Molecular Characterization and Feeding-Associated Expression Dynamics of the Period Gene Family in Channel Catfish (Ictalurus punctatus)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Sampling
2.2. Molecular Cloning and Tissue Distribution of Expression of Period Family Genes
2.3. Bioinformatic Analysis
2.4. In Silico Analyses
3. Results
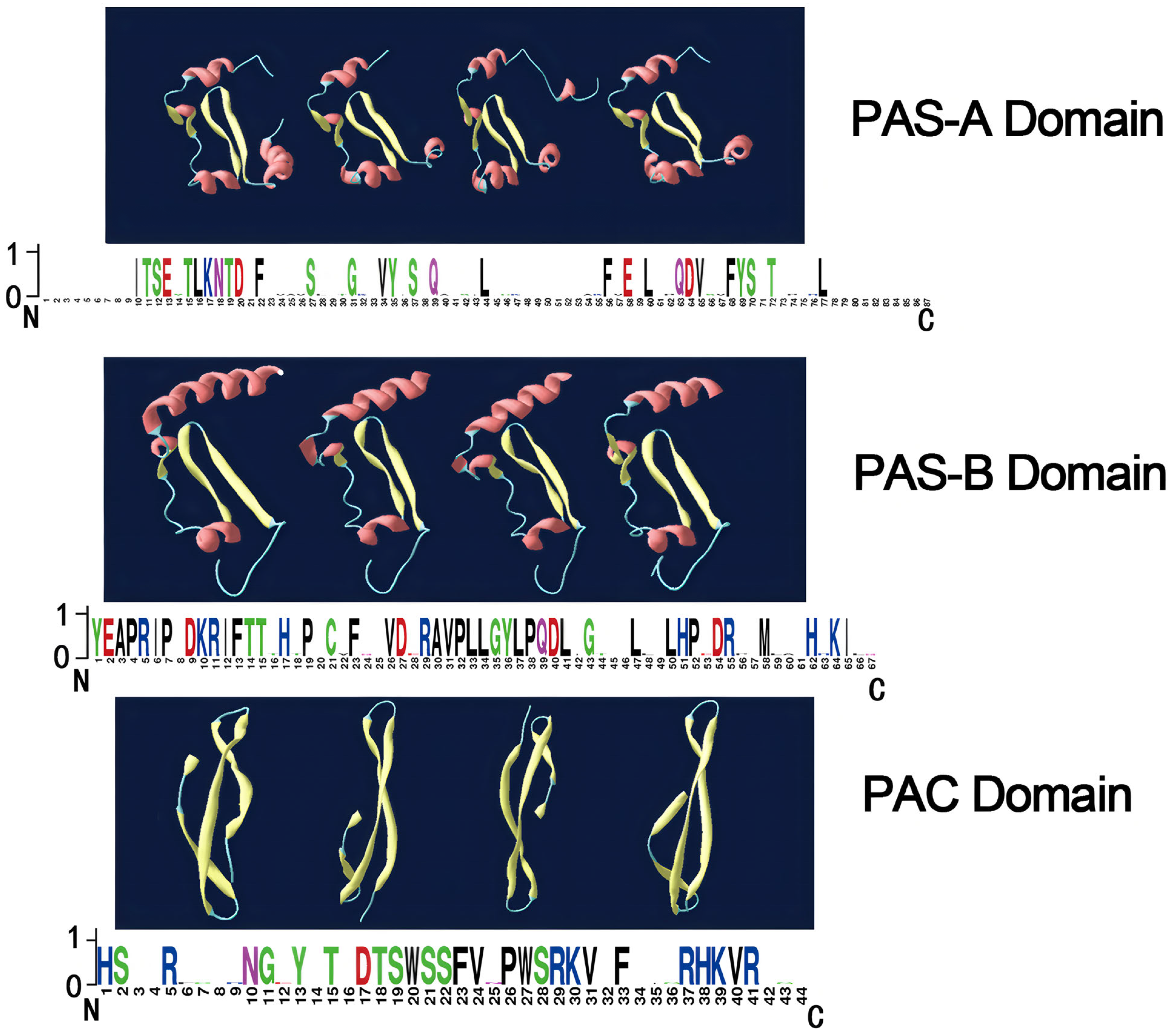
3.1. Molecular Cloning and Tissue Distribution of the Channel Catfish Period Family Genes
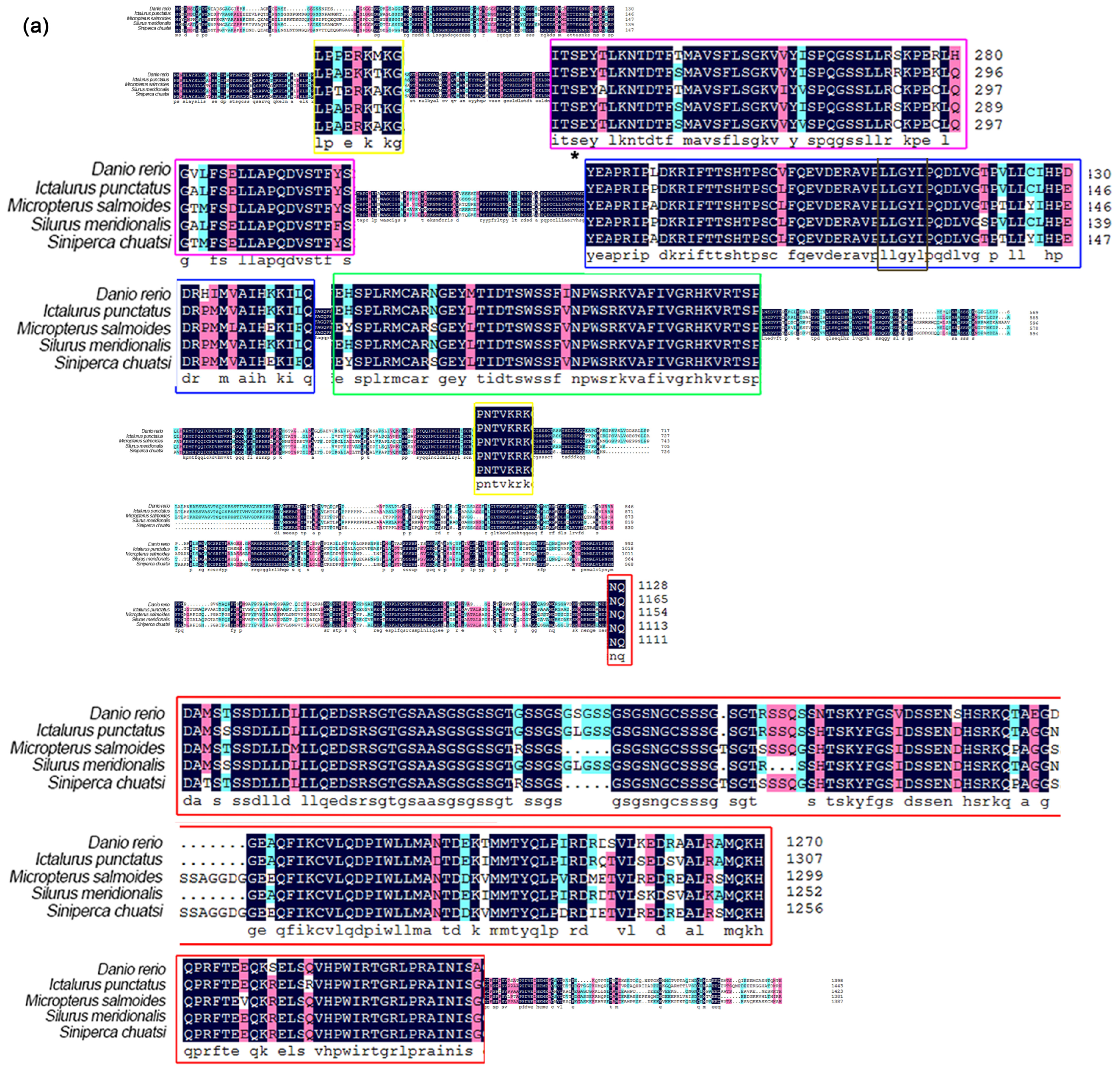
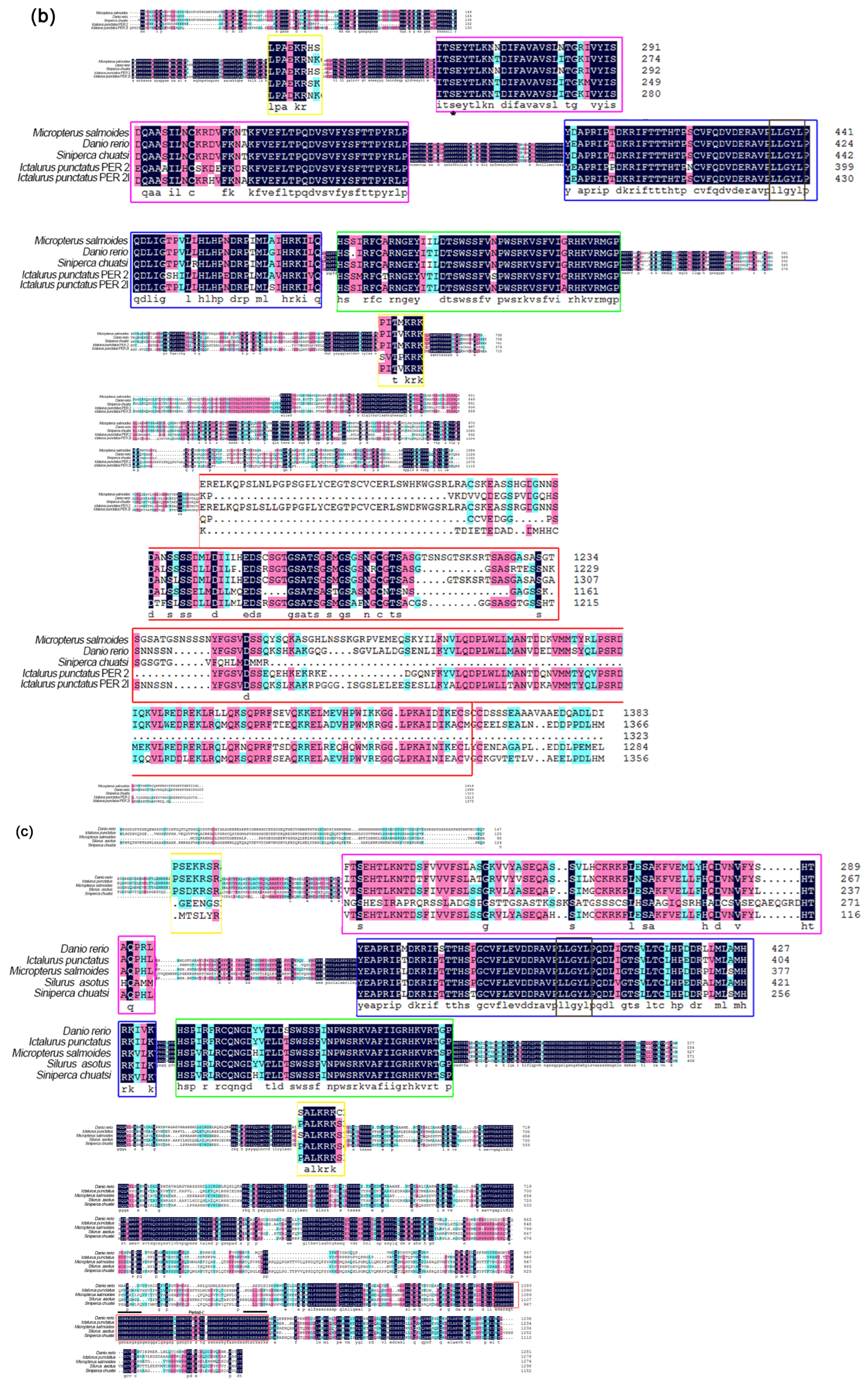
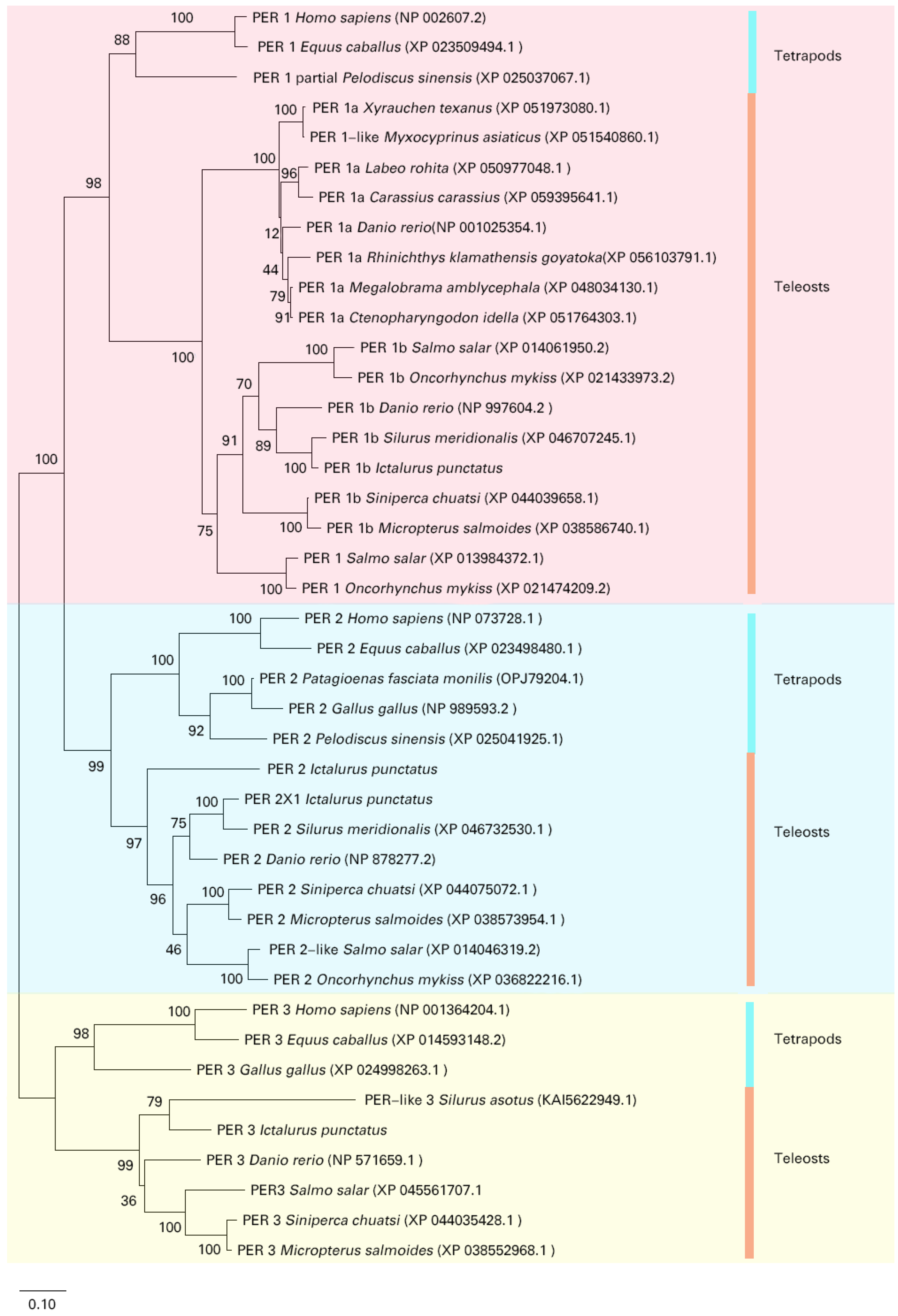
3.2. Phylogenetic Analysis and Multiple Alignment
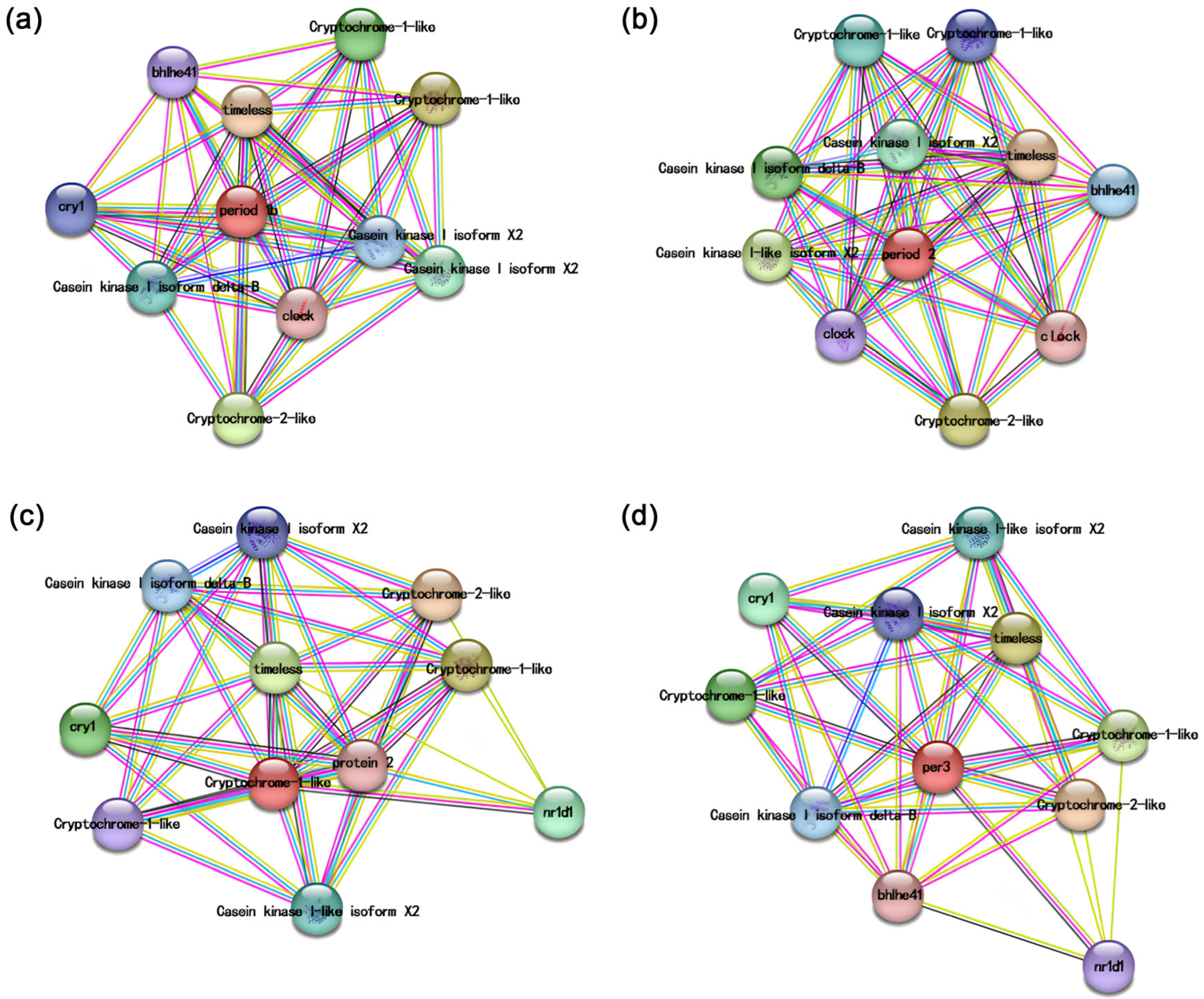
3.3. Protein–Protein Interaction Network Analysis
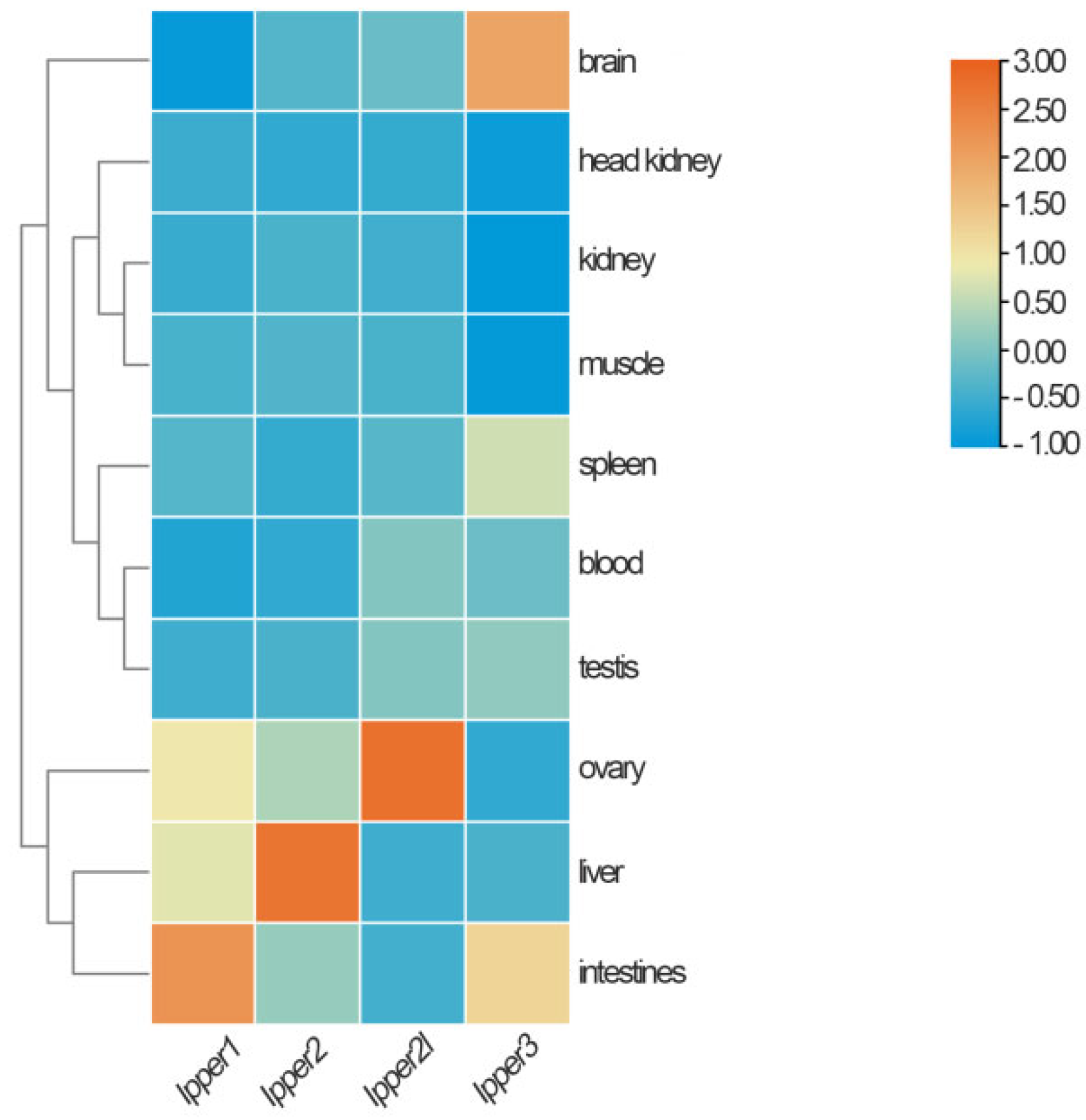
3.4. Tissue Distribution of I. punctatus Period Genes
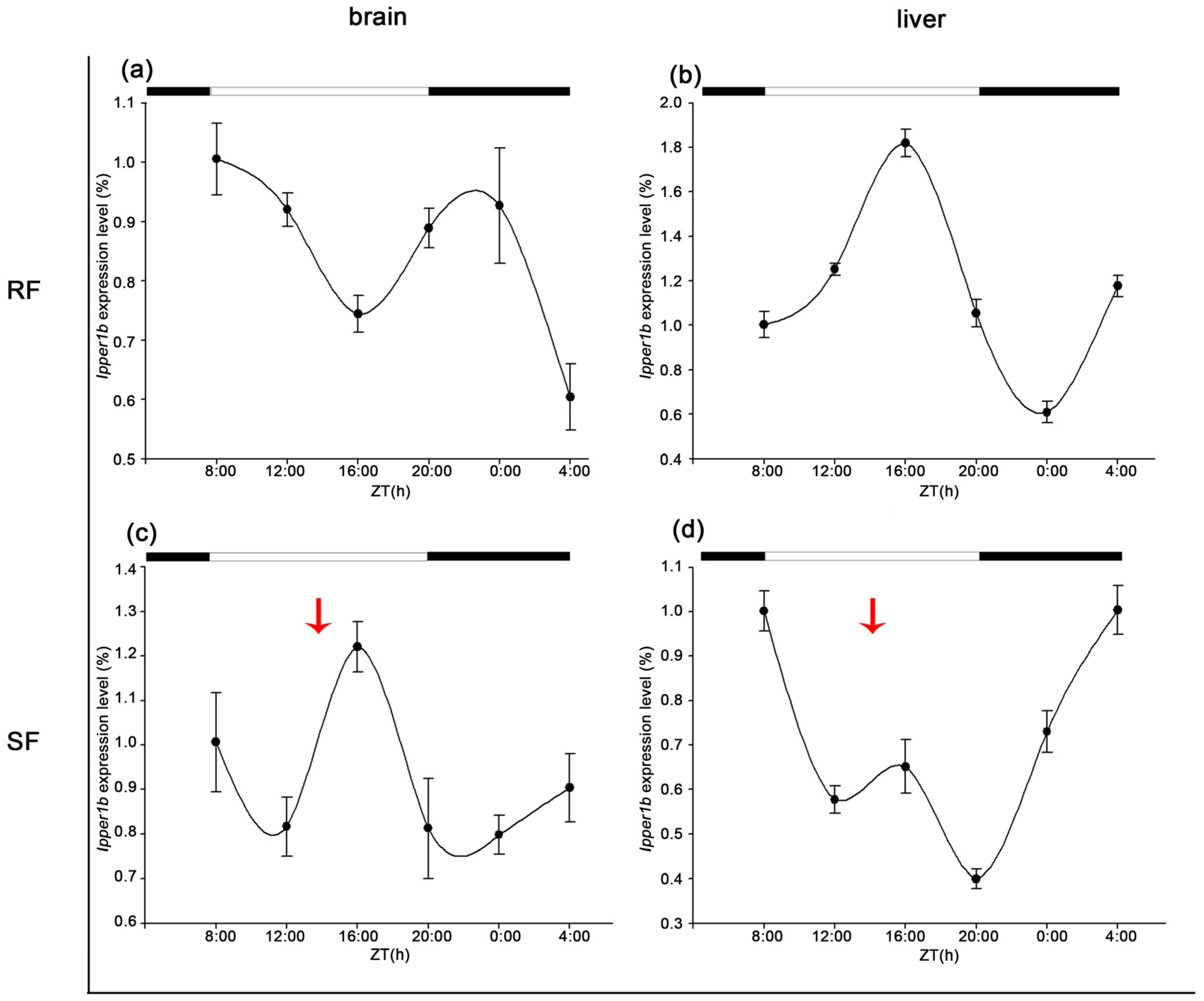
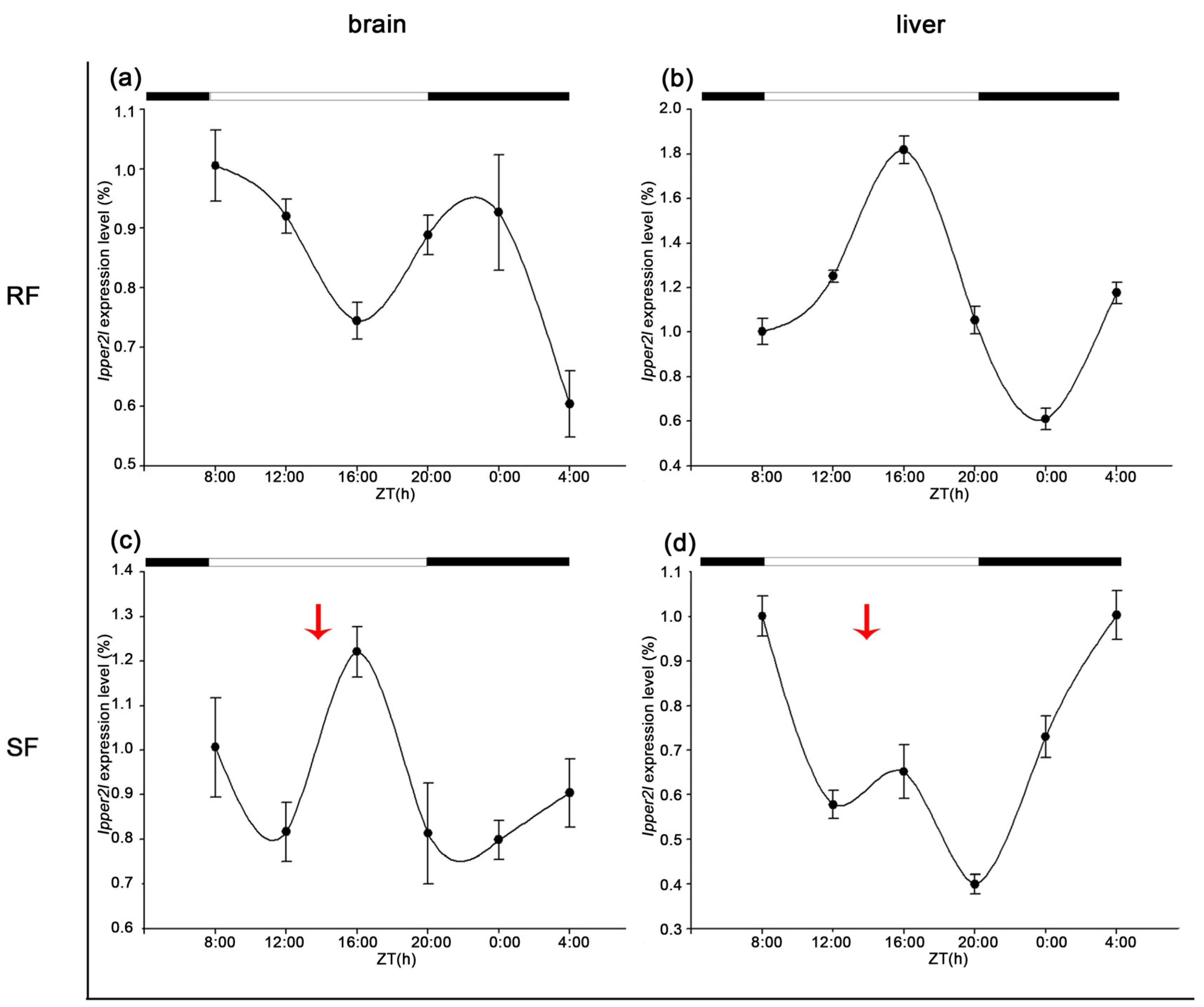
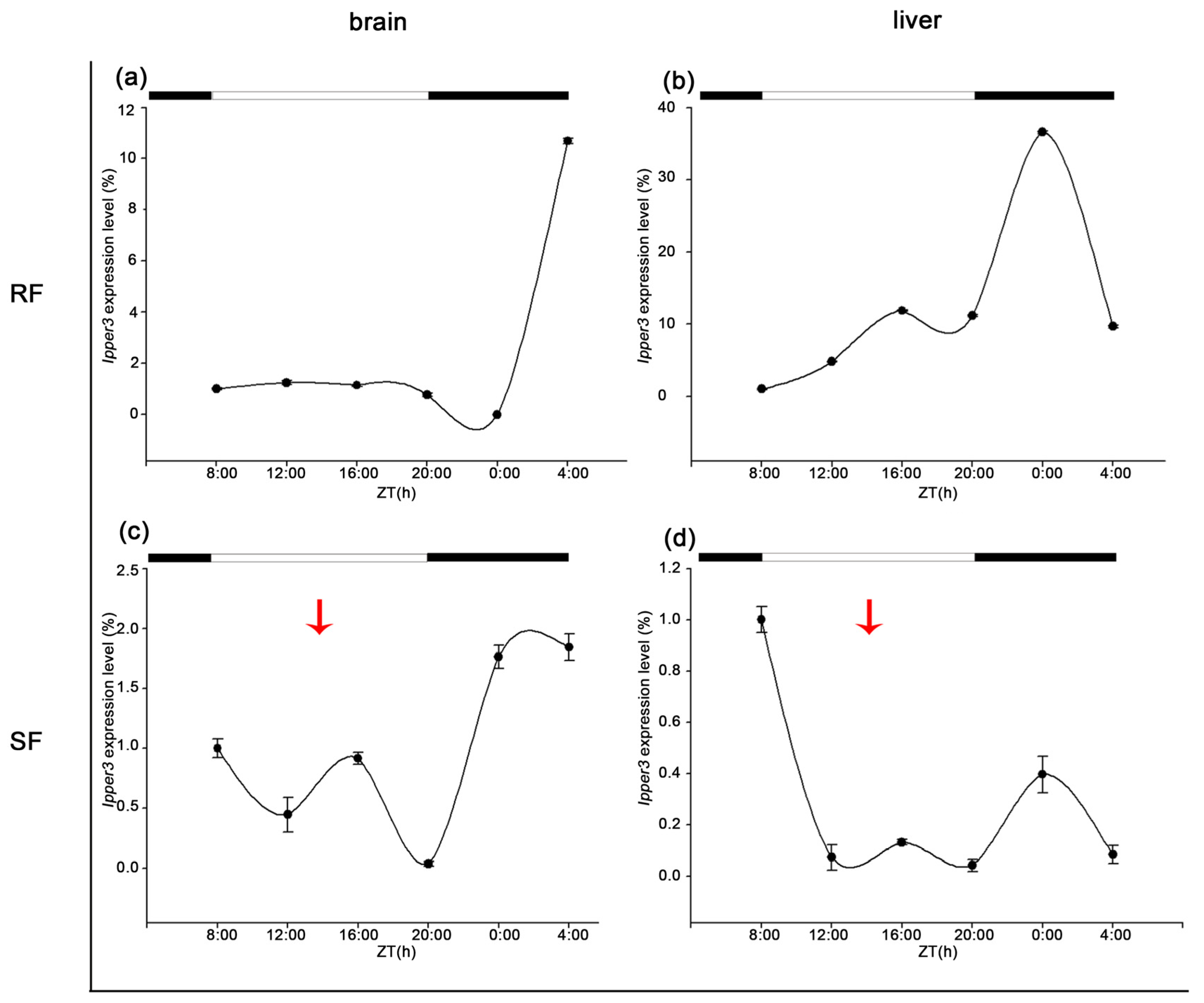
3.5. Diurnal Expression of Period Family Genes in the Brain and Liver
4. Discussion
4.1. Evolutionary Divergence and Functional Specialization of Channel Catfish Period Genes
4.2. Period Family Gene Function Is Related to Energy Metabolism and Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohawk, J.A.; Green, C.B. Central and peripheral circadian clocks in mammals. Annu. Rev. Neurosci. 2012, 35, 445–462. [Google Scholar] [CrossRef] [PubMed]
