Natl. Cancer Inst. 2001, 93, 148–150. [Google Scholar] [CrossRef]
- Turck, D.; Comité de nutrition de la Société française de pédiatrie. Allaitement maternel: Les bénéfices pour la santé de l'enfant et de sa mère [Breast feeding: Health benefits for child and mother]. Arch. De Pediatr. Organe Off. De La Soc. Fr. De Pediatr. 2005, 12 (Suppl. 3), S145–S165. [Google Scholar] [CrossRef]
- Lazar, K.; Kussmann, T.; Pawelec, G.; Pöschel, S.; Goelz, R.; Hamprecht, K.; Wistuba-Hamprecht, K. Immunomonitoring of Human Breast Milk Cells During HCMV-Reactivation. Front. Immunol. 2021, 12, 723010. [Google Scholar] [CrossRef]
- Costa, S.; Posteraro, B.; Marchetti, S.; Tamburrini, E.; Carducci, B.; Lanzone, A.; Valentini, P.; Buonsenso, D.; Sanguinetti, M.; Vento, G.; et al. Excretion of SARS-CoV-2 in human breast milk. Clin. Microbiol. Infect. 2020, 26, 1430–1432. [Google Scholar] [CrossRef]
- Pang, Z.; Hu, R.; Tian, L.; Lou, F.; Chen, Y.; Wang, S.; He, S.; Zhu, S.; An, X.; Song, L.; et al. Overview of Breastfeeding Under COVID-19 Pandemic. Front. Immunol. 2022, 13, 896068. [Google Scholar] [CrossRef]
- Vassilopoulou, E.; Feketea, G.; Koumbi, L.; Mesiari, C.; Berghea, E.C.; Konstantinou, G.N. Breastfeeding and COVID-19: From Nutrition to Immunity. Front. Immunol. 2021, 12, 661806. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Che, S.; Zhang, J.; Wang, X.; Tang, Y.; Wang, J.; Huang, L.; Wang, C.; Zhang, H.; Baskota, M.; et al. Breastfeeding of infants born to mothers with COVID-19: A rapid review. Ann Transl Med. 2020, 8, 618. [Google Scholar] [CrossRef]
- Cheema, R.; Partridge, E.; Kair, L.R.; Kuhn-Riordon, K.M.; Silva, A.I.; Bettinelli, M.E.; Chantry, C.J.; Underwood, M.A.; Lakshmin-rusimha, S.; Blumberg, D. Protecting Breastfeeding during the COVID-19 Pandemic. Am. J. Perinatol. 2023, 40, 260–266. [Google Scholar] [CrossRef]
- Tomori, C.; Gribble, K.; Palmquist, A.E.L.; Ververs, M.T.; Gross, M.S. When separation is not the answer: Breastfeeding mothers and infants affected by COVID-19. Matern Child Nutr. 2020, 16, e13033. [Google Scholar] [CrossRef] [PubMed]
- Morniroli, D.; Consales, A.; Crippa, B.L.; Vizzari, G.; Ceroni, F.; Cerasani, J.; Colombo, L.; Mosca, F.; Giannì, M.L. The Antiviral Properties of Human Milk: A Multitude of Defence Tools from Mother Nature. Nutrients 2021, 13, 694. [Google Scholar] [CrossRef]
- Vorbach, C.; Capecchi, M.R.; Penninger, J.M. Evolution of the mammary gland from the innate immune system? BioEssays 2006, 28, 606–616. [Google Scholar] [CrossRef] [PubMed]
- Henrick, B.M.; Yao, X.-D.; Nasser, L.; Roozrogousheh, A.; Rosenthal, K.L. Breastfeeding Behaviors and the Innate Immune System of Human Milk: Working Together to Protect Infants against Inflammation, HIV-1, and Other Infections. Front. Immunol. 2017, 8, 1631. [Google Scholar] [CrossRef]
