Role of the Endocannabinoid System in Fibromyalgia
Abstract
:1. Introduction
2. Overview of the Endocannabinoid System
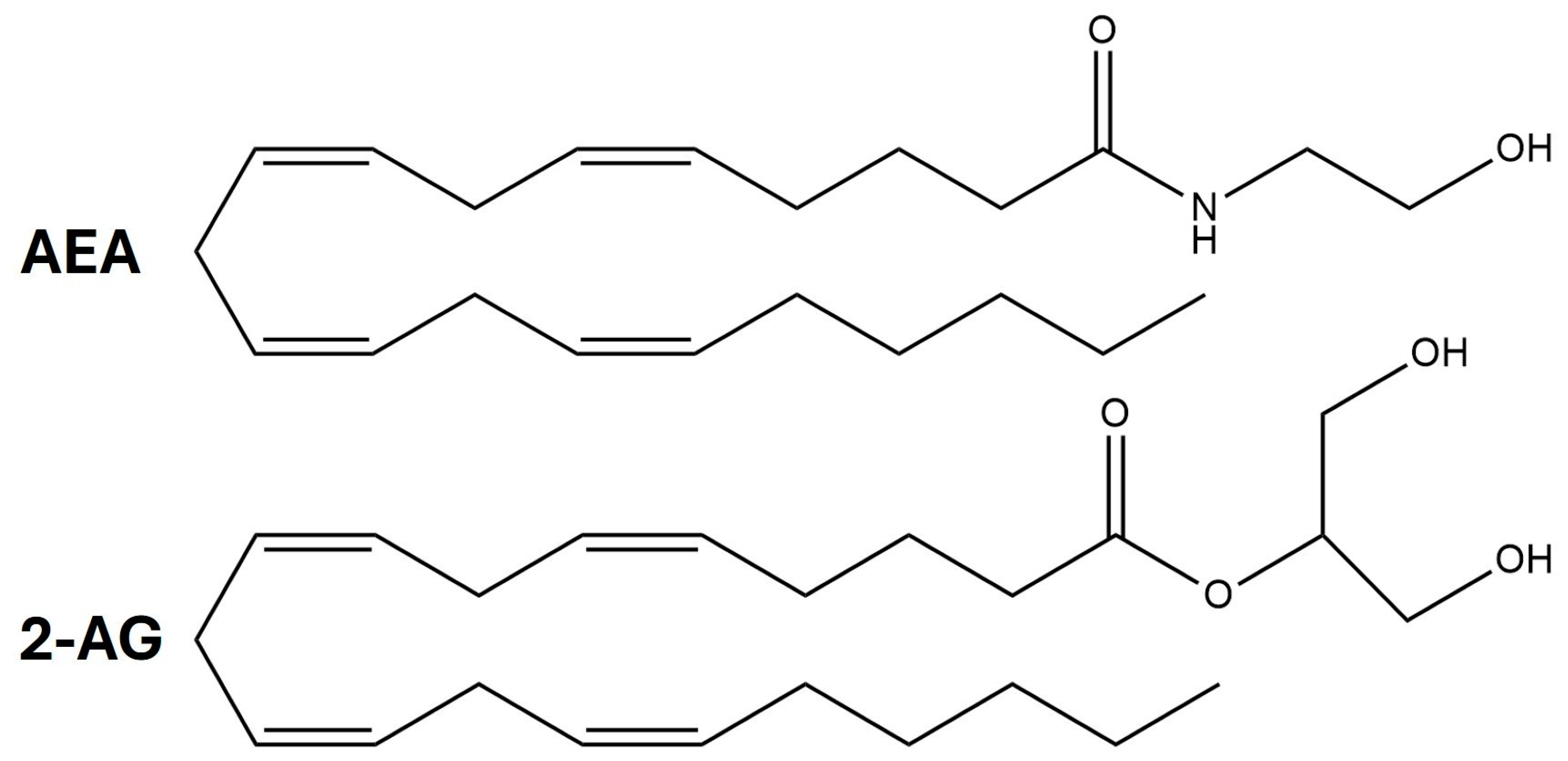
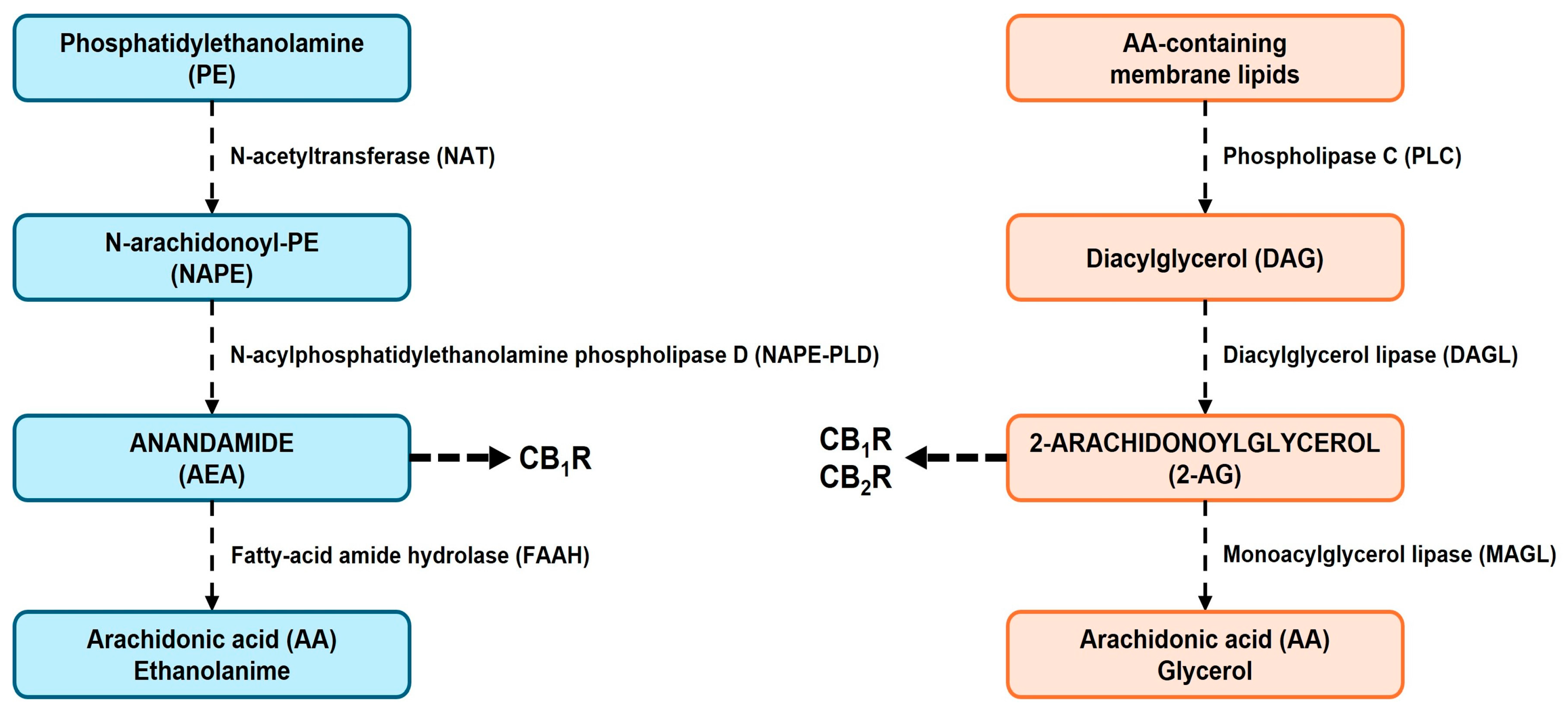
2.1. Endocannabinoid Synthesis
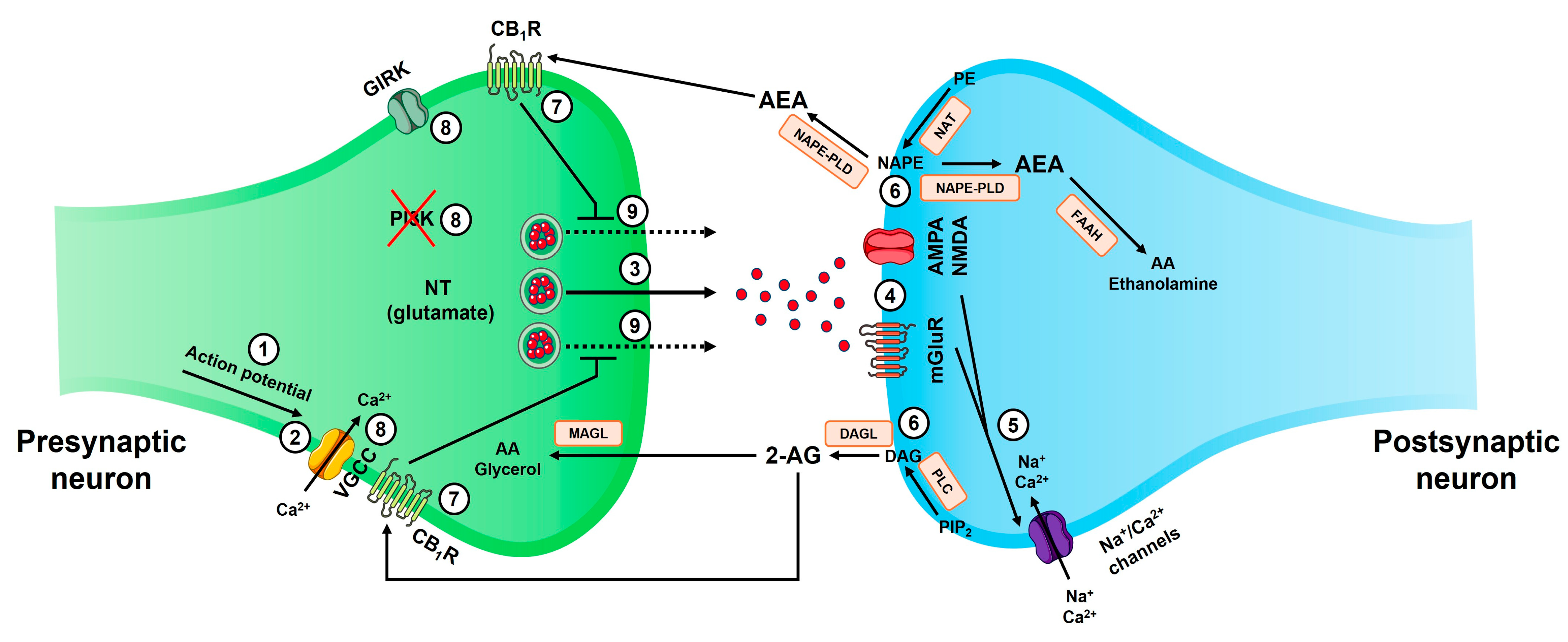
2.2. Endocannabinoid as a Retrograde Synaptic Messenger
2.3. Cannabinoid Receptors
2.4. Endocannabinoid Degradation
2.5. Endocannabinoid-Mediated Biological Processes
3. Relationship Between the ECS and Fibromyalgia
4. Cannabinoid-Based Therapies for Fibromyalgia
4.1. Current Therapies
4.2. Regulatory, Ethical, and Social Considerations in the Use of Cannabinoids in the Management of Fibromyalgia
5. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2-AG | 2-arachidonoylglycerol |
| AA | Arachidonic acid |
| ABHD4 | Abhydrolase domain containing 4, N-acyl phospholipase B |
| AC | Adenylate cyclase |
| ACR | American College of Rheumatology |
| AEA | Anandamide |
| AMPAR | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor |
| ANS | Autonomic nervous system |
| APBB2 | Amyloid beta precursor protein binding family B member 2 |
| cAMP | Cyclic adenosine monophosphate |
| CB1R | Cannabinoid receptor 1 (protein) |
| CB2R | Cannabinoid receptor 2 (protein) |
| CECD | Clinical endocannabinoid deficiency |
| CNR1 | Cannabinoid receptor 1 (gene) |
| CNR2 | Cannabinoid receptor 2 (gene) |
| CNS | Central nervous system |
| COPD | Chronic obstructive pulmonary disease |
| DAG | Diacylglycerol |
| DAGL | Diacylglycerol lipase |
| ECS | Endocannabinoid system |
| EMBASE | Excerpta medica dataBASE |
| ENS | Enteric nervous system |
| ERK1/2 | Extracellular signal-regulated kinases 1 and 2 |
| FAAH | Fatty acid amide hydrolase |
| FIQ | Fibromyalgia Impact Questionnaire |
| GATA2 | GATA-binding factor 2 |
| GIRK | G protein-coupled inwardly rectifying potassium channel |
| GPCR | G protein-coupled receptor |
| GPR18 | G protein-coupled receptor 18 |
| GPR55 | G protein-coupled receptor 55 |
| GPR119 | G protein-coupled receptor 119 |
| HDC | Histidine decarboxylase |
| IBD | Inflammatory bowel disease |
| IBS | Irritable bowel syndrome |
| LTD | Long-term depression |
| LTP | Long-term potentiation |
| MAGL | Monoacylglycerol lipase |
| MAPK | Mitogen-activated protein kinase |
| MEDLINE | Medical literature analysis and retrieval system online |
| mGluR | Metabotropic glutamate receptor |
| NAE | N-acylethanolamine |
| NAPE | N-arachidonoyl phosphatidylethanolamine |
| NAPE-PLD | N-acyl phosphatidylethanolamine phospholipase D |
| NAT | N-acyltransferase |
| NMDAR | N-methyl-D-aspartate receptor |
| NT | Neurotransmitter |
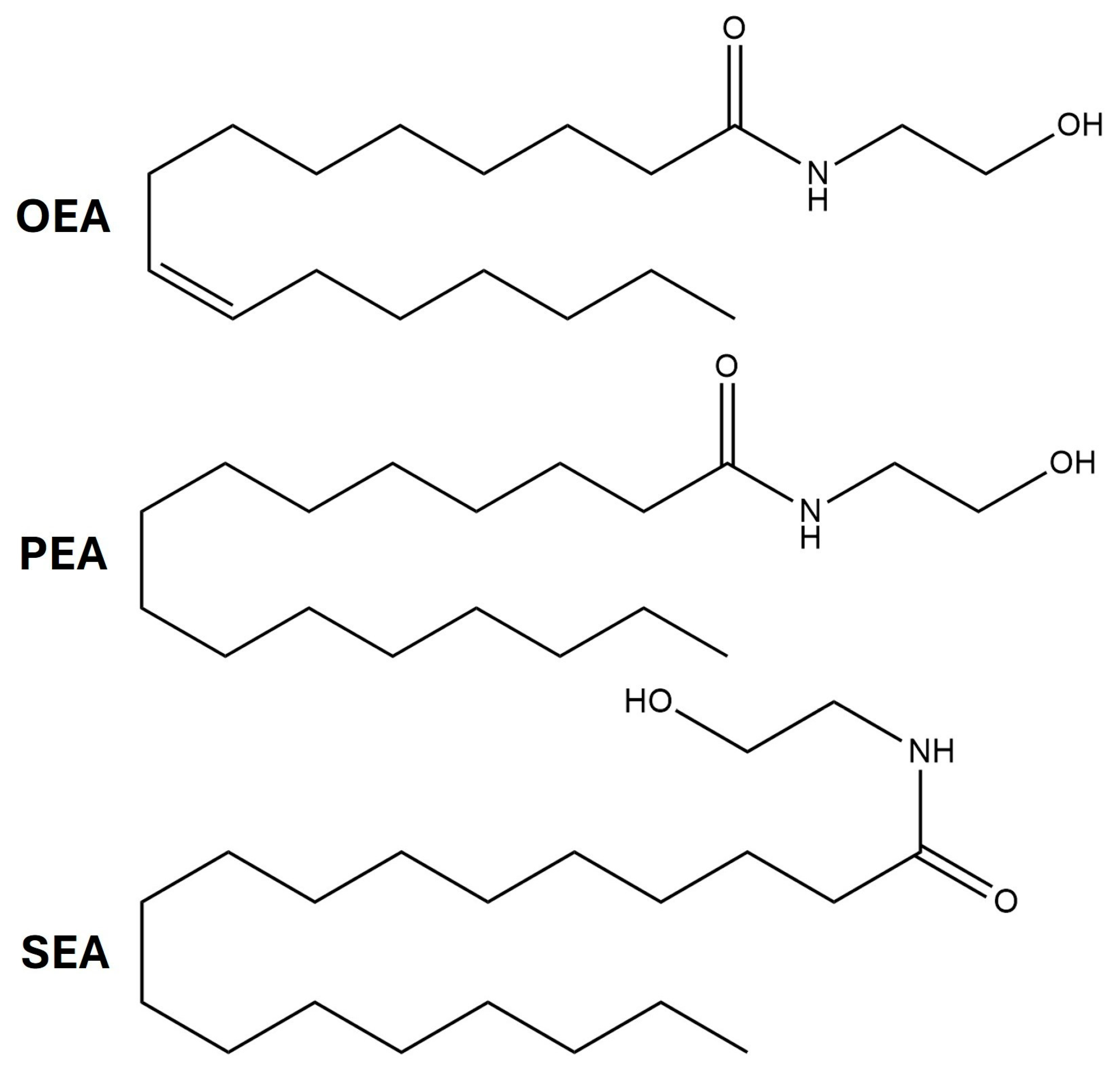
| OEA | Oleoylethanolamine |
| PE | Phosphatidyl-ethanolamine |
| PEA | Palmitoylethanolamine |
| PI3K | Phosphatidylinositide-3-kinase |
| PIP2 | Phosphatidylinositol 4,5-bisphosphate |
| PLA1 | Phospholipase A1 |
| PLC | Phospholipase C |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| SEA | N-stearoylethanolamine |
| SNP | Single nucleotide polymorphism |
| THC | Tetrahydrocannabinol |
| TNF-α | Tumor necrosis factor alpha |
| VGCC | Voltage-gated calcium channel |
References
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [PubMed]
- Cohen-Biton, L.; Buskila, D.; Nissanholtz-Gannot, R. Review of Fibromyalgia (FM) Syndrome Treatments. Int. J. Environ. Res. Public Health 2022, 19, 12106. [Google Scholar] [CrossRef]
- Vincent, A.; Lahr, B.D.; Wolfe, F.; Clauw, D.J.; Whipple, M.O.; Oh, T.H.; Barton, D.L.; St Sauver, J. Prevalence of fibromyalgia: A population-based study in Olmsted County, Minnesota, utilizing the Rochester Epidemiology Project. Arthritis Care Res. 2013, 65, 786–792. [Google Scholar]
- Heidari, F.; Afshari, M.; Moosazadeh, M. Prevalence of fibromyalgia in general population and patients, a systematic review and meta-analysis. Rheumatol. Int. 2017, 37, 1527–1539. [Google Scholar] [PubMed]
- Otón, T.; Messina, O.D.; Fernández Ávila, D.G.; Robles San Román, M.; Mata, D.; Arguissain, C.; Galindo Guzmán, J.M.; Pérez, M.; Carmona, L.; Grupo Fibrojourney Latam. The patient journey of fibromyalgia in Latin America. Reumatol. Clin. (Engl. Ed.) 2024, 20, 32–42. [Google Scholar]
- Ruschak, I.; Montesó-Curto, P.; Rosselló, L.; Aguilar Martín, C.; Sánchez-Montesó, L.; Toussaint, L. Fibromyalgia Syndrome Pain in Men and Women: A Scoping Review. Healthcare 2023, 11, 223. [Google Scholar] [CrossRef]
- Bazzichi, L.; Giorgi, V.; Di Franco, M.; Iannuccelli, C.; Bongiovanni, S.; Batticciotto, A.; Pellegrino, G.; Sarzi Puttini, P. Environmental factors and fibromyalgia syndrome: A narrative review. Clin. Exp. Rheumatol. 2024, 42, 1240–1247. [Google Scholar]
- Kurlyandchik, I.; Lauche, R.; Tiralongo, E.; Warne, L.N.; Schloss, J. Plasma and interstitial levels of endocannabinoids and N-acylethanolamines in patients with chronic widespread pain and fibromyalgia: A systematic review and meta-analysis. Pain Rep. 2022, 7, e1045. [Google Scholar]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar]
- Finn, D.P.; Haroutounian, S.; Hohmann, A.G.; Krane, E.; Soliman, N.; Rice, A.S.C. Cannabinoids, the endocannabinoid system, and pain: A review of preclinical studies. Pain 2021, 162, S5–S25. [Google Scholar]