- Reppert, S.M.; Weaver, D.R. Coordination of circadian timing in mammals. Nature 2002, 418, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular architecture of the mammalian circadian clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef]
- Reick, M.; Garcia, J.A. An analog of clock operative in the mammalian forebrain. Science 2001, 293, 506–509. [Google Scholar] [CrossRef]
- Gekakis, N.; Staknis, D. Role of the CLOCK protein in the mammalian circadian mechanism. Science 1998, 280, 1564–1569. [Google Scholar] [CrossRef]
- Garbe, D.S.; Fang, Y. Cooperative interaction between phosphorylation sites on PERIOD maintains circadian period in Drosophila. PLoS Genet. 2013, 9, e1003749. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Pelicano, H. The circadian gene Period2 plays an important role in tumor suppression and DNA damage response in vivo. Cell 2002, 111, 41–50. [Google Scholar] [CrossRef]
- Von Schantz, M.; Jenkins, A. Evolutionary history of the vertebrate period genes. J. Mol. Evol. 2006, 62, 701–707. [Google Scholar] [CrossRef]
- Wang, H. Comparative analysis of period genes in teleost fish genomes. J. Mol. Evol. 2008, 67, 29–40. [Google Scholar] [CrossRef]
- Chen, R.; Schirmer, A. Rhythmic PER abundance defines a critical nodal point for negative feedback within the circadian clock mechanism. Mol. Cell 2009, 36, 417–430. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; López-Olmeda, J.F. Environmental cycles, melatonin, and circadian control of stress response in fish. Front. Endocrinol. 2019, 10, 279. [Google Scholar] [CrossRef] [PubMed]
