Fecal Microbiota Transplant for Hematologic and Oncologic Diseases: Principle and Practice
Abstract
:Simple Summary
Abstract
1. Introduction
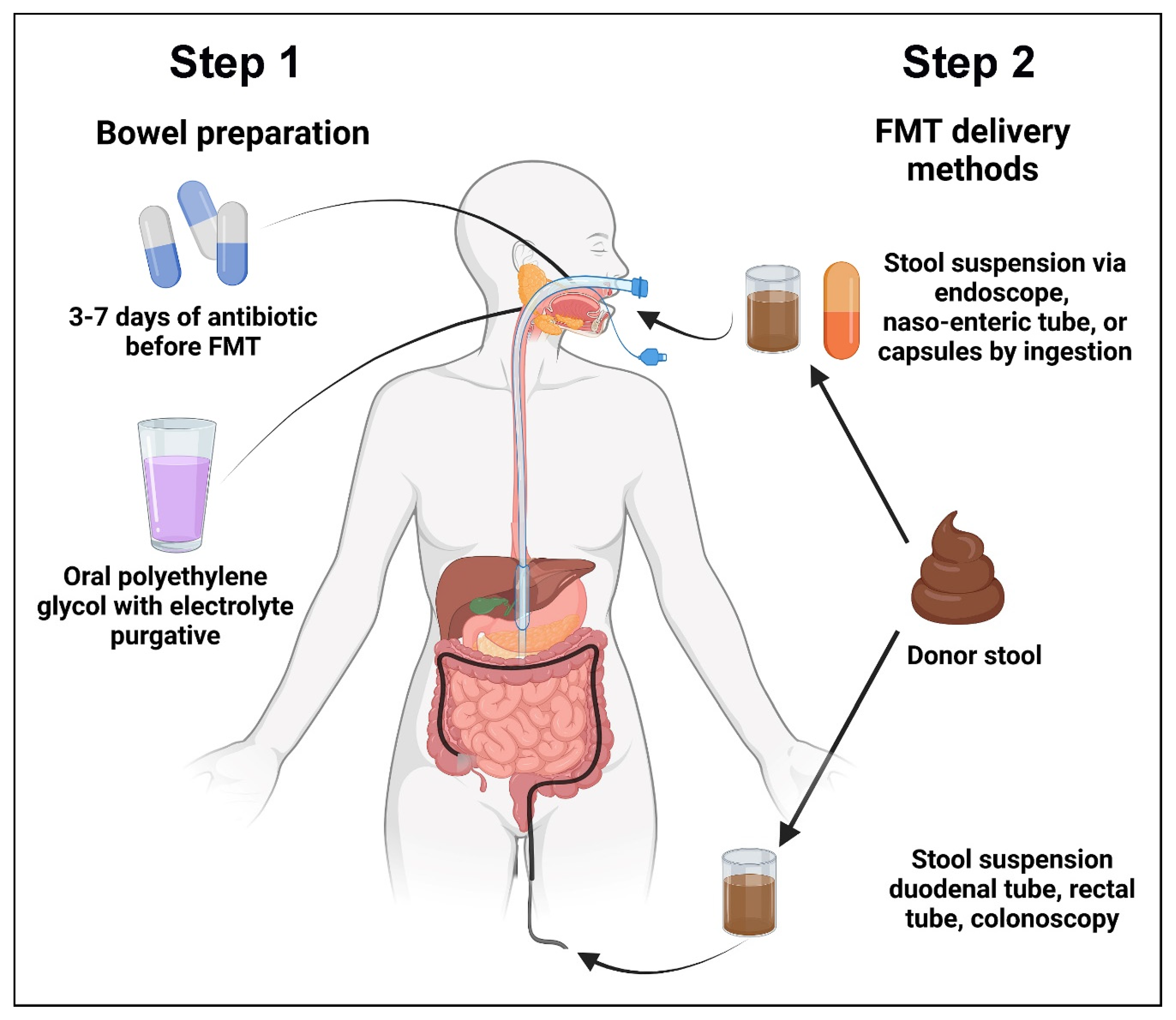
2. Steps and Biologic Effects of FMT
2.1. The Steps of FMT
2.2. Biologic Consequences of FMT
3. CDI in Patients with Hematologic and Oncologic Diseases
3.1. Factors Predisposing Patients to CDI
3.2. Use of FMT in Hematologic and Oncologic Patients outside Treatment of CDI
3.3. Ongoing FMT Studies in Patients with Hematologic and Oncologic Diseases
3.4. Harnessing the Potentials of FMT for Future Studies in Hematologic and Oncologic Diseases
4. Challenges Facing FMT Use in Hematologic and Oncologic Patients
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, L.; Duffy, A. Factors influencing the gut microbiota, inflammation, and Type 2 Diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brandt, L.J. Fecal microbiota transplant: Respice, Adspice, Prospice. J. Clin. Gastroenterol. 2015, 49, S65–S68. [Google Scholar] [CrossRef] [PubMed]
- Lewin, R.A. More on merde. Perspect. Biol. Med. 2001, 44, 594–607. [Google Scholar] [CrossRef] [PubMed]
- Eiseman, B.; Silen, W.; Bascom, G.S.; Kauvar, A.J. Fecal enema as an adjunct in the treatment of pseudomembranous enterocolitis. Surgery 1958, 44, 854–859. [Google Scholar]
- Schwan, S.; Sjolin, U.; Trottestam, B.; Aronsson, B. Relapsing Clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet 1983, 2, 845. [Google Scholar] [CrossRef]
- Mamoon, L.; Olesen, S.W. Fecal microbiota transplant annually and their positive clinical impact. Clin. Transl. Gastroenterol. 2020, 11, e00247. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Terveer, E.M.; Dahlerup, J.F.; Erikstrup, C.; Arkkila, P.; Vehreschild, M.J.; Ianiro, G.; Gasbarrini, A.; Sokol, H.; Kump, P.K.; et al. The use of Faecal Microbiota Transplantation (FMT) in Europe: A Europe-wide survey. Lancet Reg. Health Eur. 2021, 9, 100181. [Google Scholar] [CrossRef]
- Ji, S.K.; Yan, H.; Jiang, T.; Guo, C.Y.; Liu, J.J.; Dong, S.Z.; Yang, K.L.; Wang, Y.J.; Cao, Z.J.; Li, S.L. Preparing the gut with antibiotics enhances gut microbiota reprogramming efficiency by promoting Xenomicrobiota colonization. Front. Microbiol. 2017, 8, 1208. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Singh, P.; Alm, E.J.; Kelley, J.M.; Cheng, V.; Smith, M.; Kassam, Z.; Nee, J.; Iturrino, J.; Lembo, A. Effects of antibiotic pretreatment on bacterial engraftment after fecal microbiota transplant (FMT) in IBS-D. Gut Microbes 2022, 14, e2020067. [Google Scholar] [CrossRef] [PubMed]
- Fecal Microbiota Transplantation-Standardization Study Group. Nanjing consensus on methodology of washed microbiota transplantation. Chin. Med. J. 2020, 133, 2330–2332. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Xiang, J.; He, Z.; Zhang, T.; Xu, L.; Cui, B.; Li, P.; Huang, G.; Ji, G.; Nie, Y.; et al. Colonic transendoscopic enteral tubing: A novel way of transplanting fecal microbiota. Endosc. Int. Open 2016, 4, E610–E613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating Clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Luo, Y.; Walsh, S.; Grinspan, A. Oral fecal microbiota transplant capsules are safe and effective for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. J. Clin. Gastroenterol. 2021, 55, 300–308. [Google Scholar] [CrossRef]
- Baunwall, S.M.D.; Lee, M.M.; Eriksen, M.K.; Mullish, B.H.; Marchesi, J.R.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation for recurrent Clostridioides difficile infection: An updated systematic review and meta-analysis. EClinicalMedicine 2020, 29–30, 100642. [Google Scholar] [CrossRef] [PubMed]
- Spreckley, E.; Murphy, K.G. The L-cell in nutritional sensing and the regulation of appetite. Front. Nutr. 2015, 2, 23. [Google Scholar] [CrossRef] [Green Version]