- Iliff, P.J.; Piwoz, E.G.; Tavengwa, N.V.; Zunguza, C.D.; Marinda, E.T.; Nathoo, K.J.; Moulton, L.H.; Ward, B.J.; Humphrey, J.H. Early exclusive breastfeeding reduces the risk of postnatal HIV-1 transmission and increases HIV-free survival. AIDS 2005, 19, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Donalisio, M.; Rittà, M.; Tonetto, P.; Civra, A.; Coscia, A.; Giribaldi, M.; Cavallarin, L.; Moro, G.E.; Bertino, E.; Lembo, D. Anti-Cytomegalovirus Activity in Human Milk and Colostrum From Mothers of Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 654–659. [Google Scholar] [CrossRef]
- Kell, D.B.; Heyden, E.L.; Pretorius, E. The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria. Front. Immunol. 2020, 11, 1221. [Google Scholar] [CrossRef]
- Campione, E.; Cosio, T.; Rosa, L.; Lanna, C.; Di Girolamo, S.; Gaziano, R.; Valenti, P.; Bianchi, L. Lactoferrin as Protective Natural Barrier of Respiratory and Intestinal Mucosa against Coronavirus Infection and Inflammation. Int. J. Mol. Sci. 2020, 21, 4903. [Google Scholar] [CrossRef] [PubMed]
- PrabhuDas, M.; Adkins, B.; Gans, H.; King, C.; Levy, O.; Ramilo, O.; Siegrist, C.A. Challenges in infant immunity: Implications for responses to infection and vaccines. Nat. Immunol. 2011, 12, 189–194. [Google Scholar] [CrossRef] [PubMed]
- McGuire, S. FAO, IFAD.and WFP. The State of Food Insecurity in the World 2015: Meeting the 2015 International Hunger Targets: Taking Stock of Uneven Progress. Adv Nutr. 2015, 6, 623–624. [Google Scholar] [CrossRef] [PubMed]
- Collier, A.Y.; McMahan, K.; Yu, J.; Tostanoski, L.H.; Aguayo, R.; Ansel, J.; Chandrashekar, A.; Patel, S.; Apraku Bondzie, E.; Sellers, D.; et al. Immunogenicity of COVID-19 mRNA Vaccines in Pregnant and Lactating Women. JAMA 2021, 325, 2370–2380. [Google Scholar] [CrossRef]
- Newburg, D.S.; Peterson, J.A.; Ruiz-Palacios, G.M.; Matson, D.O.; Morrow, A.L.; Shults, J.; Guerrero, M.L.; Chaturvedi, P.; Newburg, S.O.; Scallan, C.D.; et al. Role of human-milk lactadherin in protection against symptomatic rotavirus infection. Lancet 1998, 35, 1160–1164. [Google Scholar] [CrossRef]
- Trofin, F.; Nastase, E.-V.; Vâță, A.; Iancu, L.S.; Luncă, C.; Buzilă, E.R.; Vlad, M.A.; Dorneanu, O.S. The Immune, Inflammatory and Hematological Response in COVID-19 Patients, According to the Severity of the Disease. Microorganisms 2023, 11, 319. [Google Scholar] [CrossRef]
- Geddes, D.T.; Gridneva, Z.; Perrella, S.L.; Mitoulas, L.R.; Kent, J.C.; Stinson, L.F.; Lai, C.T.; Sakalidis, V.; Twigger, A.-J.; Hartmann, P.E. 25 Years of Research in Human Lactation: From Discovery to Translation. Nutrients 2021, 13, 3071. [Google Scholar] [CrossRef]
- Mangan, R.J.; Stamper, L.; Ohashi, T.; Eudailey, J.A.; Go, E.P.; Jaeger, F.H.; Itell, H.L.; Watts, B.E.; Fouda, G.G.; Erickson, H.P.; et al. Determinants of Tenascin-C and HIV-1 envelope binding and neutralization. Mucosal Immunol. 2019, 12, 1004–1012. [Google Scholar] [CrossRef]
- Fouda, G.G.; Jaeger, F.H.; Amos, J.D.; Ho, C.; Kunz, E.L.; Anasti, K.; Stamper, L.W.; Liebl, B.E.; Barbas, K.H.; Ohashi, T.; et al. Tenascin-C is an innate broad-spectrum, HIV-1-neutralizing protein in breast milk. Proc. Natl. Acad. Sci. USA 2013, 110, 18220–18225. [Google Scholar] [CrossRef]
