- Review
The Structure, Pathogenesis, and Inhibition of the p53-MDM2 Pathway
- Amanda L. Brown,
- Xiaoying Lian and
- Qian Wang
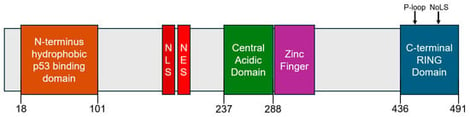
The p53 tumor suppressor protein plays a central role in maintaining genomic stability by regulating cell cycle arrest, apoptosis, and DNA repair under cellular stress. Mouse double minute 2 (MDM2), an E3 ubiquitin ligase, negatively regulates p53 via direct binding and proteasomal degradation. Overexpression or amplification of MDM2 can disrupt this pathway and promote tumorigenesis, even in cancers with wild-type p53. This review outlines the structural features of MDM2, particularly its N-terminal hydrophobic pocket and C-terminal RING domain, and their roles in p53 regulation. We further examine the pathological effects of MDM2 dysregulation and SNPs linked to increased cancer risk. Recent progress in small molecule MDM2 inhibitors is discussed, with a focus on non-covalent agents such as rhein-derived anthraquinone analogs, including AQ-101, which demonstrate promising anti-cancer activity with reduced toxicity. These findings support the continued development of non-covalent MDM2 inhibitors as a novel therapeutic approach for cancers involving both wild-type and mutant p53.
7 February 2026