- Bagdasarian, N.; Rao, K.; Malani, P.N. Diagnosis and treatment of Clostridium difficile in adults: A systematic review. JAMA 2015, 313, 398–408. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- Grigorescu, I.; Dumitrascu, D.L. Implication of gut microbiota in diabetes mellitus and obesity. Acta Endocrinol. 2016, 12, 206–214. [Google Scholar] [CrossRef]
- Alang, N.; Kelly, C.R. Weight gain after fecal microbiota transplantation. Open Forum Infect. Dis. 2015, 2, ofv004. [Google Scholar] [CrossRef] [PubMed]
- Napolitano, M.; Covasa, M. Microbiota transplant in the treatment of obesity and diabetes: Current and future perspectives. Front. Microbiol. 2020, 11, 590370. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J.; et al. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youngster, I.; Mahabamunuge, J.; Systrom, H.K.; Sauk, J.; Khalili, H.; Levin, J.; Kaplan, J.L.; Hohmann, E.L. Oral, frozen fecal microbiota transplant (FMT) capsules for recurrent Clostridium difficile infection. BMC Med. 2016, 14, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya-Kanamori, L.; Doi, S.A.; Paterson, D.L.; Helms, S.K.; Yakob, L.; McKenzie, S.J.; Garborg, K.; Emanuelsson, F.; Stollman, N.; Kronman, M.P.; et al. Upper versus lower gastrointestinal delivery for transplantation of fecal microbiota in recurrent or refractory Clostridium difficile infection: A collaborative analysis of individual patient data from 14 studies. J. Clin. Gastroenterol. 2017, 51, 145–150. [Google Scholar] [CrossRef]
- Lui, R.N.; Wong, S.H.; Lau, L.H.S.; Chan, T.T.; Cheung, K.C.Y.; Li, A.; Chin, M.L.; Tang, W.; Ching, J.Y.L.; Lam, K.L.Y.; et al. Faecal microbiota transplantation for treatment of recurrent or refractory Clostridioides difficile infection in Hong Kong. Hong Kong Med. J. 2019, 25, 178–182. [Google Scholar]
- Ponte, A.; Pinho, R.; Mota, M.; Silva, J.; Vieira, N.; Oliveira, R.; Rodrigues, J.; Sousa, M.; Sousa, I.; Carvalho, J. Fecal microbiota transplantation in refractory or recurrent Clostridium difficile infection: A real-life experience in a non-academic center. Rev. Esp. Enferm. Dig. 2018, 110, 311–315. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.R.; Yen, E.F.; Grinspan, A.M.; Kahn, S.A.; Atreja, A.; Lewis, J.D.; Moore, T.A.; Rubin, D.T.; Kim, A.M.; Serra, S.; et al. Fecal microbiota transplantation is highly effective in real-world practice: Initial results from the FMT National Registry. Gastroenterology 2021, 160, 183–192.e3. [Google Scholar] [CrossRef]
- Singh, R.; van Nood, E.; Nieuwdorp, M.; van Dam, B.; ten Berge, I.J.; Geerlings, S.E.; Bemelman, F.J. Donor feces infusion for eradication of extended spectrum beta-lactamase producing Escherichia coli in a patient with end stage renal disease. Clin. Microbiol. Infect. 2014, 20, O977–O978. [Google Scholar] [CrossRef] [Green Version]
- Dinh, A.; Fessi, H.; Duran, C.; Batista, R.; Michelon, H.; Bouchand, F.; Lepeule, R.; Vittecoq, D.; Escaut, L.; Sobhani, I.; et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: A prospective comparative study. J. Hosp. Infect. 2018, 99, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Imdad, A.; Nicholson, M.R.; Tanner-Smith, E.E.; Zackular, J.P.; Gomez-Duarte, O.G.; Beaulieu, D.B.; Acra, S. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst. Rev. 2018, 11, CD012774. [Google Scholar] [CrossRef] [PubMed]
- Paramsothy, S.; Paramsothy, R.; Rubin, D.T.; Kamm, M.A.; Kaakoush, N.O.; Mitchell, H.M.; Castaño-Rodríguez, N. Faecal microbiota transplantation for inflammatory bowel disease: A systematic review and meta-analysis. J. Crohn’s Colitis 2017, 11, 1180–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Zhang, T.; Xiao, Y.; Tian, L.; Cui, B.; Ji, G.; Liu, Y.Y.; Zhang, F. Timing for the second fecal microbiota transplantation to maintain the long-term benefit from the first treatment for Crohn’s disease. Appl. Microbiol. Biotechnol. 2019, 103, 349–360. [Google Scholar] [CrossRef] [Green Version]