- Nie, K.; Wang, K. Effects of circadian clock protein Per1b on zebrafish visual functions. Chronobiol. Int. 2018, 35, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Serin, I.; Pehlivan, S. A new clock is running for multiple myeloma: Circadian clock protein-Period 3 (PER-3) polymorphism. Balk. J. Med. Genet. 2023, 25, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Guess, J.; Burch, J.B. Circadian disruption, Per3, and human cytokine secretion. Integr. Cancer Ther. 2009, 8, 329–336. [Google Scholar] [CrossRef]
- Bishehsari, F.; Voigt, R.M. Circadian rhythms and the gut microbiota: From the metabolic syndrome to cancer. Nat. Rev. Endocrinol. 2020, 16, 731–739. [Google Scholar] [CrossRef]
- Damiola, F.; Le Minh, N. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000, 14, 2950–2961. [Google Scholar] [CrossRef]
- Wang, M.; Zhong, Z. The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor α (Rorα). J. Biol. Chem. 2015, 290, 4367–4382. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef]
- Takahashi, J.S.; Hong, H.-K. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Dunlap, J.C. Molecular bases for circadian clocks. Cell 1999, 96, 271–290. [Google Scholar] [CrossRef]
- Zeng, H.; Qian, Z. A light-entrainment mechanism for the Drosophila circadian clock. Nature 1996, 380, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Postlethwait, J.H.; Woods, I.G. Zebrafish comparative genomics and the origins of vertebrate chromosomes. Genome Res. 2000, 10, 1890–1902. [Google Scholar] [CrossRef] [PubMed]
