TP53 Mutations in Chagasic Megaesophagus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures
2.3. Statistical Analysis
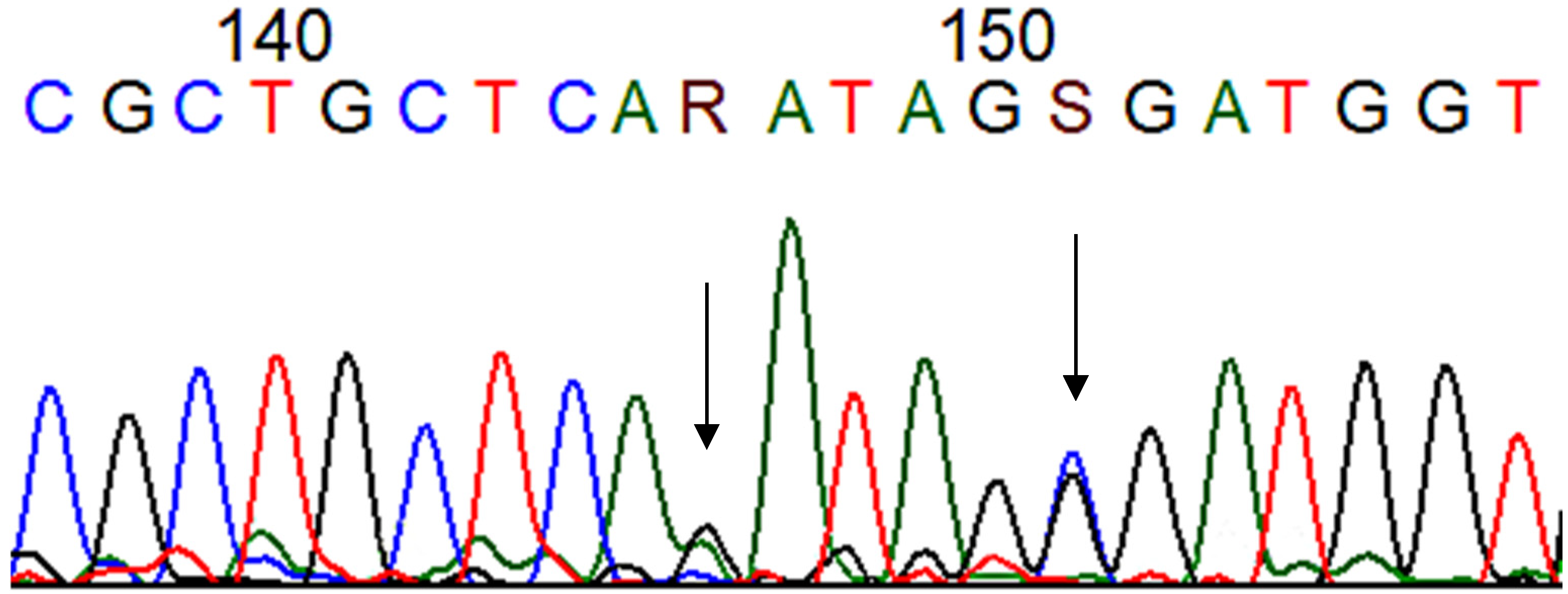
3. Results
4. Discussion
Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. 2022. Available online: https://www.who.int/health-topics/chagas-disease#tab=tab_1 (accessed on 21 June 2022).