- Mazzawi, T.; Lied, G.A.; Sangnes, D.A.; El-Salhy, M.; Hov, J.R.; Gilja, O.H.; Hatlebakk, J.G.; Hausken, T. The kinetics of gut microbial community composition in patients with irritable bowel syndrome following fecal microbiota transplantation. PLoS ONE 2018, 13, e0194904. [Google Scholar] [CrossRef] [Green Version]
- Rodiño-Janeiro, B.K.; Vicario, M.; Alonso-Cotoner, C.; Pascua-García, R.; Santos, J. A review of microbiota and irritable bowel syndrome: Future in therapies. Adv. Ther. 2018, 35, 289–310. [Google Scholar] [CrossRef] [Green Version]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota transfer therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Berer, K.; Gerdes, L.A.; Cekanaviciute, E.; Jia, X.; Xiao, L.; Xia, Z.; Liu, C.; Klotz, L.; Stauffer, U.; Baranzini, S.E.; et al. Gut microbiota from multiple sclerosis patients enables spontaneous autoimmune encephalomyelitis in mice. Proc. Natl. Acad. Sci. USA 2017, 114, 10719–10724. [Google Scholar] [CrossRef] [Green Version]
- Borody, T.; Leis, S.; Campbell, J.; Torres, M.; Nowak, A. Fecal microbiota transplantation (FMT) in multiple sclerosis (MS). Am. J. Gastroenterol. 2011, 106, S352. [Google Scholar] [CrossRef]
- Sun, M.F.; Zhu, Y.L.; Zhou, Z.L.; Jia, X.B.; Xu, Y.D.; Yang, Q.; Cui, C.; Shen, Y.Q. Neuroprotective effects of fecal microbiota transplantation on MPTP-induced Parkinson’s disease mice: Gut microbiota, glial reaction and TLR4/TNF-α signaling pathway. Brain Behav. Immun. 2018, 70, 48–60. [Google Scholar] [CrossRef]
- McGlone, S.M.; Bailey, R.R.; Zimmer, S.M.; Popovich, M.J.; Tian, Y.; Ufberg, P.; Muder, R.R.; Lee, B.Y. The economic burden of Clostridium difficile. Clin. Microbiol. Infect. 2012, 18, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willems, L.; Porcher, R.; Lafaurie, M.; Casin, I.; Robin, M.; Xhaard, A.; Andreoli, A.L.; Rodriguez-Otero, P.; Dhedin, N.; Socié, G.; et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplantation: Incidence, risk factors, and outcome. Biol. Blood Marrow Transplant. 2012, 18, 1295–1301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamboj, M.; Xiao, K.; Kaltsas, A.; Huang, Y.T.; Sun, J.; Chung, D.; Wu, S.; Sheahan, A.; Sepkowitz, K.; Jakubowski, A.A.; et al. Clostridium difficile infection after allogeneic hematopoietic stem cell transplant: Strain diversity and outcomes associated with NAP1/027. Biol. Blood Marrow Transplant. 2014, 20, 1626–1633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, A.; Glatt, A.E. Clostridium difficile infection associated with antineoplastic chemotherapy: A review. Clin. Infect. Dis. 1993, 17, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Kamthan, A.G.; Bruckner, H.W.; Hirschman, S.Z.; Agus, S.G. Clostridium difficile diarrhea induced by cancer chemotherapy. Arch. Intern. Med. 1992, 152, 1715–1717. [Google Scholar] [CrossRef] [PubMed]
- Agha, A.; Sehgal, A.; Lim, M.J.; Weber, D.; Hou, J.Z.; Farah, R.; Raptis, A.; Im, A.; Dorritie, K.; Marks, S.; et al. Peri-transplant Clostridium difficile infections in patients undergoing allogeneic hematopoietic progenitor cell transplant. Am. J. Hematol. 2016, 91, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Schmidt-Hieber, M.; Bierwirth, J.; Buchheidt, D.; Cornely, O.A.; Hentrich, M.; Maschmeyer, G.; Schalk, E.; Vehreschild, J.J.; Vehreschild, M.J.G.T.; AGIHO Working Group. Diagnosis and management of gastrointestinal complications in adult cancer patients: 2017 updated evidence-based guidelines of the Infectious Diseases Working Party (AGIHO) of the German Society of Hematology and Medical Oncology (DGHO). Ann. Hematol. 2018, 97, 31–49. [Google Scholar] [CrossRef] [Green Version]