- Cermakian, N.; Whitmore, D. Asynchronous oscillations of two zebrafish CLOCK partners reveal differential clock control and function. Proc. Natl. Acad. Sci. USA 2000, 97, 4339–4344. [Google Scholar] [CrossRef]
- Taylor, J.S.; Van de Peer, Y. Comparative genomics provides evidence for an ancient genome duplication event in fish. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 1661–1679. [Google Scholar] [CrossRef]
- Force, A.; Lynch, M. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 1999, 151, 1531–1545. [Google Scholar] [CrossRef]
- Schmutz, J.; Cannon, S.B. Genome sequence of the palaeopolyploid soybean. Nature 2010, 463, 178–183. [Google Scholar] [CrossRef]
- Kaneko, M.; Cahill, G.M. Light-dependent development of circadian gene expression in transgenic zebrafish. PLoS Biol. 2005, 3, e34. [Google Scholar] [CrossRef] [PubMed]
- Velarde, E.; Haque, R. Circadian clock genes of goldfish, Carassius auratus: cDNA cloning and rhythmic expression of period and cryptochrome transcripts in retina, liver, and gut. J. Biol. Rhythms 2009, 24, 104–113. [Google Scholar] [CrossRef]
- Di Rosa, V.; López-Olmeda, J.F. Daily rhythms of the expression of key genes involved in steroidogenesis and gonadal function in zebrafish. PLoS ONE 2016, 11, e0157716. [Google Scholar] [CrossRef]