- de Andrade, M.F.; de Almeida, V.D.; de Souza, L.M.S.; Paiva, D.C.C.; Andrade, C.M.; de Medeiros Fernandes, T.A.A. Involvement of neutrophils in Chagas disease pathology. Parasite Immunol. 2018, 40, e12593. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.F.; Silistino-Souza, R.; Varella-Garcia, M.; de Azeredo-Oliveira, M.T.V.; Silva, A.E. Biologic and Genetics Aspects of Chagas Disease at Endemic Areas. J. Trop. Med. 2012, 2012, e357948. [Google Scholar] [CrossRef] [PubMed]
- Pinotti, H.W. Megaesôfago Chagásico. In Aparelho Digestivo; Coelho, J.C.U., Ed.; Editora Atheneu: Rio de Janeiro, Brazil, 1996; pp. 61–67. [Google Scholar]
- Wong, R.K.H.; Maydonovitch, C.L. Achalasia. In The Esophagus; Castell, D.O., Richter, J.E., Eds.; Blackwell Publishing: Hoboken, NJ, USA, 1999; pp. 185–213. [Google Scholar]
- Pajecki, D.; Zilberstein, B.; Santos, M.A.A.; Quintanilha, A.G.; Cecconello, I.; Gama-Rodrigues, J. Microbiota do megaesôfago e carcinogênese. Arq. Gastroenterol. 2003, 40, 16–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Instituto Nacional de Câncer. Estimativa 2020: Incidência de Câncer No Brasil. 2022. Available online: https://www.gov.br/inca/pt-br/assuntos/noticias/2024 (accessed on 12 April 2022).
- Torres-Aguilera, M.; Remes Troche, J.M. Achalasia and esophageal cancer: Risks and links. Clin. Exp. Gastroenterol. 2018, 11, 309–316. [Google Scholar] [CrossRef]
- McCabe, M.L.; Dlamini, Z. The molecular mechanisms of oesophageal cancer. Int. Immunopharmacol. 2005, 5, 1113–1130. [Google Scholar] [CrossRef]
- Baugh, E.H.; Ke, H.; Levine, A.J.; Bonneau, R.A.; Chan, C.S. Why are there hotspot mutations in the TP53 gene in human cancers? Cell Death Differ. 2018, 25, 154–160. [Google Scholar] [CrossRef]
- De Moura-Gallo, C.V.; Azevedo, G.; Mendonça, S.; de Moraes, E.; Olivier, M.; Hainaut, P. TP53 mutations as biomarkers for cancer epidemiology in Latin America: Current knowledge and perspectives. Mut. Res. 2005, 589, 192–207. [Google Scholar] [CrossRef]
- Kuwano, H.; Kato, H.; Miyazaki, T.; Fukuchi, M.; Masuda, N.; Nakajima, M.; Fukai, Y.; Sohda, M.; Kimura, H.; Faried, A. Genetic Alterations in Esophageal Cancer. Surg. Today 2005, 35, 7–18. [Google Scholar] [CrossRef]
- Silveira, A.P.F.; Manoel-Caetano, F.D.S.; Aoki SYamasaki, L.H.T.; Rahal, P.; Silva, A.E. Gene Mutations and Polymorphisms of TP53 and FHIT in Chronic Esophagitis and Esophageal Carcinoma. Anticancer. Res. 2011, 31, 1685–1690. [Google Scholar]
- Lacerda, C.F.; Cruvinel-Carloni, A.; de Oliveira, A.T.; Scapulatempo-Neto, C.; López, R.V.; Crema, E.; Adad, S.J.; Rodrigues, M.A.; Henry, M.A.; Guimarães, D.P.; et al. Mutational profile of TP53 in esophageal squamous cell carcinoma associated with chagasic megaesophagus. Dis. Esophagus. 2017, 30, 1–9. [Google Scholar] [CrossRef]
- Hall, T.A. Bioedit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Dancey, P.C.; Reidy, J. Estatística Sem Matemática Para Psicologia; Penso: Porto Alegre, Brazil, 2006. [Google Scholar]
- Soussi, T.; Leroy, B.; Taschner, P.E.M. Recommendations for Analyzing and Reporting TP53 Gene Variants in the High-Throughput Sequencing Era. Hum. Mutat. 2014, 35, 766–768. [Google Scholar] [CrossRef] [PubMed]
- IARC TP53 Database. 2021. Available online: https://p53.iarc.fr/ (accessed on 21 June 2024).