- Yoon, Y.K.; Kim, M.J.; Sohn, J.W.; Kim, H.S.; Choi, Y.J.; Kim, J.S.; Kim, S.T.; Park, K.H.; Kim, S.J.; Kim, B.S.; et al. Predictors of mortality attributable to Clostridium difficile infection in patients with underlying malignancy. Support. Care Cancer 2014, 22, 2039–2048. [Google Scholar] [CrossRef]
- Viscoli, C.; on behalf of the EORTC International Antimicrobial Therapy Group. Management of infection in cancer patients: Studies of the EORTC International Antimicrobial Therapy Group (IATG). Eur. J. Cancer 2002, 38 (Suppl. 4), 82–87. [Google Scholar] [CrossRef]
- Krantz, E.M.; Zier, J.; Stohs, E.; Ogimi, C.; Sweet, A.; Marquis, S.; Klaassen, J.; Pergam, S.A.; Liu, C. Antibiotic prescribing and respiratory viral testing for acute upper respiratory infections among adult patients at an ambulatory cancer center. Clin. Infect. Dis. 2020, 70, 1421–1428. [Google Scholar] [CrossRef]
- Janarthanan, S.; Ditah, I.; Adler, D.G.; Ehrinpreis, M.N. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: A meta-analysis. Am. J. Gastroenterol. 2012, 107, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Staley, C.; Weingarden, A.R.; Khoruts, A.; Sadowsky, M.J. Interaction of gut microbiota with bile acid metabolism and its influence on disease states. Appl. Microbiol. Biotechnol. 2017, 101, 47–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Meng, J.; Zhang, L.; Johnson, T.; Chen, C.; Roy, S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci. Rep. 2018, 8, 3596. [Google Scholar] [CrossRef] [Green Version]
- Lichtbroun, M.; Jafri, F.; Chaudhary, R.S.; Batool, S.; Ahmed, J.; Lim, S.H. High incidence of healthcare facility-acquired Clostridium difficile infections in chronic opioid users. J. Intern. Med. 2021, 289, 129–130. [Google Scholar] [CrossRef] [PubMed]
- Eze, P.; Balsells, E.; Kyaw, M.H.; Nair, H. Risk factors for Clostridium difficile infections—An overview of the evidence base and challenges in data synthesis. J. Glob. Health. 2017, 7, 010417. [Google Scholar] [CrossRef]
- Ouellette, A.J. Paneth cell α-defensin synthesis and function. In Antimicrobial Peptides and Human Disease; Current Topics in Microbiology and Immunology; Springer: Berlin, Germany, 2006; Volume 306, pp. 1–25. [Google Scholar]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv. Immunol. 2014, 121, 91–119. [Google Scholar] [PubMed]
- Seong, S.Y.; Matzinger, P. Hydrophobicity: An ancient damage-associated molecular pattern that initiates innate immune responses. Nat. Rev. Immunol. 2004, 4, 469–478. [Google Scholar] [CrossRef]
- Kakihana, K.; Fujioka, Y.; Suda, W.; Najima, Y.; Kuwata, G.; Sasajima, S.; Mimura, I.; Morita, H.; Sugiyama, D.; Nishikawa, H.; et al. Fecal microbiota transplantation for patients with steroid-resistant acute graft-versus-host disease of the gut. Blood 2016, 128, 2083–2088. [Google Scholar] [CrossRef]
- Spindelboeck, W.; Schulz, E.; Uhl, B.; Kashofer, K.; Aigelsreiter, A.; Zinke-Cerwenka, W.; Mulabecirovic, A.; Kump, P.K.; Halwachs, B.; Gorkiewicz, G.; et al. Repeated fecal microbiota transplantations attenuate diarrhea and lead to sustained changes in the fecal microbiota in acute, refractory gastrointestinal graft-versus-host-disease. Haematologica 2017, 102, e210–e213. [Google Scholar] [CrossRef] [Green Version]
- Qi, X.; Li, X.; Zhao, Y.; Wu, X.; Chen, F.; Ma, X.; Zhang, F.; Wu, D. Treating steroid refractory intestinal acute graft-vs.-host disease with fecal microbiota transplantation: A pilot study. Front. Immunol. 2018, 9, 2195. [Google Scholar] [CrossRef]