- Okamura, H.; Tsutsi, H.; Komatsu, T.; Yutsudo, M.; Hakura, A.; Tanimoto, T.; Torigoe, K.; Okura, T.; Nukada, Y.; Hattori, K. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature 1995, 378, 88–91. [Google Scholar] [CrossRef]
- Walch, M.; Dotiwala, F.; Mulik, S.; Thiery, J.; Kirchhausen, T.; Clayberger, C.; Krensky, A.M.; Martinvalet, D.; Lieberman, J. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell 2014, 157, 1309–1323. [Google Scholar] [CrossRef]
- Ghose, P.; Ali, A.Q.; Fang, R.; Forbes, D.; Ballard, B.; Ismail, N. The interaction between IL-18 and IL-18 receptor limits the magnitude of protective immunity and enhances pathogenic responses following infection with intracellular bacteria. J. Immunol. 2011, 187, 1333–1346. [Google Scholar] [CrossRef] [PubMed]
- Twigger, A.-J.; Küffer, G.K.; Geddes, D.T.; Filgueria, L. Expression of Granulisyn, Perforin and Granzymes in Human Milk over Lactation and in the Case of Maternal Infection. Nutrients 2018, 10, 1230. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.G.; Da Silva, C.A.; Lee, J.Y.; Hartl, D.; Elias, J.A. Chitin regulation of immune responses: An old molecule with new roles. Curr. Opin. Immunol. 2008, 20, 684–689. [Google Scholar] [CrossRef]
- Zhu, Z.; Zheng, T.; Homer, R.J.; Kim, Y.K.; Chen, N.Y.; Cohn, L.; Hamid, Q.; Elias, J.A. Acidic mammalian chitinase in asthmatic Th2 inflammation and IL-13 pathway activation. Science 2004, 304, 1678–1682. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Huang, M.; Jiang, L. Potential Roles and Future Perspectives of Chitinase 3-like 1 in Macrophage Polarization and the Development of Diseases. Int. J. Mol. Sci. 2023, 24, 16149. [Google Scholar] [CrossRef]
- Breyne, K.; Steenbrugge, J.; Demeyere, K.; Lee, C.G.; Elias, J.A.; Petzl, W.; Smith, D.G.E.; Germon, P.; Meyer, E. Immunomodulation of Host Chitinase 3-Like 1 During a Mammary Pathogenic Escherichia coli Infection. Front. Immunol. 2018, 9, 1143. [Google Scholar] [CrossRef]
- Couture, F.; Kwiatkowska, A.; Dory, Y.L.; Day, R. Therapeutic Uses of Furin and Its Inhibitors: A Patent Review. Expert Opin. Ther. Pat. 2015, 25, 379–396. [Google Scholar] [CrossRef]
- Izaguirre, G. The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses 2019, 11, 837. [Google Scholar] [CrossRef]
- Devi, K.P.; Pourkarim, M.R.; Thijssen, M.; Sureda, A.; Khayatkashani, M.; Cismaru, C.A.; Neagoe, I.B.; Habtemariam, S.; Razmjouei, S.; Khayat Kashani, H.R. A Perspective on the Applications of Furin Inhibitors for the Treatment of SARS-CoV-2. Pharmacol. Rep. 2022, 74, 425–430. [Google Scholar] [CrossRef]
- Essalmani, R.; Jain, J.; Susan-Resiga, D.; Andréo, U.; Evagelidis, A.; Derbali, R.M.; Huynh, D.N.; Dallaire, F.; Laporte, M.; Delpal, A.; et al. Distinctive Roles of Furin and TMPRSS2 in SARS-CoV-2 Infectivity. J. Virol. 2022, 96, e0012822. [Google Scholar] [CrossRef] [PubMed]
- Gordon, V.M.; Klimpel, K.R.; Arora, N.; Henderson, M.A.; Leppla, S.H. Proteolytic Activation of Bacterial Toxins by Eukaryotic Cells Is Performed by Furin and by Additional Cellular Proteases. Infect. Immun. 1995, 63, 82–87. [Google Scholar] [CrossRef]