- Zou, S.; Kumar, U. Cannabinoid Receptors and the Endocannabinoid System: Signaling and Function in the Central Nervous System. Int. J. Mol. Sci. 2018, 19, 833. [Google Scholar] [CrossRef] [PubMed]
- Busquets-Garcia, A.; Bains, J.; Marsicano, G. CB1 Receptor Signaling in the Brain: Extracting Specificity from Ubiquity. Neuropsychopharmacology 2018, 43, 4–20. [Google Scholar] [PubMed]
- Hashiesh, H.M.; Sharma, C.; Goyal, S.N.; Sadek, B.; Jha, N.K.; Kaabi, J.A.; Ojha, S. A focused review on CB2 receptor-selective pharmacological properties and therapeutic potential of β-caryophyllene, a dietary cannabinoid. Biomed. Pharmacother. 2021, 140, 111639. [Google Scholar]
- Di Marzo, V. Endocannabinoids: Synthesis and degradation. Rev. Physiol. Biochem. Pharmacol. 2008, 160, 1–24. [Google Scholar] [PubMed]
- Strand, N.H.; Maloney, J.; Kraus, M.; Wie, C.; Turkiewicz, M.; Gomez, D.A.; Adeleye, O.; Harbell, M.W. Cannabis for the Treatment of Fibromyalgia: A Systematic Review. Biomedicines 2023, 11, 1621. [Google Scholar] [CrossRef]
- Hourfane, S.; Mechqoq, H.; Bekkali, A.Y.; Rocha, J.M.; El Aouad, N. A Comprehensive Review on Cannabis sativa Ethnobotany, Phytochemistry, Molecular Docking and Biological Activities. Plants 2023, 12, 1245. [Google Scholar] [CrossRef]
- Devane, W.A.; Dysarz, F.A., 3rd; Johnson, M.R.; Melvin, L.S.; Howlett, A.C. Determination and characterization of a cannabinoid receptor in rat brain. Mol. Pharmacol. 1988, 34, 605–613. [Google Scholar]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular characterization of a peripheral receptor for cannabinoids. Nature 1993, 365, 61–65. [Google Scholar]
- Cabral, G.A.; Griffin-Thomas, L. Emerging role of the cannabinoid receptor CB2 in immune regulation: Therapeutic prospects for neuroinflammation. Expert. Rev. Mol. Med. 2009, 11, e3. [Google Scholar]
- Turcotte, C.; Blanchet, M.R.; Laviolette, M.; Flamand, N. The CB2 receptor and its role as a regulator of inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar]
- Fezza, F.; Bari, M.; Florio, R.; Talamonti, E.; Feole, M.; Maccarrone, M. Endocannabinoids, related compounds and their metabolic routes. Molecules 2014, 19, 17078–17106. [Google Scholar] [CrossRef] [PubMed]
- Simard, M.; Archambault, A.S.; Lavoie, J.C.; Dumais, É.; Di Marzo, V.; Flamand, N. Biosynthesis and metabolism of endocannabinoids and their congeners from the monoacylglycerol and N-acyl-ethanolamine families. Biochem. Pharmacol. 2022, 205, 115261. [Google Scholar] [CrossRef] [PubMed]
- Albarran, E.; Sun, Y.; Liu, Y.; Raju, K.; Dong, A.; Li, Y.; Wang, S.; Südhof, T.C.; Ding, J.B. Postsynaptic synucleins mediate endocannabinoid signaling. Nat. Neurosci. 2023, 26, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Dudok, B.; Fan, L.Z.; Farrell, J.S.; Malhotra, S.; Homidan, J.; Kim, D.K.; Wenardy, C.; Ramakrishnan, C.; Li, Y.; Deisseroth, K.; et al. Retrograde endocannabinoid signaling at inhibitory synapses in vivo. Science 2024, 383, 967–970. [Google Scholar] [CrossRef]
- Cuddihey, H.; MacNaughton, W.K.; Sharkey, K.A. Role of the Endocannabinoid System in the Regulation of Intestinal Homeostasis. Cell. Mol. Gastroenterol. Hepatol. 2022, 14, 947–963. [Google Scholar] [CrossRef]
- Maccarrone, M. Metabolism of the Endocannabinoid Anandamide: Open Questions after 25 Years. Front. Mol. Neurosci. 2017, 10, 166. [Google Scholar] [CrossRef]
- Murataeva, N.; Straiker, A.; Mackie, K. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. Br. J. Pharmacol. 2014, 171, 1379–1391. [Google Scholar] [CrossRef]
- Simon, G.M.; Cravatt, B.F. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 2006, 281, 26465–26472. [Google Scholar] [CrossRef]
- Briand-Mésange, F.; Gennero, I.; Salles, J.; Trudel, S.; Dahan, L.; Ausseil, J.; Payrastre, B.; Salles, J.P.; Chap, H. From Classical to Alternative Pathways of 2-Arachidonoylglycerol Synthesis: AlterAGs at the Crossroad of Endocannabinoid and Lysophospholipid Signaling. Molecules 2024, 29, 3694. [Google Scholar] [CrossRef]
- Kuipers, E.N.; Kantae, V.; Maarse, B.C.E.; van den Berg, S.M.; van Eenige, R.; Nahon, K.J.; Reifel-Miller, A.; Coskun, T.; de Winther, M.P.J.; Lutgens, E.; et al. High Fat Diet Increases Circulating Endocannabinoids Accompanied by Increased Synthesis Enzymes in Adipose Tissue. Front. Physiol. 2019, 9, 1913. [Google Scholar] [CrossRef]
- Basavarajappa, B.S. Critical enzymes involved in endocannabinoid metabolism. Protein Pept. Lett. 2007, 14, 237–246. [Google Scholar] [PubMed]
- Blankman, J.L.; Cravatt, B.F. Chemical probes of endocannabinoid metabolism. Pharmacol. Rev. 2013, 65, 849–871. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [PubMed]
- Ohno-Shosaku, T.; Tanimura, A.; Hashimotodani, Y.; Kano, M. Endocannabinoids and retrograde modulation of synaptic transmission. Neuroscientist 2012, 18, 119–132. [Google Scholar] [PubMed]
- Kruk-Slomka, M.; Dzik, A.; Budzynska, B.; Biala, G. Endocannabinoid System: The Direct and Indirect Involvement in the Memory and Learning Processes-a Short Review. Mol. Neurobiol. 2017, 54, 8332–8347. [Google Scholar]
- Zogopoulos, P.; Vasileiou, I.; Patsouris, E.; Theocharis, S.E. The role of endocannabinoids in pain modulation. Fundam. Clin. Pharmacol. 2013, 27, 64–80. [Google Scholar] [CrossRef]