- Shouval, R.; Geva, M.; Nagler, A.; Youngster, I. Fecal microbiota transplantation for treatment of acute graft-versus-host disease. Clin. Hematol. Int. 2019, 1, 28–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Lier, Y.F.; Davids, M.; Haverkate, N.J.E.; de Groot, P.F.; Donker, M.L.; Meijer, E.; Heubel-Moenen, F.C.J.I.; Nur, E.; Zeerleder, S.S.; Nieuwdorp, M.; et al. Donor fecal microbiota transplantation ameliorates intestinal graft-versus-host disease in allogeneic hematopoietic cell transplant recipients. Sci. Transl. Med. 2020, 12, eaaz8926. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.; Zhou, Y.; Gao, J.; Jiao, Y.; Zhu, B.; Wu, D.; Qi, X. Safety and efficacy of fecal microbiota transplantation for Grade IV steroid refractory GI-GvHD patients: Interim results from FMT2017002 Trial. Front. Immunol. 2021, 12, 678476. [Google Scholar] [CrossRef] [PubMed]
- Goeser, F.; Sifft, B.; Stein-Thoeringer, C.; Farowski, F.; Strassburg, C.P.; Brossart, P.; Higgins, P.G.; Scheid, C.; Wolf, D.; Holderried, T.A.W.; et al. Fecal microbiota transfer for refractory intestinal graft-versus-host disease—Experience from two German tertiary centers. Eur. J. Haematol. 2021, 107, 229–245. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Jiang, Q.; Sun, Y.; Mao, Y.; Guo, L.; Zhang, Y.; Man, M.; Ouyang, G.; Sheng, L. Treatment of intestinal graft-versus-host disease with unrelated donor fecal microbiota transplantation capsules. Medicine 2020, 99, e22129. [Google Scholar] [CrossRef] [PubMed]
- DeFilipp, Z.; Bloom, P.P.; Torres Soto, M.; Mansour, M.K.; Sater, M.R.A.; Huntley, M.H.; Turbett, S.; Chung, R.T.; Chen, Y.B.; Hohmann, E.L. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. N. Engl. J. Med. 2019, 381, 2043–2050. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef] [Green Version]
- Derosa, L.; Hellmann, M.D.; Spaziano, M.; Halpenny, D.; Fidelle, M.; Rizvi, H.; Long, N.; Plodkowski, A.J.; Arbour, K.C.; Chaft, J.E.; et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann. Oncol. 2018, 29, 1437–1444. [Google Scholar] [CrossRef]
- Pinato, D.J.; Howlett, S.; Ottaviani, D.; Urus, H.; Patel, A.; Mineo, T.; Brock, C.; Power, D.; Hatcher, O.; Falconer, A.; et al. Association of prior antibiotic treatment with survival and response to immune checkpoint inhibitor therapy in patients with cancer. JAMA Oncol. 2019, 5, 1774–1778. [Google Scholar] [CrossRef]
- Ahmed, J.; Kumar, A.; Parikh, K.; Anwar, A.; Knoll, B.M.; Puccio, C.; Chun, H.; Fanucchi, M.; Lim, S.H. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology 2018, 7, e1507670. [Google Scholar] [CrossRef] [Green Version]
- Baruch, E.N.; Youngster, I.; Ben-Betzalel, G.; Ortenberg, R.; Lahat, A.; Katz, L.; Adler, K.; Dick-Necula, D.; Raskin, S.; Bloch, N.; et al. Fecal microbiota transplant promotes response in immunotherapy-refractory melanoma patients. Science 2021, 371, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Dzutsev, A.K.; McCulloch, J.A.; Rodrigues, R.R.; Chauvin, J.M.; Morrison, R.M.; Deblasio, R.N.; Menna, C.; Ding, Q.; Pagliano, O.; et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021, 371, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Wardill, H.R.; Bowen, J.M.; Al-Dasooqi, N.; Sultani, M.; Bateman, E.; Stansborough, R.; Shirren, J.; Gibson, R.J. Irinotecan disrupts tight junction proteins within the gut: Implications for chemotherapy-induced gut toxicity. Cancer Biol. Ther. 2014, 15, 236–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsirigotis, P.; Triantafyllou, K.; Girkas, K.; Giannopoulou, V.; Ioannidou, E.; Chondropoulos, S.; Kalli, T.; Papaxoinis, G.; Pappa, V.; Papageorgiou, E.; et al. Keratinocyte growth factor is effective in the prevention of intestinal mucositis in patients with hematological malignancies treated with high-dose chemotherapy and autologous hematopoietic SCT: A video-capsule endoscopy study. Bone Marrow Transplant. 2008, 42, 337–343. [Google Scholar] [CrossRef]