- Remacle, A.G.; Gawlik, K.; Golubkov, V.S.; Cadwell, G.W.; Liddington, R.C.; Cieplak, P.; Millis, S.Z.; Desjardins, R.; Routhier, S.; Yuan, X.W.; et al. Selective and Potent Furin Inhibitors Protect Cells from Anthrax without Significant Toxicity. Int. J. Biochem. Cell Biol. 2010, 42, 987–995. [Google Scholar] [CrossRef]
- Garred, Ø.; van Deurs, B.; Sandvig, K. Furin-Induced Cleavage and Activation of Shiga Toxin. J. Biol. Chem. 1995, 270, 10817–10821. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, T.; Fukui, A.; Kashimoto, T.; Nagao, K.; Oka, K.; Miyake, M.; Horiguchi, Y. Bordetella Dermonecrotic Toxin Undergoes Proteolytic Processing to Be Translocated from a Dynamin-Related Endosome into the Cytoplasm in an Acidification-Independent Manner. J. Biol. Chem. 2004, 279, 2866–2872. [Google Scholar] [CrossRef]
- Ivachtchenko, A.V.; Khvat, A.V.; Shkil, D.O. Development and Prospects of Furin Inhibitors for Therapeutic Applications. Int. J. Mol. Sci. 2024, 25, 9199. [Google Scholar] [CrossRef] [PubMed]
- Chou, J.; Platt, C.D.; Habiballah, S.; Nguyen, A.A.; Elkins, M.; Weeks, S.; Peters, Z.; Day-Lewis, M.; Novak, T.; Armant, M.; et al. Mechanisms underlying genetic susceptibility to multisystem inflammatory syndrome in children (MIS-C). J. Allergy Clin. Immunol. 2021, 148, 732–738. [Google Scholar] [CrossRef]
- Chou, J.; Thomas, P.G.; Randolph, A.G. Immunology of SARS-CoV-2 infection in children. Nat. Immunol. 2022, 23, 177–185. [Google Scholar] [CrossRef]
- Jenkins, T.C.; McGuire, M.A. Major advances in nutrition: Impact on milk composition. J. Dairy Sci. 2006, 89, 1302–1310. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Bidirectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon? Immunol. Today 1993, 14, 353–356. [Google Scholar] [CrossRef]
- Aagaard-Tillery, K.M.; Silver, R.; Dalton, J. Immunology of normal pregnancy. Semin. Fetal Neonatal Med. 2006, 11, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Larsen, B.; Hwang, J. Hormonal regulation of uterine chemokines and immune cell recruitment during pregnancy. Cell. Mol. Immunol. 2011, 8, 499–510. [Google Scholar]
- McGuire, M.K.; McGuire, M.A. Human milk: Mother nature’s prototypical probiotic food? Adv Nutr. 2015, 6, 112–123. [Google Scholar] [CrossRef]
- Hassiotou, F.; Geddes, D.; Hartmann, P. Cells in human milk: State of the science. J. Hum. Lact. 2013, 29, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, M.C.; McManaman, J.L.; Phang, T.; Russell, T.; Kominsky, D.J.; Serkova, N.J.; Stein, T.; Anderson, S.M.; Neville, M.C. Metabolic regulation in the lactating mammary gland: A lipid synthesizing machine. Physiol Genom. 2007, 28, 323–336. [Google Scholar] [CrossRef]
- Victora, C.G.; Bahl, R.; Barros, A.J.D.; França, G.V.; Horton, S.; Krasevec, J.; Murch, S.; Sankar, M.J.; Walker, N.; Rollins, N.C.; et al. Breastfeeding in the 21st century: Epidemiology, mechanisms, and lifelong effect. Lancet 2016, 387, 475–490. [Google Scholar] [CrossRef]