- Xu, P.; Wang, Y. Genome sequence and genetic diversity of the common carp, Cyprinus carpio. Nat. Genet. 2014, 46, 1212–1219. [Google Scholar] [CrossRef]
- Toloza-Villalobos, J.; Arroyo, J.I.; Opazo, J.C. The circadian clock of teleost fish: A comparative analysis reveals distinct fates for duplicated genes. J. Mol. Evol. 2015, 80, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Nisembaum, L.G.; Velarde, E. Light-dark cycle and feeding time differentially entrains the gut molecular clock of the goldfish (Carassius auratus). Chronobiol. Int. 2012, 29, 665–673. [Google Scholar] [CrossRef]
- Sánchez-Vázquez, F.J.; Madrid, J.A. Demand feeding and locomotor circadian rhythms in the goldfish, Carassius auratus: Dual and independent phasing. Physiol. Behav. 1996, 60, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Volkoff, H.; Rønnestad, I. Effects of temperature on feeding and digestive processes in fish. Temperature 2020, 7, 307–320. [Google Scholar] [CrossRef] [PubMed]
- Paredes, M.F.; Sorrells, S.F. Brain size and limits to adult neurogenesis. J. Comp. Neurol. 2016, 524, 646–664. [Google Scholar] [CrossRef]
- López-Olmeda, J.F.; Tartaglione, E.V. Feeding entrainment of food-anticipatory activity and Per1 expression in the brain and liver of zebrafish under different lighting and feeding conditions. Chronobiol. Int. 2010, 27, 1380–1400. [Google Scholar] [CrossRef]
- Kalsbeek, A.; la Fleur, S. Circadian disruption and SCN control of energy metabolism. FEBS Lett. 2011, 585, 1412–1426. [Google Scholar] [CrossRef]