- Poplawska, M.; Dutta, D.; Jayaram, M.; Salifu, M.; Chong, N.S.; Lim, S.H. Intestinal pathophysiological abnormalities in steady state and after vaso-occlusive crisis in murine sickle cell disease. Br. J. Haematol. 2021. online ahead of print. [Google Scholar] [CrossRef]
- Xu, C.; Lee, S.K.; Zhang, D.; Frenette, P.S. The gut microbiome regulates psychological-stress-induced inflammation. Immunity 2020, 53, 417–428.e4. [Google Scholar] [CrossRef]
- Dutta, D.; Methe, B.A.; Amar, S.; Morris, A.; Lim, S.H. Intestinal injury and gut permeability in sickle cell disease. J. Transl. Med. 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Dutta, D. Clinicopathologic consequences following discontinuation of rifaximin in patients with sickle cell disease. Am. J. Hematol. 2020, 95, E151–E153. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Morris, A.; Li, K.; Fitch, A.C.; Fast, L.; Goldberg, L.; Quesenberry, M.; Sprinz, P.; Methé, B. Intestinal microbiome analysis revealed dysbiosis in sickle cell disease. Am. J. Hematol. 2018, 93, E91–E93. [Google Scholar] [CrossRef] [Green Version]
- Dutta, D.; Li, K.; Methe, B.; Lim, S.H. Rifaximin on intestinally-related pathologic changes in sickle cell disease. Am. J. Hematol. 2020, 95, E83–E86. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.H.; Dutta, D.; Moore, J. Rifaximin in sickle cell disease. Am. J. Hematol. 2019, 94, E325–E328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tariq, R.; Saha, S.; Solanky, D.; Pardi, D.S.; Khanna, S. Predictors and management of failed fecal microbiota transplantation for recurrent Clostridioides difficile infection. J. Clin. Gastroenterol. 2021, 55, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Marcella, C.; Cui, B.; Kelly, C.R.; Ianiro, G.; Cammarota, G.; Zhang, F. Systematic review: The global incidence of faecal microbiota transplantation-related adverse events from 2000 to 2020. Aliment. Pharmacol. Ther. 2021, 53, 33–42. [Google Scholar] [PubMed]
- Hengel, R.L.; Ritter, T.E.; Nathan, R.V.; Van Anglen, L.J.; Schroeder, C.P.; Dillon, R.J.; Marcella, S.W.; Garey, K.W. Real-world Experience of Bezlotoxumab for Prevention of Clostridioides difficile Infection: A Retrospective Multicenter Cohort Study. Open Forum Infect. Dis. 2020, 7, ofaa097. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Reference | Data Source | Number of Patients (n) | Outcome | Adverse Events |
|---|---|---|---|---|
| Youngster et al. [24] | A prospective study | 180 using oral frozen capsules | CDI resolved in 82% of patients after a single treatment, rising to a 91% cure rate with two treatments. | Three cases of Grade 2 or above adverse reactions deemed related to the FMT were reported: One transient high fever, two new endoscopic diagnoses of ulcerative colitis. |
| Furuya-Kanamori et al. [25] | A collaborative analysis of patient data from 14 studies | 305 (207 by lower and 98 by upper gastrointestinal route) | Risk of clinical failure was 5.6% and 17.9% in those treated by upper gastrointestinal route, and 4.9% and 8.5% in those treated by lower gastrointestinal route at Day 30 and 90, respectively. | Not reported. |
| Liu et al. [26] | Single center retrospective data | 25 procedures (via feeding tube (n = 11), upper gastrointestinal endoscopy (n = 8), or colonoscopy (n = 6) in 24 patients) | Symptoms resolved in 21 of 24 patients (87.5%). Three patients who did not respond underwent a second FMT and all three responded | No serious adverse reactions were attributed to FMT. |
| Ponte et al. [27] | Single center retrospective study | 34 (via upper gastrointestinal endoscopy (n = 30) or colonoscopy (n = 4) | Cure after one FMT in 22/25 (88%) and after two or more FMT in another 2/25 (8%). | No serious adverse reactions were reported. |