- Silva-Cruz, A.; Carlström, M.; Ribeiro, J.A.; Sebastião, A.M. Dual Influence of Endocannabinoids on Long-Term Potentiation of Synaptic Transmission. Front. Pharmacol. 2017, 8, 921. [Google Scholar]
- Peñasco, S.; Rico-Barrio, I.; Puente, N.; Gómez-Urquijo, S.M.; Fontaine, C.J.; Egaña-Huguet, J.; Achicallende, S.; Ramos, A.; Reguero, L.; Elezgarai, I.; et al. Endocannabinoid long-term depression revealed at medial perforant path excitatory synapses in the dentate gyrus. Neuropharmacology 2019, 153, 32–40. [Google Scholar] [CrossRef]
- Huang, W.J.; Chen, W.W.; Zhang, X. Endocannabinoid system: Role in depression, reward and pain control (Review). Mol. Med. Rep. 2016, 14, 2899–2903. [Google Scholar]
- Ruehle, S.; Rey, A.A.; Remmers, F.; Lutz, B. The endocannabinoid system in anxiety, fear memory and habituation. J. Psychopharmacol. 2012, 26, 23–39. [Google Scholar]
- Cheung, K.A.K.; Peiris, H.; Wallace, G.; Holland, O.J.; Mitchell, M.D. The Interplay between the Endocannabinoid System, Epilepsy and Cannabinoids. Int. J. Mol. Sci. 2019, 20, 6079. [Google Scholar] [CrossRef]
- Matsuda, L.A.; Lolait, S.J.; Brownstein, M.J.; Young, A.C.; Bonner, T.I. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 1990, 346, 561–564. [Google Scholar] [CrossRef]
- Onaivi, E.S.; Ishiguro, H.; Gu, S.; Liu, Q.R. CNS effects of CB2 cannabinoid receptors: Beyond neuro-immuno-cannabinoid activity. J. Psychopharmacol. 2012, 26, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Howlett, A.C.; Blume, L.C.; Dalton, G.D. CB1 cannabinoid receptors and their associated proteins. Curr. Med. Chem. 2010, 17, 1382–1393. [Google Scholar] [CrossRef] [PubMed]
- Rezende, B.; Alencar, A.K.N.; de Bem, G.F.; Fontes-Dantas, F.L.; Montes, G.C. Endocannabinoid System: Chemical Characteristics and Biological Activity. Pharmaceuticals 2023, 16, 148. [Google Scholar] [CrossRef]
- Lutz, B. Molecular biology of cannabinoid receptors. Prostaglandins Leukot. Essent. Fat. Acids 2002, 66, 123–142. [Google Scholar] [CrossRef]
- Svízenská, I.; Dubový, P.; Sulcová, A. Cannabinoid receptors 1 and 2 (CB1 and CB2), their distribution, ligands and functional involvement in nervous system structures—A short review. Pharmacol. Biochem. Behav. 2008, 90, 501–511. [Google Scholar] [CrossRef]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef]
- Tsou, K.; Brown, S.; Sañudo-Peña, M.C.; Mackie, K.; Walker, J.M. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience 1998, 83, 393–411. [Google Scholar] [CrossRef] [PubMed]
- Eggan, S.M.; Lewis, D.A. Immunocytochemical distribution of the cannabinoid CB1 receptor in the primate neocortex: A regional and laminar analysis. Cereb. Cortex 2007, 17, 175–191. [Google Scholar] [CrossRef]
- Pugh, G., Jr.; Smith, P.B.; Dombrowski, D.S.; Welch, S.P. The role of endogenous opioids in enhancing the antinociception produced by the combination of delta 9-tetrahydrocannabinol and morphine in the spinal cord. J. Pharmacol. Exp. Ther. 1996, 279, 608–616. [Google Scholar]
- Hohmann, A.G.; Briley, E.M.; Herkenham, M. Pre- and postsynaptic distribution of cannabinoid and mu opioid receptors in rat spinal cord. Brain Res. 1999, 822, 17–25. [Google Scholar] [PubMed]
- Abrams, D.I.; Couey, P.; Shade, S.B.; Kelly, M.E.; Benowitz, N.L. Cannabinoid-opioid interaction in chronic pain. Clin. Pharmacol. Ther. 2011, 90, 844–851. [Google Scholar] [PubMed]
- Parnell, J.; Martin, N.; Dedek, A.; Rudyk, C.; Landrigan, J.; Bellavance, J.; VanDerLoo, S.; Tsai, E.C.; Hildebrand, M.E. Cannabinoid CB1 Receptor Expression and Localization in the Dorsal Horn of Male and Female Rat and Human Spinal Cord. Can. J. Pain 2023, 7, 2264895. [Google Scholar] [PubMed]
- Freundt-Revilla, J.; Kegler, K.; Baumgärtner, W.; Tipold, A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE 2017, 12, e0181064. [Google Scholar]
- Duncan, M.; Davison, J.S.; Sharkey, K.A. Review article: Endocannabinoids and their receptors in the enteric nervous system. Aliment. Pharmacol. Ther. 2005, 22, 667–683. [Google Scholar]
- Galligan, J.J. Cannabinoid signalling in the enteric nervous system. Neurogastroenterol. Motil. 2009, 21, 899–902. [Google Scholar]
- Ishac, E.J.; Jiang, L.; Lake, K.D.; Varga, K.; Abood, M.E.; Kunos, G. Inhibition of exocytotic noradrenaline release by presynaptic cannabinoid CB1 receptors on peripheral sympathetic nerves. Br. J. Pharmacol. 1996, 118, 2023–2028. [Google Scholar] [CrossRef]
- Bellocchio, L.; Soria-Gómez, E.; Quarta, C.; Metna-Laurent, M.; Cardinal, P.; Binder, E.; Cannich, A.; Delamarre, A.; Häring, M.; Martín-Fontecha, M.; et al. Activation of the sympathetic nervous system mediates hypophagic and anxiety-like effects of CB1 receptor blockade. Proc. Natl. Acad. Sci. USA 2013, 110, 4786–4791. [Google Scholar]
- Mendizabal-Zubiaga, J.; Melser, S.; Bénard, G.; Ramos, A.; Reguero, L.; Arrabal, S.; Elezgarai, I.; Gerrikagoitia, I.; Suarez, J.; Rodríguez De Fonseca, F.; et al. Cannabinoid CB1 Receptors Are Localized in Striated Muscle Mitochondria and Regulate Mitochondrial Respiration. Front. Physiol. 2016, 7, 476. [Google Scholar]
- Zduniak, K.; Ziółkowski, P.; Regnell, P.; Tollet-Egnell, P.; Åkesson, L.; Cooper, M.E. Immunohistochemical analysis of cannabinoid receptor 1 expression in steatotic rat livers. Exp. Ther. Med. 2016, 11, 1227–1230. [Google Scholar] [CrossRef]