- Mistlberger, R.E. Food-anticipatory circadian rhythms: Concepts and method. Eur. J. Neurosci. 2009, 30, 1718–1729. [Google Scholar] [CrossRef]
- Costa, L.S.; Serrano, I. Circadian rhythms of clock gene expression in Nile tilapia (Oreochromis niloticus) central and peripheral tissues: Influence of different lighting and feeding conditions. J. Comp. Physiol. B 2016, 186, 775–785. [Google Scholar] [CrossRef]
- Escobar, C.; Martínez-Merlos, M.T.; Angeles-Castellanos, M.; del Carmen Miñana, M.; Buijs, R.M. Unpredictable feeding schedules unmask a system for daily resetting of behavioural and metabolic food entrainment. Eur. J. Neurosci. 2007, 26, 2804–2814. [Google Scholar] [CrossRef]
- Davidson, A.J.; Castanon-Cervantes, O. Visualizing jet lag in the mouse suprachiasmatic nucleus and peripheral circadian timing system. Eur. J. Neurosci. 2009, 29, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Zavala, J.B.; Molina-Aguilar, C. Daytime restricted feeding modifies the daily regulation of fatty acid β-oxidation and the lipoprotein profile in rats. Br. J. Nutr. 2017, 117, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Asher, G.; Schibler, U. Crosstalk between components of circadian and metabolic cycles in mammals. Cell Metab. 2011, 13, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Metabolism and cancer: The circadian clock connection. Nat. Rev. Cancer 2009, 9, 886–896. [Google Scholar] [CrossRef]