- Geddes, D.T.; Prescott, S.L. Developmental origins of health and disease: The role of human milk in preventing disease in the 21st century. J. Hum. Lact. 2013, 29, 123–127. [Google Scholar] [CrossRef]



| Components | Function | References | |
|---|---|---|---|
| Immunoglobulins | IgA/sIgA | Antimicrobial | [3,4,5,6,7,8,9,10] |
| IgG | Antimicrobial | [4,6,8,9,10,11,12] | |
| IgM | Antimicrobial | [3,6,9] | |
| Oligosaccharides and glycan | HMOs | Prebiotics | [9,11,13,14,15] |
| Mucins | MUC1 | Antimicrobial | [16] |
| MUC4 | Antimicrobial | [16] | |
| Cytokine inhibitors | TNF-R I and II | Anti-inflammatory | [16] |
| Antimicrobial factors | Lactoferrin | Antimicrobial, anti-oxidant, anti-inflammatory | [16,17] |
| Lactadherin | Antimicrobial, anti-oxidant, anti-inflammatory | [16,18] | |
| Metabolic factors | Adiponectin | Anti-inflammatory | [19] |
| Leptin | Regulation of energy conversion and infant weight; Regulation of appetite | [19] | |
| Ghrelin | Regulation of energy conversion; Control of infant weight; Role in maintaining harmonious development. | [19] | |
| Inclusion criteria | Breastfeeding mothers |
| Immunization with two doses of mRNA vaccine after birth OR | |
| Onset of SARS-CoV-2 infection postbirth in the last month prior to study inclusion OR | |
| Positive result for RT-PCR/antigen postbirth in the last month prior to study inclusion OR | |
| Absence of anti-SARS-CoV-2 IgG antibodies in breast milk | |
| Apparent good health at the time of milk sample collection | |
| At any time from the start of breastfeeding | |
| Direct nursing or expressed breast milk | |
| Providing and signing informed consent | |
| Exclusion criteria | Participants without verified vaccination records |
| Participants without records of RT-PCR/antigen results | |
| Unrelated acute illnesses (e.g., mastitis) | |
| Clear signs of infection or inflammatory diseases | |
| Insufficient or contaminated milk samples | |
| Samples collected outside the designated time frame | |
| Improper methods of sampling | |
| Vaccination or infection at inappropriate time | |
| Incomplete vaccination scheme | |
| COVID-19 vaccination or infection during pregnacy |
| Study Group | Entire Group | Infected Mothers | Immunized Mothers | Control Group | |||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Mother’s Age (Years) | Child’s Age (Months) | Mother’s Age (Years) | Child’s Age (Months) | Mother’s Age (Years) | Child’s Age (Months) | Mother’s Age (Years) | Child’s Age (Months) | |
| Median | 32.50 | 11.50 | 32.00 | 12.00 | 33.00 | 12.00 | 32.50 | 9.00 | |
| Std. Deviation | 2.58 | 9.06 | 2.90 | 8.68 | 2.44 | 10.21 | 2.13 | 6.62 | |
| Variance | 6.66 | 82.12 | 8.41 | 75.28 | 5.98 | 104.27 | 4.54 | 43.78 | |
| Range | 10 | 33 | 10 | 31 | 9 | 33 | 7 | 22 | |
| Percentiles | 25 | 31.00 | 5.25 | 31.00 | 6.25 | 31.00 | 4.00 | 30.00 | 4.50 |
| 50 | 32.50 | 11.50 | 32.00 | 12.00 | 33.00 | 12.00 | 32.50 | 9.00 | |