- Pagano Zottola, A.C.; Severi, I.; Cannich, A.; Ciofi, P.; Cota, D.; Marsicano, G.; Giordano, A.; Bellocchio, L. Expression of Functional Cannabinoid Type-1 (CB1) Receptor in Mitochondria of White Adipocytes. Cells 2022, 11, 2582. [Google Scholar] [CrossRef]
- Liao, Y.; Bin, J.; Luo, T.; Zhao, H.; Ledent, C.; Asakura, M.; Xu, D.; Takashima, S.; Kitakaze, M. CB1 cannabinoid receptor deficiency promotes cardiac remodeling induced by pressure overload in mice. Int. J. Cardiol. 2013, 167, 1936–1944. [Google Scholar] [CrossRef]
- Barajas-Martínez, A.; Bermeo, K.; de la Cruz, L.; Martínez-Vargas, M.; Martínez-Tapia, R.J.; García, D.E.; Navarro, L. Cannabinoid receptors are differentially regulated in the pancreatic islets during the early development of metabolic syndrome. Islets 2020, 12, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Reggio, P.H. Endocannabinoid binding to the cannabinoid receptors: What is known and what remains unknown. Curr. Med. Chem. 2010, 17, 1468–1486. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef]
- Leo, L.M.; Abood, M.E. CB1 Cannabinoid Receptor Signaling and Biased Signaling. Molecules 2021, 26, 5413. [Google Scholar] [CrossRef]
- Sarkar, A.; Mitra, A.; Borics, A. All-Atom Molecular Dynamics Simulations Indicated the Involvement of a Conserved Polar Signaling Channel in the Activation Mechanism of the Type I Cannabinoid Receptor. Int. J. Mol. Sci. 2023, 24, 4232. [Google Scholar] [CrossRef]
- Dhopeshwarkar, A.; Mackie, K. Functional Selectivity of CB2 Cannabinoid Receptor Ligands at a Canonical and Noncanonical Pathway. J. Pharmacol. Exp. Ther. 2016, 358, 342–351. [Google Scholar] [CrossRef]
- Cinar, R.; Gochuico, B.R.; Iyer, M.R.; Jourdan, T.; Yokoyama, T.; Park, J.K.; Coffey, N.J.; Pri-Chen, H.; Szanda, G.; Liu, Z.; et al. Cannabinoid CB1 receptor overactivity contributes to the pathogenesis of idiopathic pulmonary fibrosis. JCI Insight 2017, 2, e92281. [Google Scholar] [PubMed]
- Simard, M.; Rakotoarivelo, V.; Di Marzo, V.; Flamand, N. Expression and Functions of the CB2 Receptor in Human Leukocytes. Front. Pharmacol. 2022, 13, 826400. [Google Scholar] [CrossRef] [PubMed]
- Saroz, Y.; Kho, D.T.; Glass, M.; Graham, E.S.; Grimsey, N.L. Cannabinoid Receptor 2 (CB2) Signals via G-alpha-s and Induces IL-6 and IL-10 Cytokine Secretion in Human Primary Leukocytes. ACS Pharmacol. Transl. Sci. 2019, 2, 414–428. [Google Scholar] [PubMed]
- Rayman, N.; Lam, K.H.; Laman, J.D.; Simons, P.J.; Löwenberg, B.; Sonneveld, P.; Delwel, R. Distinct expression profiles of the peripheral cannabinoid receptor in lymphoid tissues depending on receptor activation status. J. Immunol. 2004, 172, 2111–2117. [Google Scholar]
- Huang, Z.; Wang, H.; Wang, J.; Zhao, M.; Sun, N.; Sun, F.; Shen, J.; Zhang, H.; Xia, K.; Chen, D.; et al. Cannabinoid receptor subtype 2 (CB2R) agonist, GW405833 reduces agonist-induced Ca2+ oscillations in mouse pancreatic acinar cells. Sci. Rep. 2016, 6, 29757. [Google Scholar] [CrossRef]
- Ruhl, T.; Karthaus, N.; Kim, B.S.; Beier, J.P. The endocannabinoid receptors CB1 and CB2 affect the regenerative potential of adipose tissue MSCs. Exp. Cell. Res. 2020, 389, 111881. [Google Scholar]
- Heinemann, J.C.; Duerr, G.D.; Keppel, K.; Breitbach, M.; Fleischmann, B.K.; Zimmer, A.; Wehner, S.; Welz, A.; Dewald, O. CB2 receptor-mediated effects of pro-inflammatory macrophages influence survival of cardiomyocytes. Life Sci. 2015, 138, 18–28. [Google Scholar]
- Schley, M.; Ständer, S.; Kerner, J.; Vajkoczy, P.; Schüpfer, G.; Dusch, M.; Schmelz, M.; Konrad, C. Predominant CB2 receptor expression in endothelial cells of glioblastoma in humans. Brain Res. Bull. 2009, 79, 333–337. [Google Scholar]
- Correia-Sá, I.; Carvalho, C.A.; Machado, V.; Carvalho, S.; Serrão, P.; Marques, M.; Vieira-Coelho, M.A. Targeting cannabinoid receptor 2 (CB2) limits collagen production-An in vitro study in a primary culture of human fibroblasts. Fundam. Clin. Pharmacol. 2022, 36, 89–99. [Google Scholar]
- Ibsen, M.S.; Finlay, D.B.; Patel, M.; Javitch, J.A.; Glass, M.; Grimsey, N.L. Cannabinoid CB1 and CB2 Receptor-Mediated Arrestin Translocation: Species, Subtype, and Agonist-Dependence. Front. Pharmacol. 2019, 10, 350. [Google Scholar]
- Irving, A.; Abdulrazzaq, G.; Chan, S.L.F.; Penman, J.; Harvey, J.; Alexander, S.P.H. Cannabinoid Receptor-Related Orphan G Protein-Coupled Receptors. Adv. Pharmacol. 2017, 80, 223–247. [Google Scholar] [PubMed]
- McHugh, D. GPR18 in microglia: Implications for the CNS and endocannabinoid system signalling. Br. J. Pharmacol. 2012, 167, 1575–1582. [Google Scholar] [CrossRef]
- Sharir, H.; Console-Bram, L.; Mundy, C.; Popoff, S.N.; Kapur, A.; Abood, M.E. The endocannabinoids anandamide and virodhamine modulate the activity of the candidate cannabinoid receptor GPR55. J. Neuroimmune Pharmacol. 2012, 7, 856–865. [Google Scholar] [CrossRef]
- Syed, S.K.; Bui, H.H.; Beavers, L.S.; Farb, T.B.; Ficorilli, J.; Chesterfield, A.K.; Kuo, M.S.; Bokvist, K.; Barrett, D.G.; Efanov, A.M. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E1469–E1478. [Google Scholar] [CrossRef]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed]