| Kelly et al. [28] | FMT National Registry Data | 222 had follow-up at 1 month and 123 at 6 months. | 90% cure rate at 1 month and 96% cure rate at 6 months. | At 1 month, 1% had hospitalization for diarrhea and severe abdominal pain, felt probably related to FMT; at 6 months, 1% developed irritable bowel syndrome and 1% inflammatory bowel disease. |
| Reference | Data Source | Number of Patients (n) | Outcome | Adverse Events |
|---|---|---|---|---|
| Kakihana et al. [58] | Single center prospective study | 4 (received a total of 7 FMT by nasogastric administration) | 3 CR and 1 PR | 1 case of lower gastrointestinal bleed and hypoxemia, may not be related to FMT |
| Spindelboeck et al. [59] | Retrospective case series | 3 (received a total of 9 FMT by colonoscopy) | 2 CR and 1 PR | None reported |
| Qi et al. [60] | Single center prospective study | 8 (received a total of 12 FMT by nasogastric administration) | 5 CR and 1 PR | None reported |
| Shouval et al. [61] | Single center prospective study | 7 (received a total of 15 FMT by capsule administration) | 2 CR | 2 episodes of bacteremia, deemed unrelated to FMT |
| van Lier et al. [62] | Single center prospective study | 15 (received a total of 15 FMT by nasoduodenal tube administration) | 10 CR | None reported |
| Zhao et al. [63] | Single center open-label Phase I/II study | 41 (23 assigned to FMT and 18 to control. FMT administered by nasojejunal or gastric tube) | Overall response rate of 82.6% (52.2% CR and 30.4% PR) in the FMT group and 39% (all PR) in the control group on Day 14 after FMT, and an overall response rate of 69.5% (56.5% CR and 13% PR) in the FMT group and 50% (16% CR and 34% PR) in the control group on Day 21 after FMT | No difference in the adverse events between the FMT group and the control group. |
| Goeser et al. [64] | Two-center retrospective study | 11 (9 by capsule and 2 by nasojejunal tube administration) | Attenuation of stool volume and frequency was observed in all 11 patients | Abdominal pain occurred in 3 patients and vomiting in 1 patient |
| Mao et al. [65] | Case report | 1 (received two cycles of FMT administered by capsules) | CR | None reported |
| NCT# | Study | Primary Outcome Measurements | Number of Patients (n) |
|---|---|---|---|
| 02928523 | PreventiOn of DYSbioSis Complications With Autologous FMT in AML Patients (ODYSSEE) | Evaluation of efficacy in dysbiosis correction and multidrug resistant bacteria based on bacterial culture | 20 |
| 03678493 | A Study of FMT in Patients With AML Allo HSCT in Recipients | Efficacy of FMT in AML patients and allo-HSCT recipients in reducing the incidence of infections | 120 |
| 04935684 | Faecal Microbiota Transplantation After Allogeneic Stem Cell Transplantation (TMF-Allo) | GVHD and relapse-free survival rate after allogeneic hematopoietic stem cell transplantation | 150 |
| 04269850 | Fecal Microbiota Transplantation With Ruxolitinib and Steroids as an Upfront Treatment of Severe Acute Intestinal GVHD (JAK-FMT) | Overall survival | 20 |
| 05094765 | Fecal Microbiota Transplant (FMT) Capsule for Improving the Efficacy of GI-aGVHD | Overall survival and Grade 3 or above adverse events | 15 |
| 02269150 | Autologous Fecal Microbiota Transplantation (Auto-FMT) for Prophylaxis of Clostridium Difficile Infection in Recipients of Allogeneic Hematopoietic Stem Cell Transplantation | CDI up to one year after entry into study | 59 |
| 03214289 | Fecal Microbiota Transplantation for Steroid Resistant and Steroid Dependent Gut Acute Graft Versus Host Disease | Serious adverse events | 4 |