- Chen, F.M.; Hou, M.F.; Wang, J.Y.; Chen, T.C.; Chen, D.C.; Huang, S.Y.; Chung, Y.S.; Lin, S.R. High frequency of G/C transversion on p53 gene alterations in breast cancers from Taiwan. Cancer Lett. 2004, 207, 59–67. [Google Scholar] [CrossRef]
- de Moura-Gallo, C.V.; Simão, T.A.; Ribeiro, F.S.; Andrada-Serpa, M.J.; Cardoso, L.E.B.; Mendonça, G.A. Mutações no gene TP53 em tumores malignos de mama: Associação com fatores de risco e características clínico-patológicas, inclusive risco de óbito, em pacientes residentes no Rio de Janeiro. Rev. Bras. Epidemiol. 2004, 7, 167–175. [Google Scholar] [CrossRef]
- Manoel-Caetano, F.S.; Silveira, A.F.P.; Silva, A.E. Gene Mutations in Esophageal Mucosa of Chagas Disease Patients. Anticancer. Res. 2009, 29, 1243–1248. [Google Scholar]
- Martins, M.C.L.; Miyazaki, D.L.; Gabiatti, C.C.T.; Silva, L.P.; Macedo, L.T.; Siqueira, N.S.; Andreollo, N.A.; Carvalheira, J.B.C. Chagasic Megaesophagus–Associated Carcinoma: Clinical Pattern and Outcomes. J. Glob. Oncol. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Gasser, R.B.; Hu, M.; Chilton, N.B.; Campbell, B.E.; Jex, A.J.; Otranto, D.; Cafarchia, C.; Beveridge, I.; Zhu, X. Single-strand conformation polymorphism (SSCP) for the analysis of genetic variation. Nat. Protoc. 2006, 1, 3121–3128. [Google Scholar] [CrossRef]
- Kannen, V.; de Oliveira, E.C.; Motta, B.Z.; Chaguri, A.J.; Brunaldi, M.O.; Garcia, S.B. Trypanosomiasis-Induced Megacolon Illustrates How Myenteric Neurons Modulate the Risk for Colon Cancer in Rats and Humans. PLoS Negl. Trop. Dis. 2015, 9, e0003744. [Google Scholar] [CrossRef]
- Murtaza, M.; Dawson, S.J.; Tsui, D.W.; Gale, D.; Forshew, T.; Piskorz, A.M.; Parkinson, C.; Chin, S.F.; Kingsbury, Z.; Wong, A.S.; et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 2013, 497, 108–112. [Google Scholar] [CrossRef]
- Bettegowda, C.; Sausen, M.; Leary, R.J.; Kinde, I.; Wang, Y.; Agrawal, N.; Bartlett, B.R.; Wang, H.; Luber, B.; Alani, R.M.; et al. Detection of circulating tumor DNA in early-and late- stage human malignancies. Sci. Transl. Med. 2014, 6, 224ra24. [Google Scholar] [CrossRef]
- Wang, L.; Jia, Y.M.; Zuo, J.; Wang, Y.D.; Fan, Z.S.; Feng, L.; Zhang, X.; Han, J.; Lyu, W.J.; Ni, Z.Y.; et al. Gene mutations of esophageal squamous cell carcinoma based on next-generation sequencing. Chin. Med. J. 2021, 134, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Naseri, A.; Salehi-Pourmehr, H.; Majidazar, R.; Seraji, P.; Rezazadeh-Gavgani, E.; Zehtabi, M.; Kiani-Kezbin, H.; Salehnia, F.; Hassannezhad, S.; Hajikamanj, A.; et al. Systematic Review and Meta-analysis of the Most Common Genetic Mutations in Esophageal Squamous Cell Carcinoma. J. Gastrointest. Cancer 2022, 53, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
| G1 N = 74 (%) | G2 N = 40 | G3 N = 40 | p-Value | |
|---|---|---|---|---|
| Mean Age (± SD) | 67.03 ± 11.09 | 75.95 ± 12.20 | 46.32 ± 11.00 | p < 0.001 |