- Guerrero-Alba, R.; Barragán-Iglesias, P.; González-Hernández, A.; Valdez-Moráles, E.E.; Granados-Soto, V.; Condés-Lara, M.; Rodríguez, M.G.; Marichal-Cancino, B.A. Some Prospective Alternatives for Treating Pain: The Endocannabinoid System and Its Putative Receptors GPR18 and GPR55. Front. Pharmacol. 2019, 9, 1496. [Google Scholar] [CrossRef]
- Kim, J.; Alger, B.E. Reduction in endocannabinoid tone is a homeostatic mechanism for specific inhibitory synapses. Nat. Neurosci. 2010, 13, 592–600. [Google Scholar] [CrossRef]
- Biernacki, M.; Skrzydlewska, E. Metabolism of endocannabinoids. Postepy. Hig. Med. Dosw. (Online) 2016, 70, 830–843. [Google Scholar] [CrossRef] [PubMed]
- Haj-Dahmane, S.; Shen, R.Y. Endocannabinoids suppress excitatory synaptic transmission to dorsal raphe serotonin neurons through the activation of presynaptic CB1 receptors. J. Pharmacol. Exp. Ther. 2009, 331, 186–196. [Google Scholar] [CrossRef]
- Xu, J.Y.; Chen, C. Endocannabinoids in synaptic plasticity and neuroprotection. Neuroscientist. 2015, 21, 152–168. [Google Scholar] [CrossRef]
- Lipina, C.; Hundal, H.S. Modulation of cellular redox homeostasis by the endocannabinoid system. Open Biol. 2016, 6, 150276. [Google Scholar]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [PubMed]
- Marsicano, G.; Lafenêtre, P. Roles of the endocannabinoid system in learning and memory. Curr. Top. Behav. Neurosci. 2009, 1, 201–230. [Google Scholar] [PubMed]
- Pinna, G. Endocannabinoids and Precision Medicine for Mood Disorders and Suicide. Front. Psychiatry 2021, 12, 658433. [Google Scholar]
- Russo, E.B. Clinical Endocannabinoid Deficiency Reconsidered: Current Research Supports the Theory in Migraine, Fibromyalgia, Irritable Bowel, and Other Treatment-Resistant Syndromes. Cannabis Cannabinoid. Res. 2016, 1, 154–165. [Google Scholar] [PubMed]
- Welberg, L. Endocannabinoids: A protective receptor pool. Nat. Rev. Neurosci. 2014, 15, 426. [Google Scholar]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef]
- Rathod, S.S.; Agrawal, Y.O.; Nakhate, K.T.; Meeran, M.F.N.; Ojha, S.; Goyal, S.N. Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System. Biomedicines 2023, 11, 2642. [Google Scholar] [CrossRef]
- Berry, A.J.; Zubko, O.; Reeves, S.J.; Howard, R.J. Endocannabinoid system alterations in Alzheimer’s disease: A systematic review of human studies. Brain Res. 2020, 1749, 147135. [Google Scholar]
- Urmeneta-Ortíz, M.F.; Tejeda-Martínez, A.R.; González-Reynoso, O.; Flores-Soto, M.E. Potential Neuroprotective Effect of the Endocannabinoid System on Parkinson’s Disease. Parkinsons Dis. 2024, 2024, 5519396. [Google Scholar]
- Rahaman, O.; Ganguly, D. Endocannabinoids in immune regulation and immunopathologies. Immunology 2021, 164, 242–252. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M.; Aguirre, A.; Lastra, A.; Hidalgo, A.; Baamonde, A.; Menéndez, L. The Systemic Administration of the Chemokine CCL1 Evokes Thermal Analgesia in Mice Through the Activation of the Endocannabinoid System. Cell. Mol. Neurobiol. 2019, 39, 1115–1124. [Google Scholar] [PubMed]
- O’Sullivan, S.E. Endocannabinoids and the Cardiovascular System in Health and Disease. Handb. Exp. Pharmacol. 2015, 231, 393–422. [Google Scholar]
- Wiese, B.M.; Alvarez Reyes, A.; Vanderah, T.W.; Largent-Milnes, T.M. The endocannabinoid system and breathing. Front. Neurosci. 2023, 17, 1126004. [Google Scholar] [CrossRef] [PubMed]
- Pandey, S.; Kashif, S.; Youssef, M.; Sarwal, S.; Zraik, H.; Singh, R.; Rutkofsky, I.H. Endocannabinoid system in irritable bowel syndrome and cannabis as a therapy. Complement. Ther. Med. 2020, 48, 102242. [Google Scholar] [CrossRef]
- Bourke, S.L.; Schlag, A.K.; O’Sullivan, S.E.; Nutt, D.J.; Finn, D.P. Cannabinoids and the endocannabinoid system in fibromyalgia: A review of preclinical and clinical research. Pharmacol. Ther. 2022, 240, 108216. [Google Scholar]
- Smith, S.C.; Wagner, M.S. Clinical endocannabinoid deficiency (CECD) revisited: Can this concept explain the therapeutic benefits of cannabis in migraine, fibromyalgia, irritable bowel syndrome and other treatment-resistant conditions? Neuro Endocrinol. Lett. 2014, 35, 198–201. [Google Scholar]
- Walitt, B.; Klose, P.; Fitzcharles, M.A.; Phillips, T.; Häuser, W. Cannabinoids for fibromyalgia. Cochrane Database Syst. Rev. 2016, 7, CD011694. [Google Scholar]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Expression of the endocannabinoid receptors in human fascial tissue. Eur. J. Histochem. 2016, 60, 2643. [Google Scholar] [CrossRef]
- Hanlon, E.C.; Tasali, E.; Leproult, R.; Stuhr, K.L.; Doncheck, E.; de Wit, H.; Hillard, C.J.; Van Cauter, E. Circadian rhythm of circulating levels of the endocannabinoid 2-arachidonoylglycerol. J. Clin. Endocrinol. Metab. 2015, 100, 220–226. [Google Scholar]
- McPartland, J.M. The endocannabinoid system: An osteopathic perspective. J. Am. Osteopath. Assoc. 2008, 108, 586–600. [Google Scholar] [PubMed]
- Mechoulam, R.; Fride, E.; Hanus, L.; Sheskin, T.; Bisogno, T.; Di Marzo, V.; Bayewitch, M.; Vogel, Z. Anandamide may mediate sleep induction. Nature 1997, 389, 25–26. [Google Scholar] [PubMed]
- Dunnett, A.J.; Roy, D.; Stewart, A.; McPartland, J.M. The diagnosis of fibromyalgia in women may be influenced by menstrual cycle phase. J. Bodyw. Mov. Ther. 2007, 11, 99–105. [Google Scholar]