| 02733744 | Fecal Microbiota Transplantation After HSCT | Feasibility on the number of participants able to ingest 15 FMT capsules over a 2-day period | 18 |
| 03359980 | Treatment of Steroid Refractory Gastro-intestinal Acute GVHD afteR AllogeneiC HSCT With fEcal Microbiota tranSfer (HERACLES) | Efficacy of FMT in steroid refractory -gastro-intestinal acute GVHD at Day 28 | 24 |
| 03819803 | Fecal Microbiota Transplantation in aGvHD After ASCT | Remission at Day 90 | 15 |
| 04038619 | Fecal Microbiota Transplantation in Treating Immune-Checkpoint Inhibitor Induced-Diarrhea or Colitis in Genitourinary Cancer Patients | Tolerability and response | 40 |
| 02770326 | Safety of Stool Transplant for Patients With Difficult to Treat C. Difficile Infection | Incidence of CDI | 10 |
| 04116775 | Fecal Microbiota Transplant and Pembrolizumab for Men With Metastatic Castration Resistant Prostate Cancer. | Anticancer effect of FMT from responders to pembrolizumab to non-responders. | 32 |
| 04040712 | Fecal Microbiota Transplantation in Diarrhea Induced by Tyrosine-kinase Inhibitors | Resolution of diarrhea four weeks after FMT | 20 |
| 03819296 | Role of Gut Microbiome and Fecal Transplant on Medication-Induced GI Complications in Patients With Cancer | Differences in stool microbiome pattern and adverse events | 800 |
| 04951583 | Fecal Microbial Transplantation Non-Small Cell Lung Cancer and Melanoma (FMT-LUMINATE) | Overall response rate | 70 |
| 04521075 | A Phase Ib Trial to Evaluate the Safety and Efficacy of FMT and Nivolumab in Subjects With Metastatic or Inoperable Melanoma, MSI-H, dMMR or NSCLC | Overall response rate and adverse events | 42 |
| 04163289 | Preventing Toxicity in Renal Cancer Patients Treated With Immunotherapy Using Fecal Microbiota Transplantation (PERFORM) | Rate of immune-related colitis associated with ipilimumab/nivolumab treatment | 20 |
| 04729322 | Fecal Microbiota Transplant and Re-introduction of Anti-PD-1 Therapy (Pembrolizumab or Nivolumab) for the Treatment of Metastatic Colorectal Cancer in Anti-PD-1 Non-responders | Overall response rate | 15 |
| 04924374 | Microbiota Transplant in Advanced Lung Cancer Treated With Immunotherapy | Measurements of safety | 20 |
| 03341143 | Fecal Microbiota Transplant (FMT) in Melanoma Patients | Overall response rate | 18 |
| 03353402 | Fecal Microbiota Transplantation (FMT) in Metastatic Melanoma Patients Who Failed Immunotherapy | Rate of adverse events and engraftment | 40 |
| 04988841 | Assessing the Tolerance and Clinical Benefit of feCAl tranSplantation in patientS With melanOma (PICASSO) | Safety and tolerability | 60 |
| 04577729 | The IRMI-FMT Trial | Progression-free survival | 60 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bou Zerdan, M.; Niforatos, S.; Nasr, S.; Nasr, D.; Ombada, M.; John, S.; Dutta, D.; Lim, S.H. Fecal Microbiota Transplant for Hematologic and Oncologic Diseases: Principle and Practice. Cancers 2022, 14, 691. https://doi.org/10.3390/cancers14030691
Bou Zerdan M, Niforatos S, Nasr S, Nasr D, Ombada M, John S, Dutta D, Lim SH. Fecal Microbiota Transplant for Hematologic and Oncologic Diseases: Principle and Practice. Cancers. 2022; 14(3):691. https://doi.org/10.3390/cancers14030691
Chicago/Turabian StyleBou Zerdan, Maroun, Stephanie Niforatos, Sandy Nasr, Dayana Nasr, Mulham Ombada, Savio John, Dibyendu Dutta, and Seah H. Lim. 2022. "Fecal Microbiota Transplant for Hematologic and Oncologic Diseases: Principle and Practice" Cancers 14, no. 3: 691. https://doi.org/10.3390/cancers14030691
APA StyleBou Zerdan, M., Niforatos, S., Nasr, S., Nasr, D., Ombada, M., John, S., Dutta, D., & Lim, S. H. (2022). Fecal Microbiota Transplant for Hematologic and Oncologic Diseases: Principle and Practice. Cancers, 14(3), 691. https://doi.org/10.3390/cancers14030691