| 75 | 35.00 | 19.75 | 36.00 | 20.75 | 35.00 | 20.50 | 33.25 | 13.75 | |
| IQR | 4.00 | 14.50 | 5.00 | 14.25 | 4.00 | 16.50 | 3.25 | 9.25 | |
| p | 0.002 | 0.039 | 0.002 | 0.004 | 0.001 | 0.000 | 0.001 | 0.000 | |
| Study Group | Entire Group Nb (Percent) | Vaccinated Mothers Nb (Percent) | Infected Mothers Nb (Percent) | Control Group Nb (Percent) | |
|---|---|---|---|---|---|
| Births number | Primiparous | 27 (45%) | 17 (65.4%) | 8 (33.3%) | 2 (20%) |
| Multiparous | 33 (55%) | 9 (34.6%) | 16 (66.7%) | 8 (80%) | |
| Birth type | Natural birth | 21 (35%) | 7 (26.9%) | 8 (33.3%) | 4 (40%) |
| Cesarean section | 39 (65%) | 19 (73.1%) | 16 (66.7%) | 6 (60%) | |
| Vaccine type | Pfizer-BioNTech vaccine | 23 (88.5%) | 16 (66.7%) | ||
| Moderna vaccine | 3 (11.5%) | ||||
| No vaccine | 8 (33.3%) | 10 (100%) | |||
| Post-vaccination adverse reactions | Mild | 17 (65.4%) | |||
| Moderate | 4 (15.4%) | ||||
| None | 5 (19.2%) | ||||
| Severity form | Mild | 13 (54.2%) | |||
| Moderate | 9 (37.5%) | ||||
| Asymptomatic | 2 (8.3%) | ||||
| Statistical Parameters | C3-1 1 (pg/mL) | C3-1 2 (pg/mL) | F 1 (pg/mL) | F 2 (pg/mL) | GB 1 (pg/mL) | GB 2 (pg/mL) | LF 1 (pg/mL) | LF 2 (pg/mL) | LD 1 (pg/mL) | LD 2 (pg/mL) | TC 1 (pg/mL) | TC 2 (pg/mL) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median | 115,790 | 116,316 | 5393 | 5169 | 26.82 | 21.99 | 16,815 | 17,464 | 11,993 | 11,743 | 3867 | 3584 | |
| Range | 3,319,616 | 687,667 | 79,688 | 76,589 | 200 | 309 | 4771 | 4244 | 7292 | 6995 | 123,726 | 123,818 | |
| P | 25 | 96,805 | 84,984 | 3674 | 3547 | 18.20 | 16.95 | 16,535 | 16,623 | 11,116 | 10,527 | 2139 | 2342 |
| 50 | 115,790 | 116,316 | 5393 | 5169 | 26.82 | 21.99 | 16,815 | 17,464 | 11,993 | 11,743 | 3867 | 3584 | |
| 75 | 243,135 | 231,832 | 8852 | 9936 | 48.84 | 37.80 | 17,104 | 19,805 | 13,415 | 12,710 | 10,223 | 8050 | |
| IQR | 146,329 | 146,847 | 5177 | 6388 | 30 | 20 | 568 | 3182 | 2299 | 2184 | 8084 | 5708 | |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.02 | 0.02 | 0.000 | 0.000 | |
| Study Groups | Statistical Parameters | C3-1 1 | C3-1 2 | F 1 | F 2 | GB 1 | GB 2 | LF 1 | LF 2 | LD 1 | LD 2 | TC 1 | TC 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | Median | 146,495 | 128,216 | 6618 | 5055 | 26 | 23 | 16,679 | 19,779 | 11,442 | 10,858 | 3688 | 3810 | |
| P | 25 | 91,505 | 69,433 | 3393 | 3410 | 18 | 17 | 16,388 | 19,286 | 10,345 | 10,253 | 2404 | 2624 | |
| 50 | 146,495 | 128,216 | 6618 | 5055 | 26 | 23 | 16,679 | 19,779 | 11,442 | 10,858 | 3688 | 3810 | ||
| 75 | 299,741 | 253,665 | 10,559 | 10,099 | 42 | 40 | 16,823 | 19,954 | 12,729 | 11,866 | 7778 | 6871 | ||
| G2 | Median | 115,790 | 115,790 | 5142 | 5687 | 23 | 22 | 17,014 | 16,651 | 13,299 | 12,515 | 3749 | 3397 | |
| P | 25 | 97,936 | 115,790 | 3812 | 3712 | 16 | 18 | 16,774 | 16,346 | 11,727 | 11,651 | 1751 | 1900 | |
| 50 | 115,790 | 115,790 | 5142 | 5687 | 23 | 22 | 17,014 | 16,651 | 13,299 | 12,515 | 3749 | 3397 | ||
| 75 | 217,500 | 214,867 | 8842 | 14,411 | 47 | 45 | 17,247 | 16,799 | 14,344 | 13,296 | 7548 | 10,020 | ||