- Johnson, D.R.; Stebulis, J.A.; Rossetti, R.G.; Burstein, S.H.; Zurier, R.B. Suppression of fibroblast metalloproteinases by ajulemic acid, a nonpsychoactive cannabinoid acid. J. Cell. Biochem. 2007, 100, 184–190. [Google Scholar] [PubMed]
- Mbvundula, E.C.; Bunning, R.A.; Rainsford, K.D. Effects of cannabinoids on nitric oxide production by chondrocytes and proteoglycan degradation in cartilage. Biochem. Pharmacol. 2005, 69, 635–640. [Google Scholar]
- Jones, K.D.; Gelbart, T.; Whisenant, T.C.; Waalen, J.; Mondala, T.S.; Iklé, D.N.; Salomon, D.R.; Bennett, R.M.; Kurian, S.M. Genome-wide expression profiling in the peripheral blood of patients with fibromyalgia. Clin. Exp. Rheumatol. 2016, 34, S89–S98. [Google Scholar]
- Smith, S.B.; Maixner, D.W.; Fillingim, R.B.; Slade, G.; Gracely, R.H.; Ambrose, K.; Zaykin, D.V.; Hyde, C.; John, S.; Tan, K.; et al. Large candidate gene association study reveals genetic risk factors and therapeutic targets for fibromyalgia. Arthritis Rheum. 2012, 64, 584–593. [Google Scholar]
- Gerra, M.C.; González-Villar, A.; Arendt-Nielsen, L.; Søkilde Pedersen, I.; Triñanes, Y.; Donnini, C.; Manfredini, M.; Walther, D.; Moeller, G.L.; Pidal-Miranda, M.; et al. A family-based study to identify genetic biomarkers of fibromyalgia: Consideration of patients’ subgroups. Clin. Exp. Rheumatol. 2021, 39 (Suppl. S130), 144–152. [Google Scholar]
- Lopez-Cortes, O.D.; Trujillo-Sánchez, F.; Sierra-Ruelas, E.; Martinez-Lopez, E.; Di Marzo, V.; Vizmanos, B. Association between the FAAH C385A variant (rs324420) and obesity-related traits: A systematic review. Int. J. Obes. 2024, 48, 188–201. [Google Scholar]
- Silva, H.H.; Tavares, V.; Neto, B.V.; Cerqueira, F.; Medeiros, R.; Silva, M.G. FAAH rs324420 Polymorphism: Biological Pathways, Impact on Elite Athletic Performance and Insights for Sport Medicine. Genes 2023, 14, 1946. [Google Scholar] [CrossRef]
- Chadwick, A.; Frazier, A.; Khan, T.W.; Young, E. Understanding the Psychological, Physiological, and Genetic Factors Affecting Precision Pain Medicine: A Narrative Review. J. Pain Res. 2021, 14, 3145–3161. [Google Scholar] [PubMed]
- Kaufmann, I.; Schelling, G.; Eisner, C.; Richter, H.P.; Krauseneck, T.; Vogeser, M.; Hauer, D.; Campolongo, P.; Chouker, A.; Beyer, A.; et al. Anandamide and neutrophil function in patients with fibromyalgia. Psychoneuroendocrinology 2008, 33, 676–685. [Google Scholar] [PubMed]
- Stensson, N.; Gerdle, B.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Ghafouri, B. Increased Anandamide and Decreased Pain and Depression after Exercise in Fibromyalgia. Med. Sci. Sports Exerc. 2020, 52, 1617–1628. [Google Scholar]
- Stensson, N.; Ghafouri, B.; Gerdle, B.; Ghafouri, N. Alterations of anti-inflammatory lipids in plasma from women with chronic widespread pain—A case control study. Lipids Health Dis. 2017, 16, 112. [Google Scholar]
- Stensson, N.; Ghafouri, N.; Ernberg, M.; Mannerkorpi, K.; Kosek, E.; Gerdle, B.; Ghafouri, B. The Relationship of Endocannabinoidome Lipid Mediators with Pain and Psychological Stress in Women with Fibromyalgia: A Case-Control Study. J. Pain 2018, 19, 1318–1328. [Google Scholar] [PubMed]
- Dalle Carbonare, M.; Del Giudice, E.; Stecca, A.; Colavito, D.; Fabris, M.; D’Arrigo, A.; Bernardini, D.; Dam, M.; Leon, A. A saturated N-acylethanolamine other than N-palmitoyl ethanolamine with anti-inflammatory properties: A neglected story. J. Neuroendocrinol. 2008, 20 (Suppl. S1), 26–34. [Google Scholar]
- Suardíaz, M.; Estivill-Torrús, G.; Goicoechea, C.; Bilbao, A.; Rodríguez de Fonseca, F. Analgesic properties of oleoylethanolamide (OEA) in visceral and inflammatory pain. Pain 2007, 133, 99–110. [Google Scholar]
- Johnson, B.W.; Strand, N.H.; Raynak, J.C.; Jara, C.; Habtegiorgis, K.; Hand, B.A.; Hong, S.; Maloney, J.A. Cannabinoids in Chronic Pain Management: A Review of the history, efficacy, applications, and risks. Biomedicines 2025, 13, 530. [Google Scholar] [CrossRef]
- Lopera, V.; Restrepo, J.C.; Amariles, P. Effectiveness and safety of cannabis-based products for medical use in patients with fibromyalgia syndrome: A systematic review. Explor. Res. Clin. Soc. Pharm. 2024, 16, 100524. [Google Scholar]
- David, P.; Mohsen, A.; Amital, H. Is Medical Cannabis a Solution for Controlling Fibromyalgia Symptoms? Mayo Clin. Proc. 2024, 99, 524–526. [Google Scholar]
- Sagy, I.; Bar-Lev Schleider, L.; Abu-Shakra, M.; Novack, V. Safety and Efficacy of Medical Cannabis in Fibromyalgia. J. Clin. Med. 2019, 8, 807. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.A.; Keefe, J.; Winnick, A.; Gilbert, E.; Eskander, J.P.; Yazdi, C.; Kaye, A.D.; Viswanath, O.; Urits, I. Cannabis and cannabidiol (CBD) for the treatment of fibromyalgia. Best. Pract. Res. Clin. Anaesthesiol. 2020, 34, 617–631. [Google Scholar]
- Blebea, N.M.; Pricopie, A.I.; Vlad, R.A.; Hancu, G. Phytocannabinoids: Exploring Pharmacological Profiles and Their Impact on Therapeutical Use. Int. J. Mol. Sci. 2024, 25, 4204. [Google Scholar] [CrossRef] [PubMed]
- Roque-Bravo, R.; Silva, R.S.; Malheiro, R.F.; Carmo, H.; Carvalho, F.; da Silva, D.D.; Silva, J.P. Synthetic Cannabinoids: A Pharmacological and Toxicological Overview. Annu. Rev. Pharmacol. Toxicol. 2023, 63, 187–209. [Google Scholar]