- Peek, C.B.; Levine, D.C. Circadian clock interaction with HIF1α mediates oxygenic metabolism and anaerobic glycolysis in skeletal muscle. Cell Metab. 2017, 25, 86–92. [Google Scholar] [CrossRef]
- Delaunay, F.; Thisse, C.; Thisse, B.; Laudet, V. Differential regulation of period 2 and period 3 expression during development of the zebrafish circadian clock. Gene Expr. Patterns 2003, 3, 319–324. [Google Scholar] [CrossRef]
- Vera, L.M.; Negrini, P. Light and feeding entrainment of the molecular circadian clock in a marine teleost (Sparus aurata). Chronobiol. Int. 2013, 30, 649–661. [Google Scholar] [CrossRef]
- Hatori, M.; Vollmers, C. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012, 15, 848–860. [Google Scholar] [CrossRef]
- Ali, M.; Nicieza, A. Compensatory growth in fishes: A response to growth depression. Fish Fish. 2003, 4, 147–190. [Google Scholar] [CrossRef]


| Properties | IpPER1b | IpPER2 | IpPER2l | IpPER3 |
|---|---|---|---|---|
| M (kDa) | 154.56 | 143.85 | 149.31 | 138.48 |
| pI | 5.81 | 5.98 | 5.97 | 5.75 |
| GRAVY | −0.573 | −0.699 | −0.615 | −0.604 |
| Amino acid number | 1443 | 1313 | 1373 | 1278 |
| Amino acid types | Percentage (%) | |||
| Aliphatic | 30.15 | 27.04 | 29.35 | 30.36 |
| Aromatic | 4.57 | 6.40 | 5.97 | 5.16 |
| Sulphur | 4.50 | 4.57 | 3.71 | 4.38 |
| Basic | 11.16 | 12.11 | 12.02 | 12.28 |
| Acidic | 19.33 | 21.86 | 22.00 | 20.81 |
| Aliphatic hydroxyl | 21.07 | 19.50 | 20.03 | 18.15 |