| Max | 64.44 | 71.99 | 42.06 | |
| Median | 67.00 | 78.00 | 44.50 | |
| Min | 42.00 | 43.00 | 29.00 | |
| Female | 41 (56.16) | 23 (58.97) | 14 (36.84) | p = 0.162 |
| Male | 32 (43.84) | 16 (41.03) | 24 (63.16) | |
| Mut pD184N (%) | 11 (14.8%) a | 4 (10%) b | 2 (5%) c | p = 0.56 a,b p = 0.21 a,c p = 0.67 b,c |
| Mut pS185R (%) | 11 (14.8%) a | 4 (10%) b | 3 (7.5%) c | p = 0.56 a,b p = 0.37 a,c p = 1.00 b,c |
| Mut G > T intron 7 (%) | 3 (4%) a | 3 (7.5%) b | 0 c | p = 0.32 a,b p = 1.00 a,c p = 0.23 b,c |
| Codons | Type | Classification | Aa * | Transcriptional Activity | SIFT Class § | Splice Site | CpG Site | Hot Spot | DNA Binding |
|---|---|---|---|---|---|---|---|---|---|
| 184 | G:C > A:T | Transition | Asp > Asn | Partially Functional | Tolerant | - | No | Yes | |
| 185 | C:G > G:C | Transversion | Ser > Arg | Functional | Tolerant | - | Yes | No | Yes |
| Intron 7 | G:C > T:A | Transition | No | No | No |
| Kappa Values | Significancy | Pearson Qui-Square | p-Value | |
|---|---|---|---|---|
| SSCP vs. Mutation 184—exon 5 | 0.97 | 0.00 | 142 | 0.00 |
| SSCP vs. Mutation 185—exon 5 | 1.00 | 0.00 | 154 | 0.00 |
| SSCP vs. Mutation—intron 7 | 1.00 | 0.00 | 308 | 0.00 |
| Grade I N(%) | Grade II | Grade II/III | Grade III | Grade III/IV | Grade IV | p-Value | |
|---|---|---|---|---|---|---|---|
| pD184N pos | 1 | 4 (14%) | 0 | 2 | 3 (15.8%) | 1 | 0.97 |
| pD184N neg | 3 | 24 | 1 | 7 | 16 | 7 | |
| Overall | 4 | 28 | 1 | 9 | 19 | 8 | |
| pS185R pos | 1 | 4 (14%) | 0 | 2 | 3 (15.8%) | 1 | 0.97 |
| pS185R neg | 3 | 24 | 1 | 7 | 16 | 7 | |
| Overall | 4 | 28 | 1 | 9 | 19 | 8 | |
| G > T intron 7 pos | 0 | 2 (7%) | 0 | 0 | 0 | 0 | 0.69 |
| G > T intron 7 neg | 4 | 26 | 1 | 9 | 19 | 8 | |
| Overall | 4 | 28 | 1 | 9 | 19 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, A.P.F.; Sartori, R.Q.; Castiglioni, L.; Alves da Cruz, H.L.; Junior, E.d.C.; Brandão, C.C.; de Mattos, L.C. TP53 Mutations in Chagasic Megaesophagus. Curr. Issues Mol. Biol. 2025, 47, 229. https://doi.org/10.3390/cimb47040229
Silveira APF, Sartori RQ, Castiglioni L, Alves da Cruz HL, Junior EdC, Brandão CC, de Mattos LC. TP53 Mutations in Chagasic Megaesophagus. Current Issues in Molecular Biology. 2025; 47(4):229. https://doi.org/10.3390/cimb47040229
Chicago/Turabian StyleSilveira, Aparecida Perpetuo Fedossi, Ricardo Quiterio Sartori, Lilian Castiglioni, Heidi Lacerda Alves da Cruz, Eumildo de Campos Junior, Cinara Cássia Brandão, and Luiz Carlos de Mattos. 2025. "TP53 Mutations in Chagasic Megaesophagus" Current Issues in Molecular Biology 47, no. 4: 229. https://doi.org/10.3390/cimb47040229
APA StyleSilveira, A. P. F., Sartori, R. Q., Castiglioni, L., Alves da Cruz, H. L., Junior, E. d. C., Brandão, C. C., & de Mattos, L. C. (2025). TP53 Mutations in Chagasic Megaesophagus. Current Issues in Molecular Biology, 47(4), 229. https://doi.org/10.3390/cimb47040229