- Mazza, M. Medical cannabis for the treatment of fibromyalgia syndrome: A retrospective, open-label case series. J. Cannabis Res. 2021, 3, 4. [Google Scholar] [PubMed]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar]
- Safi, K.; Sobieraj, J.; Błaszkiewicz, M.; Żyła, J.; Salata, B.; Dzierżanowski, T. Tetrahydrocannabinol and Cannabidiol for Pain Treatment-An Update on the Evidence. Biomedicines 2024, 12, 307. [Google Scholar] [CrossRef]
- Fiz, J.; Durán, M.; Capellà, D.; Carbonell, J.; Farré, M. Cannabis use in patients with fibromyalgia: Effect on symptoms relief and health-related quality of life. PLoS ONE 2011, 6, e18440. [Google Scholar]
- Habib, G.; Avisar, I. The Consumption of Cannabis by Fibromyalgia Patients in Israel. Pain Res. Treat. 2018, 2018, 7829427. [Google Scholar]
- van de Donk, T.; Niesters, M.; Kowal, M.A.; Olofsen, E.; Dahan, A.; van Velzen, M. An experimental randomized study on the analgesic effects of pharmaceutical-grade cannabis in chronic pain patients with fibromyalgia. Pain 2019, 160, 860–869. [Google Scholar]
- Schley, M.; Legler, A.; Skopp, G.; Schmelz, M.; Konrad, C.; Rukwied, R. Delta-9-THC based monotherapy in fibromyalgia patients on experimentally induced pain, axon reflex flare, and pain relief. Curr. Med. Res. Opin. 2006, 22, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Yassin, M.; Oron, A.; Robinson, D. Effect of adding medical cannabis to analgesic treatment in patients with low back pain related to fibromyalgia: An observational cross-over single centre study. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S116), 13–20. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, H.; Qureshi, I.A.; Jahan, N.; Went, T.R.; Sultan, W.; Sapkota, A.; Alfonso, M. A Systematic Review of Fibromyalgia and Recent Advancements in Treatment: Is Medicinal Cannabis a New Hope? Cureus 2021, 13, e17332. [Google Scholar] [CrossRef]
- van Dam, C.J.; Kramers, C.; Schellekens, A.; Bouvy, M.; van Dorp, E.; Kowal, M.A.; Olofsen, E.; Dahan, A.; Niesters, M.; van Velzen, M. Cannabis combined with oxycodone for pain relief in fibromyalgia pain: A randomized clinical self-titration trial with focus on adverse events. Front. Pain Res. 2024, 5, 1497111. [Google Scholar] [CrossRef]
- Giardina, A.; Palmieri, R.; Ponticelli, M.; Antonelli, C.; Carlucci, V.; Colangelo, M.; Benedetto, N.; Di Fazio, A.; Milella, L. Is a Low Dosage of Medical Cannabis Effective for Treating Pain Related to Fibromyalgia? A Pilot Study and Systematic Review. J. Clin. Med. 2024, 13, 4088. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Erridge, S.; Holvey, C.; Coomber, R.; Holden, W.; Rucker, J.J.; Platt, M.; Sodergren, M.H. Comparison of Cannabis-Based Medicinal Product Formulations for Fibromyalgia: A Cohort Study. J. Pain Palliat. Care. Pharmacother. 2024, 1–14. [Google Scholar] [CrossRef]
- Skrabek, R.Q.; Galimova, L.; Ethans, K.; Perry, D. Nabilone for the treatment of pain in fibromyalgia. J. Pain 2008, 9, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Ware, M.A.; Fitzcharles, M.A.; Joseph, L.; Shir, Y. The effects of nabilone on sleep in fibromyalgia: Results of a randomized controlled trial. Anesth. Analg. 2010, 110, 604–610. [Google Scholar] [CrossRef]
- Stockings, E.; Campbell, G.; Hall, W.D.; Nielsen, S.; Zagic, D.; Rahman, R.; Murnion, B.; Farrell, M.; Weier, M.; Degenhardt, L. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: A systematic review and meta-analysis of controlled and observational studies. Pain 2018, 159, 1932–1954. [Google Scholar] [CrossRef]
- Tsang, C.C.; Giudice, M.G. Nabilone for the Management of Pain. Pharmacotherapy 2016, 36, 273–286. [Google Scholar] [CrossRef]
- Ambrosio, A.L.B.; Dias, S.M.G.; Polikarpov, I.; Zurier, R.B.; Burstein, S.H.; Garratt, R.C. Ajulemic acid, a synthetic nonpsychoactive cannabinoid acid, bound to the ligand binding domain of the human peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2007, 282, 18625–18633. [Google Scholar] [PubMed]
- Tepper, M.A.; Zurier, R.B.; Burstein, S.H. Ultrapure ajulemic acid has improved CB2 selectivity with reduced CB1 activity. Bioorg. Med. Chem. 2014, 22, 3245–3251. [Google Scholar]
- Burstein, S.H. Ajulemic acid: Potential treatment for chronic inflammation. Pharmacol. Res. Perspect. 2018, 6, e00394. [Google Scholar]
- Schimrigk, S.; Marziniak, M.; Neubauer, C.; Kugler, E.M.; Werner, G.; Abramov-Sommariva, D. Dronabinol Is a Safe Long-Term Treatment Option for Neuropathic Pain Patients. Eur. Neurol. 2017, 78, 320–329. [Google Scholar] [PubMed]
- Kitdumrongthum, S.; Trachootham, D. An Individuality of Response to Cannabinoids: Challenges in Safety and Efficacy of Cannabis Products. Molecules 2023, 28, 2791. [Google Scholar] [CrossRef]
- Cooper, Z.D.; Abrams, D.I.; Gust, S.; Salicrup, A.; Throckmorton, D.C. Challenges for Clinical Cannabis and Cannabinoid Research in the United States. J. Natl. Cancer Inst. Monogr. 2021, 2021, 114–122. [Google Scholar]
- Clobes, T.A.; Palmier, L.A.; Gagnon, M.; Klaiman, C.; Arellano, M. The impact of education on attitudes toward medical cannabis. PEC Innov. 2021, 1, 100009. [Google Scholar] [PubMed]