| tRNA synthetase class I | 40.06 | 41.20 | 40.06 | 41.08 |
| tRNA synthetase class II | 59.94 | 58.80 | 59.94 | 58.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Zhang, S.; Chen, X.; Wang, M.; Zhong, L.; Duan, Y.; Xie, B.; Tang, L.; Cheng, Y. Molecular Characterization and Feeding-Associated Expression Dynamics of the Period Gene Family in Channel Catfish (Ictalurus punctatus). Curr. Issues Mol. Biol. 2025, 47, 438. https://doi.org/10.3390/cimb47060438
Liu H, Zhang S, Chen X, Wang M, Zhong L, Duan Y, Xie B, Tang L, Cheng Y. Molecular Characterization and Feeding-Associated Expression Dynamics of the Period Gene Family in Channel Catfish (Ictalurus punctatus). Current Issues in Molecular Biology. 2025; 47(6):438. https://doi.org/10.3390/cimb47060438
Chicago/Turabian StyleLiu, Hongyan, Shiyong Zhang, Xiaohui Chen, Minghua Wang, Liqiang Zhong, Yongqiang Duan, Bingjie Xie, Luyu Tang, and Yi Cheng. 2025. "Molecular Characterization and Feeding-Associated Expression Dynamics of the Period Gene Family in Channel Catfish (Ictalurus punctatus)" Current Issues in Molecular Biology 47, no. 6: 438. https://doi.org/10.3390/cimb47060438
APA StyleLiu, H., Zhang, S., Chen, X., Wang, M., Zhong, L., Duan, Y., Xie, B., Tang, L., & Cheng, Y. (2025). Molecular Characterization and Feeding-Associated Expression Dynamics of the Period Gene Family in Channel Catfish (Ictalurus punctatus). Current Issues in Molecular Biology, 47(6), 438. https://doi.org/10.3390/cimb47060438