| G3 | Median | 115,790 | 4581 | 43 | 16,977 | 11,717 | 8766 | |||||||
| P | 25 | 74,457 | 3414 | 20 | 16,584 | 10,950 | 1248 | |||||||
| 50 | 115,790 | 4581 | 43 | 16,977 | 11,717 | 8766 | ||||||||
| 75 | 259,512 | 6396 | 88 | 17,321 | 13,562 | 21,537 | ||||||||
| Parameters | C3-1 1 | C3-1 2 | F 1 | F 2 | GB 1 | GB 2 | LF 1 | LF 2 | LD 1 | LD 2 | TC 1 | TC 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IL-6 | ρ | 0.206 | 0.175 | 0.319 | 0.314 | 0.442 | 0.476 | 0.087 | −0.182 | −0.031 | 0.021 | 0.361 | 0.320 |
| p | 0.120 | 0.238 | 0.015 | 0.032 | 0.001 | 0.001 | 0.515 | 0.222 | 0.817 | 0.890 | 0.005 | 0.028 | |
| Antibodies | ρ | −0.036 | −0.168 | 0.195 | 0.098 | −0.052 | 0.124 | ||||||
| p | 0.783 | 0.200 | 0.135 | 0.456 | 0.695 | 0.345 | |||||||
| Affiliation with the study subgroup | ρ | −0.116 | 0.076 | −0.171 | 0.098 | 0.109 | 0.021 | 0.337 | 0.726 | 0.264 | 0.475 | 0.036 | −0.098 |
| p | 0.378 | 0.612 | 0.193 | 0.513 | 0.407 | 0.891 | 0.008 | 0.000 | 0.041 | 0.001 | 0.783 | 0.513 | |
| Child’s age | ρ | 0.227 | 0.318 | 0.387 | 0.447 | −0.208 | −0.090 | 0.183 | 0.161 | 0.263 | 0.166 | −0.439 | −0.344 |
| p | 0.081 | 0.029 | 0.002 | 0.002 | 0.110 | 0.545 | 0.161 | 0.278 | 0.042 | 0.265 | 0.000 | 0.018 | |
| Parity | ρ | −0.190 | −0.399 | −0.320 | −0.224 | −0.053 | 0.067 | 0.063 | −0.171 | −0.067 | 0.009 | 0.052 | 0.064 |
| p | 0.147 | 0.005 | 0.013 | 0.129 | 0.686 | 0.652 | 0.633 | 0.250 | 0.612 | 0.950 | 0.692 | 0.668 | |
| Mother’s age | ρ | −0.053 | −0.218 | −0.092 | −0.095 | −0.065 | 0.038 | 0.052 | 0.067 | −0.097 | −0.127 | −0.003 | 0.093 |
| p | 0.686 | 0.141 | 0.486 | 0.526 | 0.621 | 0.799 | 0.696 | 0.655 | 0.461 | 0.394 | 0.983 | 0.534 | |
| Birth | ρ | 0.040 | 0.138 | 0.013 | −0.015 | 0.007 | 0.145 | 0.017 | −0.062 | −0.017 | 0.077 | 0.021 | 0.151 |
| p | 0.764 | 0.354 | 0.921 | 0.920 | 0.957 | 0.332 | 0.897 | 0.678 | 0.897 | 0.605 | 0.872 | 0.310 | |
| Vaccine | ρ | 0.006 | 0.019 | −0.151 | 0.047 | 0.116 | 0.115 | 0.233 | −0.381 | 0.015 | 0.118 | 0.042 | −0.047 |
| p | 0.962 | 0.900 | 0.249 | 0.754 | 0.379 | 0.441 | 0.073 | 0.008 | 0.911 | 0.431 | 0.750 | 0.754 | |
| Symptoms | ρ | −0.061 | 0.030 | −0.090 | −0.046 | −0.043 | −0.004 | −0.010 | −0.257 | 0.137 | 0.288 | 0.047 | −0.127 |
| p | 0.675 | 0.842 | 0.536 | 0.759 | 0.764 | 0.976 | 0.943 | 0.081 | 0.344 | 0.049 | 0.748 | 0.395 | |
| p | Mean Difference | |
|---|---|---|
| Chitinase 3-like 1-1 | 0.025 | 134,661.53 |
| Chitinase 3-like 1-2 | 0.003 | 76,933.47 |
| Furin-1 | 0.000 | −18,226.66 |
| Furin-2 | 0.000 | −15,196.28 |
| Granzyme B-1 | 0.000 | −4328.40 |
| Granzyme B-2 | 0.000 | −4326.83 |
| Standard Deviation | p | |
|---|---|---|
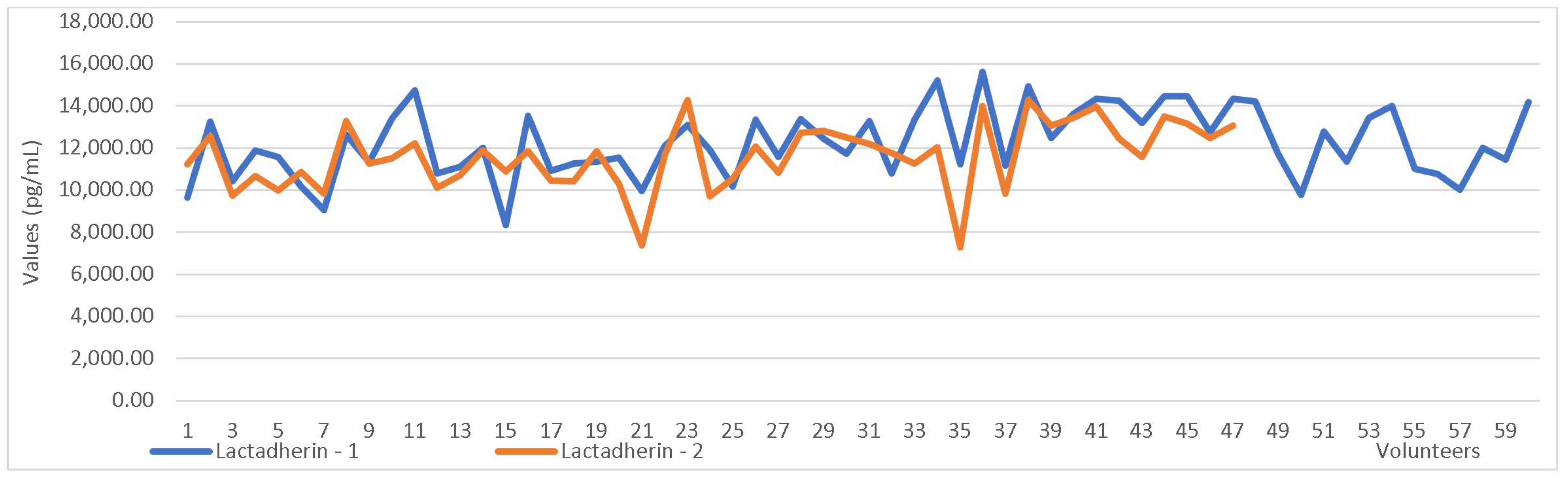
| Lactoferrin-1 vs. Lactoferrin-2 | 1742.30 | 0.000 |
| Lactadherin 1 vs. Lactadherin-2 | 1262.30 | 0.000 |
| Grouping Variable | Dependent Variables | p |
|---|---|---|
| Mother’s parity | Furin | 0.021 |
| Chitinase 3-like 1 | 0.017 | |
| Delivery mode | Chitinase 3-like 1 | 0.027 |