- Shi, Y.; Wu, W. Multimodal non-invasive non-pharmacological therapies for chronic pain: Mechanisms and progress. BMC Med. 2023, 21, 372. [Google Scholar]
- Sagy, I.; Peleg-Sagy, T.; Barski, L.; Zeller, L.; Jotkowitz, A. Ethical issues in medical cannabis use. Eur. J. Intern. Med. 2018, 49, 20–22. [Google Scholar]
- Pacula, R.L.; Smart, R. Medical Marijuana and Marijuana Legalization. Annu. Rev. Clin. Psychol. 2017, 13, 397–419. [Google Scholar] [CrossRef]
- Szaflarski, M.; Sirven, J.I. Social factors in marijuana use for medical and recreational purposes. Epilepsy Behav. 2017, 70, 280–287. [Google Scholar]
- Hall, W.; Lynskey, M. Assessing the public health impacts of legalizing recreational cannabis use: The US experience. World Psychiatry 2020, 19, 179–186. [Google Scholar] [PubMed]
- Morris, M.; Chye, R.; Liu, Z.; Agar, M.; Razmovski-Naumovski, V. A Retrospective Medical Record Review of Adults with Non-Cancer Diagnoses Prescribed Medicinal Cannabis. J. Clin. Med. 2023, 12, 1483. [Google Scholar] [CrossRef]
- Philpot, L.M.; Ebbert, J.O.; Hurt, R.T. A survey of the attitudes, beliefs and knowledge about medical cannabis among primary care providers. BMC Fam. Pract. 2019, 20, 17. [Google Scholar]
- Hossain, M.K.; Chae, H.J. Medical cannabis: From research breakthroughs to shifting public perceptions and ensuring safe use. Integr. Med. Res. 2024, 13, 101094. [Google Scholar]
- Li, T.; Wang, G.S.; Brooks-Russell, A.; Tung, G.; Leslie, L.; Rittiphairoj, T.; Oberste, J.P.; Yim, T.W.; Bero, L.; Samet, J.M. Methodological challenges and actionable recommendations in studying the health effects of high-concentration THC products. Am. J. Epidemiol. 2024, kwae421. [Google Scholar] [CrossRef]
- Wheeldon, J.; Heidt, J. Cannabis, research ethics, and a duty of care. Res. Ethics 2023, 19, 250–287. [Google Scholar]
- Martin, J.H.; Hill, C.; Walsh, A.; Efron, D.; Taylor, K.; Kennedy, M.; Galettis, R.; Lightfoot, P.; Hanson, J.; Irving, H.; et al. Clinical trials with cannabis medicines—Guidance for ethics committees, governance officers and researchers to streamline ethics applications and ensuring patient safety: Considerations from the Australian experience. Trials 2021, 21, 932. [Google Scholar]
- Cohen, K.; Weizman, A.; Weinstein, A. Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin. Pharmacol. Ther. 2019, 105, 1139–1147. [Google Scholar]
- Hernandez, M.; Franks, A.M.; Payakachat, N. Changes in Arkansans’ attitudes toward pharmacist involvement and regulation of medical cannabis following its availability in Arkansas. J. Am. Pharm. Assoc. 2003, 63, 1131–1137.e4. [Google Scholar]
- Abu Baker, D.; Cruz Rivera, P.N.; Narasimhan, R.; Nguyen, N.; Tibiriçá, L.; Kepner, W.E.; O’Malley, P.; Nguyen, A.L.; Moore, A.A. Older Adults’ Use of Cannabis and Attitudes Around Disclosing Medical Cannabis Use to Their Healthcare Providers in California: A Mixed Methods Study. Drugs Real World Outcomes 2024, 11, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Prasad, S.; Jain, E.; Dogrul, B.N.; Al-Oleimat, A.; Pokhrel, B.; Chowdhury, S.; Co, E.L.; Mitra, S.; Quinonez, J.; et al. Medical Cannabis for Chronic Nonmalignant Pain Management. Curr. Pain Headache Rep. 2023, 27, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Simiyu, D.C.; Jang, J.H.; Lee, O.R. Understanding Cannabis sativa L.: Current Status of Propagation, Use, Legalization, and Haploid-Inducer-Mediated Genetic Engineering. Plants 2022, 11, 1236. [Google Scholar] [CrossRef] [PubMed]
- Connor, J.P.; Stjepanović, D.; Le Foll, B.; Hoch, E.; Budney, A.J.; Hall, W.D. Cannabis use and cannabis use disorder. Nat. Rev. Dis. Primers 2021, 7, 16. [Google Scholar]
- Hall, W.; Stjepanović, D.; Dawson, D.; Leung, J. The implementation and public health impacts of cannabis legalization in Canada: A systematic review. Addiction 2023, 118, 2062–2072. [Google Scholar]
| Cannabinoid Receptor | Gene Localization | Tissue Localization | References |
|---|---|---|---|
| CB1R | Human: chromosome 6, Mouse: chromosome 4, Rat: chromosome 5 | Cerebral cortex, basal ganglia, periaqueductal gray, hypothalamus, cerebellum, amygdala, brainstem medullary nuclei, spinal/ventral dorsal horn, autonomic nervous system, enteric nervous system, muscle, liver, adipose tissue, heart, pancreas, and lungs. | [42,43,44,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70] |
| CB2R | Human: chromosome 6, Mouse: chromosome 4, Rat: chromosome 5 | Immune cells, lymphoid tissues, pancreatic acinar cells, adipocytes, cardiomyocytes, endothelial cells, fibroblasts, and osteoclasts. | [18,43,45,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Domínguez, M. Role of the Endocannabinoid System in Fibromyalgia. Curr. Issues Mol. Biol. 2025, 47, 230. https://doi.org/10.3390/cimb47040230
García-Domínguez M. Role of the Endocannabinoid System in Fibromyalgia. Current Issues in Molecular Biology. 2025; 47(4):230. https://doi.org/10.3390/cimb47040230
Chicago/Turabian StyleGarcía-Domínguez, Mario. 2025. "Role of the Endocannabinoid System in Fibromyalgia" Current Issues in Molecular Biology 47, no. 4: 230. https://doi.org/10.3390/cimb47040230
APA StyleGarcía-Domínguez, M. (2025). Role of the Endocannabinoid System in Fibromyalgia. Current Issues in Molecular Biology, 47(4), 230. https://doi.org/10.3390/cimb47040230