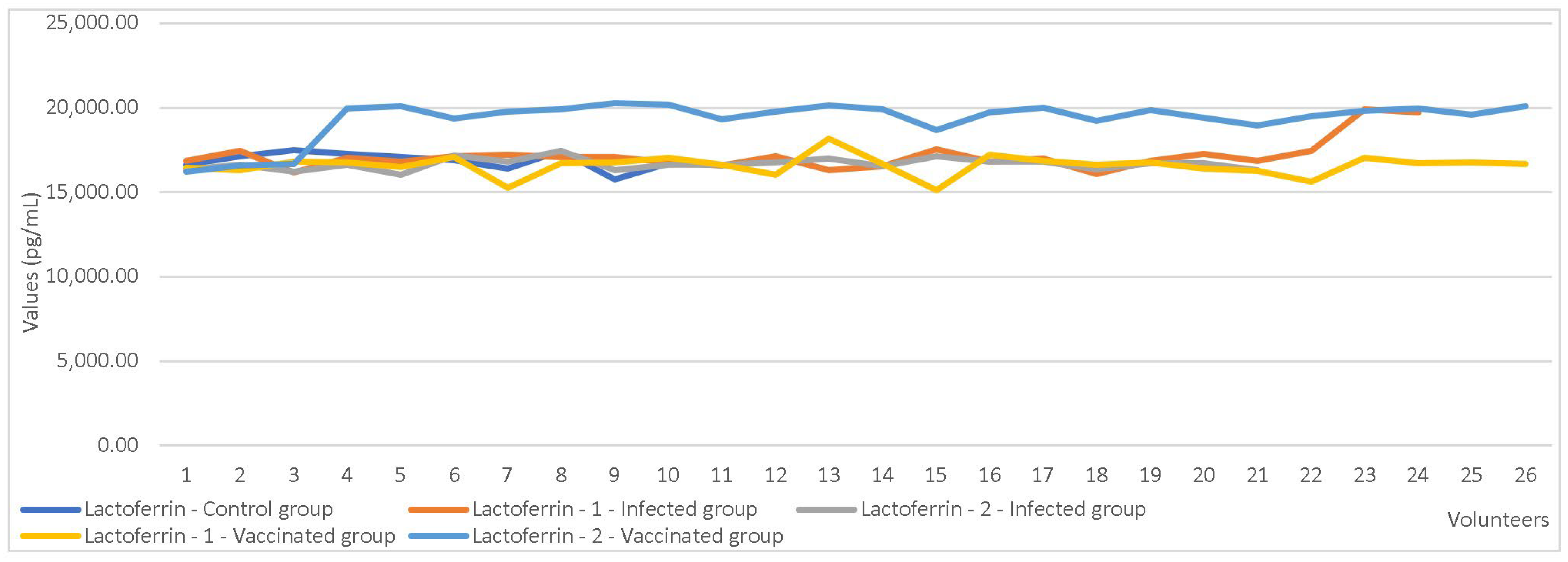
| Immunization status | Lactoferrin | 0.000 |
| Vaccinated mother group vs. infected mother group | Lactoferrin | 0.000 |
| Lactadherin | 0.001 |
| Groups | Action | Parameters | Statistical Test | p |
|---|---|---|---|---|
| Vaccinated mothers vs. infected mothers vs. control group | Values decrease from vaccinated to control group | Lactoferrin | Spearman’s correlation | <0.05 |
| Vaccinated mothers vs. infected mothers vs. control group | Values decrease from vaccinated to control group | Lactadherin | Spearman’s correlation | <0.05 |
| Primiparous vs. multiparous mothers | Depending on mother’s parity the parameters decrease | Furin Chitinase 3-like 1 | Independent sample t-test | <0.05 |
| Natural births vs. C-section | Depending on delivery mode the parameters increase | Chitinase 3-like 1 | Independent sample t-test | <0.05 |
| Vaccinated mothers vs. non-vaccinated mothers | Depending on immunization status the parameters increase | Lactoferrin | Independent sample t-test | <0.05 |
| Vaccinated mother group vs. infected mothers groups | Depending on group assignment the parameters increase | Lactoferrin Lactadherin | Independent sample t-test | <0.05 |
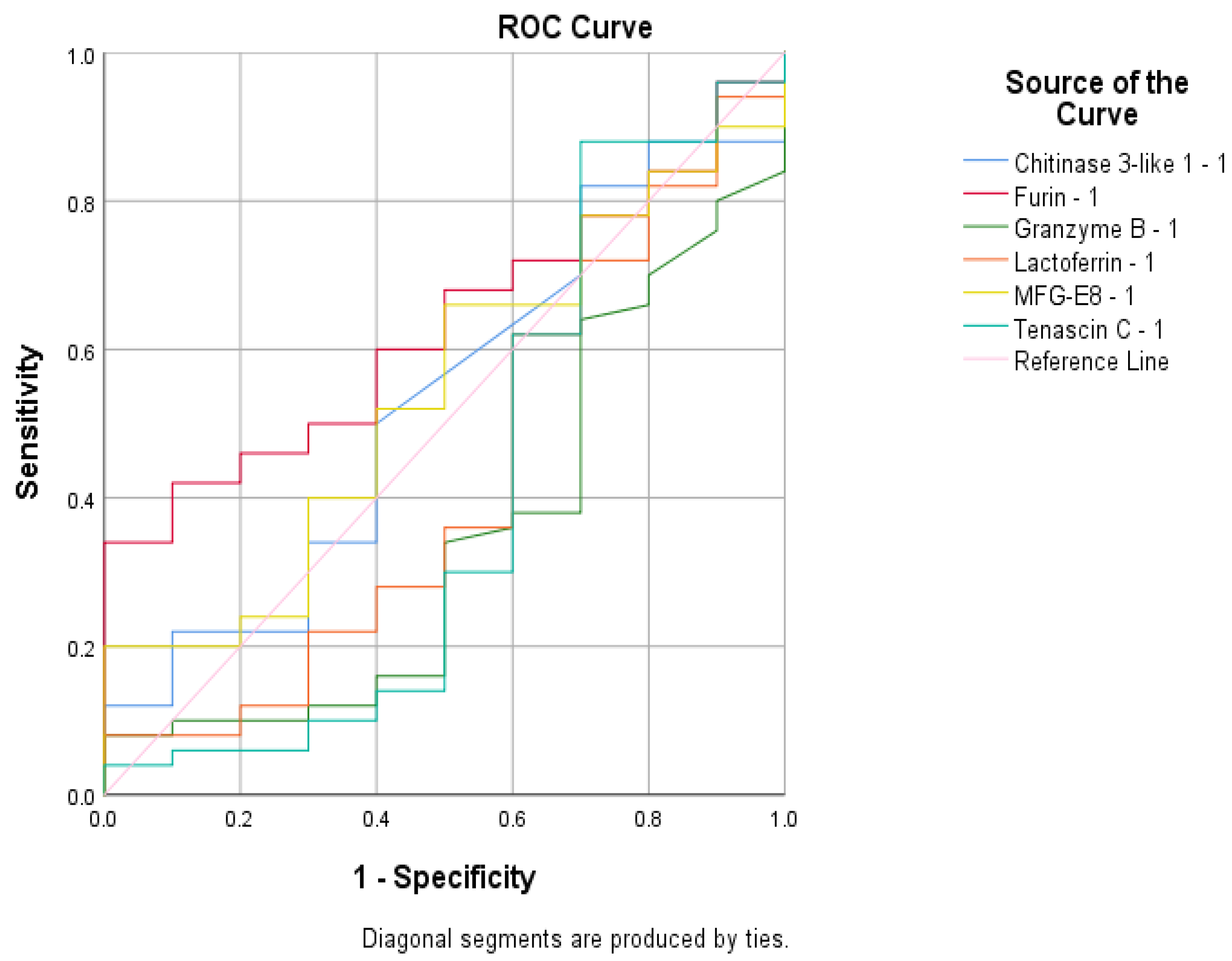
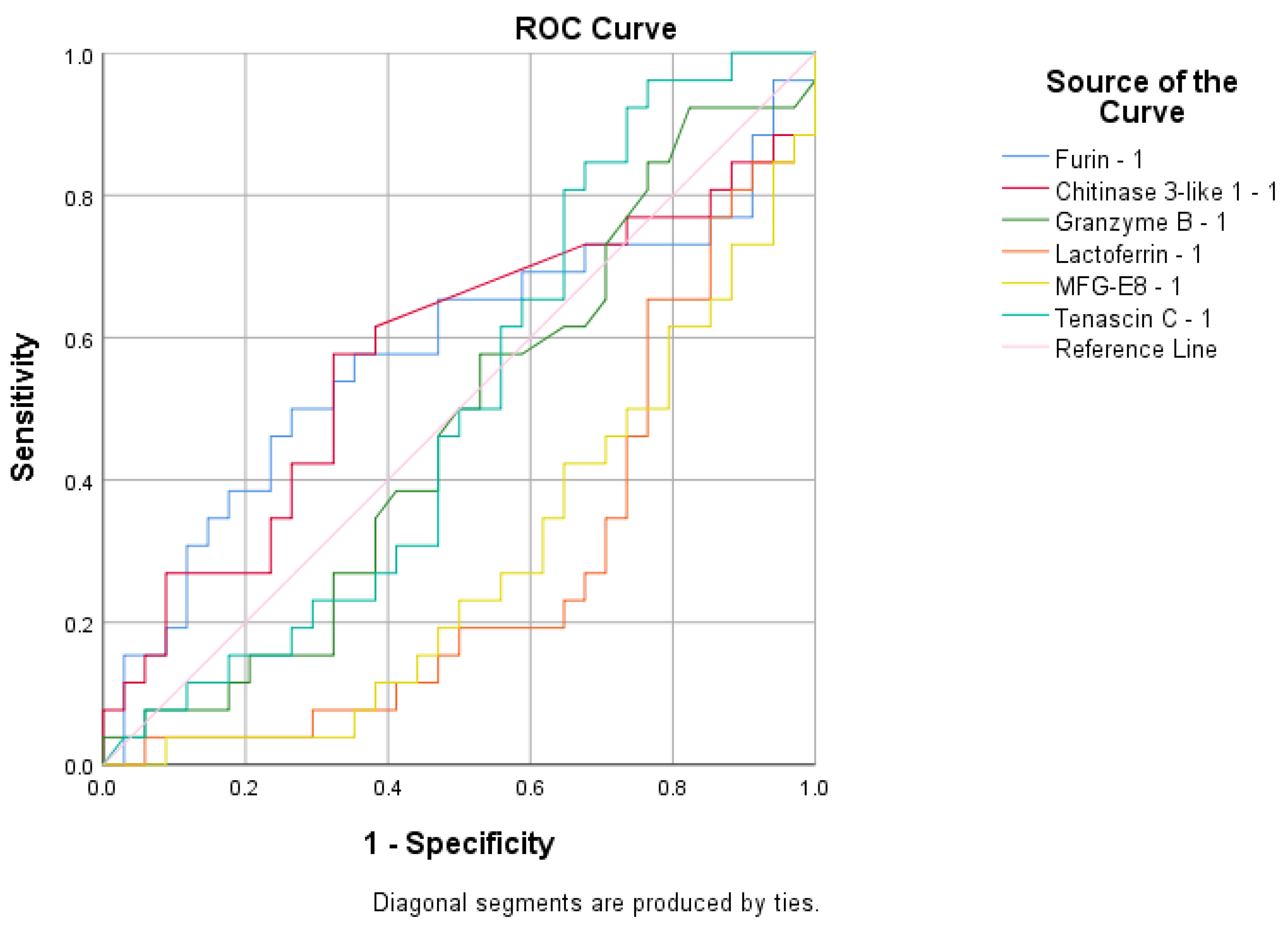
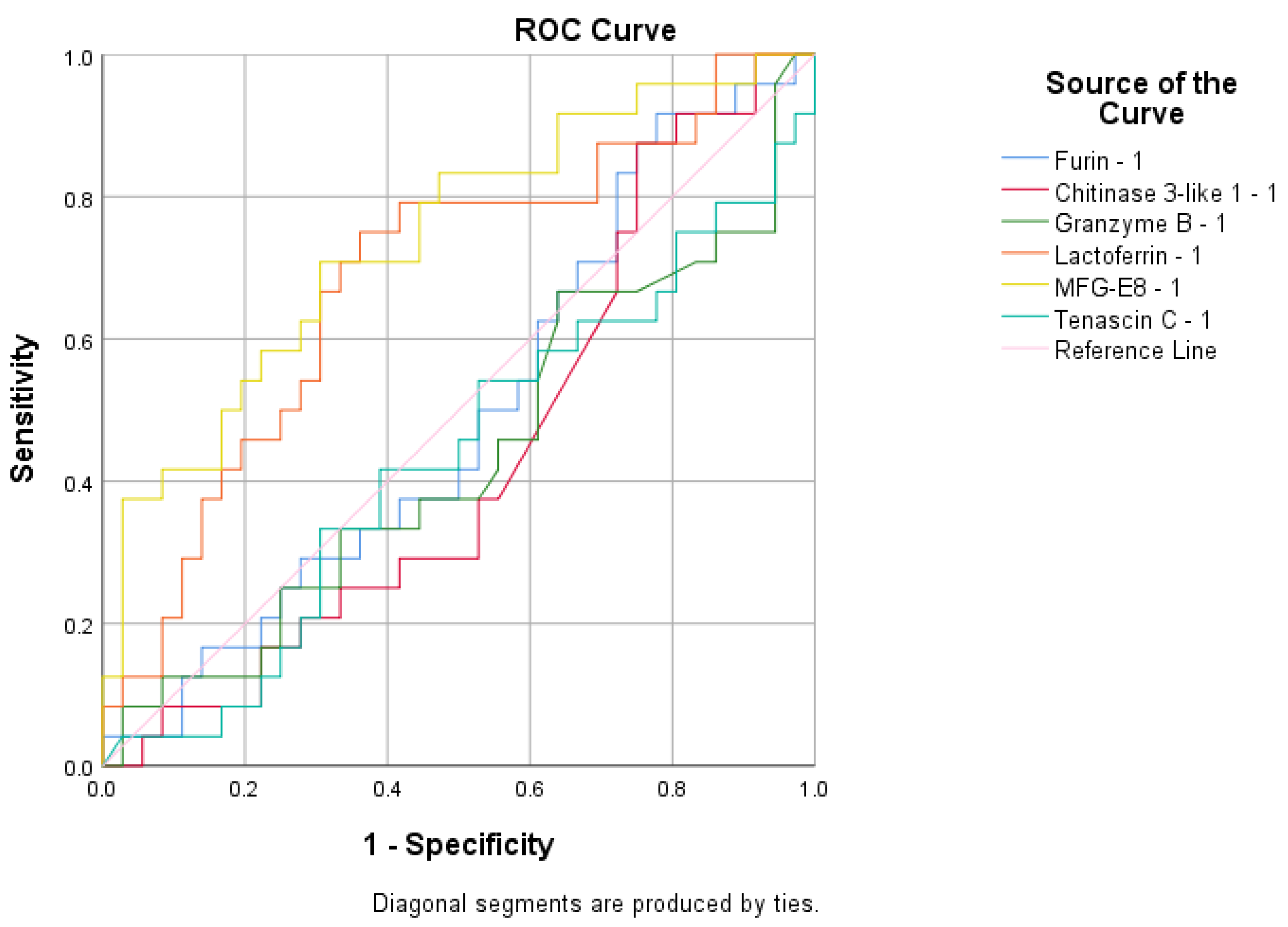
| Vaccinated and infected mothers vs. control group | Values decrease in control group | Furin | ROC analyses | - |
| Infected mothers group vs. vaccinated mothers and control group | Values decrease in vaccinated mothers and control group | Lactadherin | ROC analyses | = |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofin, F.; Cianga, P.; Constantinescu, D.; Iancu, L.S.; Iancu, R.I.; Păduraru, D.; Nastase, E.V.; Buzilă, E.R.; Luncă, C.; Cianga, C.M.; et al. The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection. Curr. Issues Mol. Biol. 2025, 47, 182. https://doi.org/10.3390/cimb47030182
Trofin F, Cianga P, Constantinescu D, Iancu LS, Iancu RI, Păduraru D, Nastase EV, Buzilă ER, Luncă C, Cianga CM, et al. The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection. Current Issues in Molecular Biology. 2025; 47(3):182. https://doi.org/10.3390/cimb47030182
Chicago/Turabian StyleTrofin, Felicia, Petru Cianga, Daniela Constantinescu, Luminița Smaranda Iancu, Roxana Irina Iancu, Diana Păduraru, Eduard Vasile Nastase, Elena Roxana Buzilă, Cătălina Luncă, Corina Maria Cianga, and et al. 2025. "The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection" Current Issues in Molecular Biology 47, no. 3: 182. https://doi.org/10.3390/cimb47030182
APA StyleTrofin, F., Cianga, P., Constantinescu, D., Iancu, L. S., Iancu, R. I., Păduraru, D., Nastase, E. V., Buzilă, E. R., Luncă, C., Cianga, C. M., & Dorneanu, O. S. (2025). The Legacy of COVID-19 in Breast Milk: The Association of Elevated Anti-Inflammatory and Antimicrobial Proteins with Vaccination or Infection. Current Issues in Molecular Biology, 47(3), 182. https://doi.org/10.3390/cimb47030182






