Dissipation and Residue Pattern of Dinotefuran, Fluazinam, Indoxacarb, and Thiacloprid in Fresh and Processed Persimmon Using LC-MS/MS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Trials
2.2. Reagents and Materials
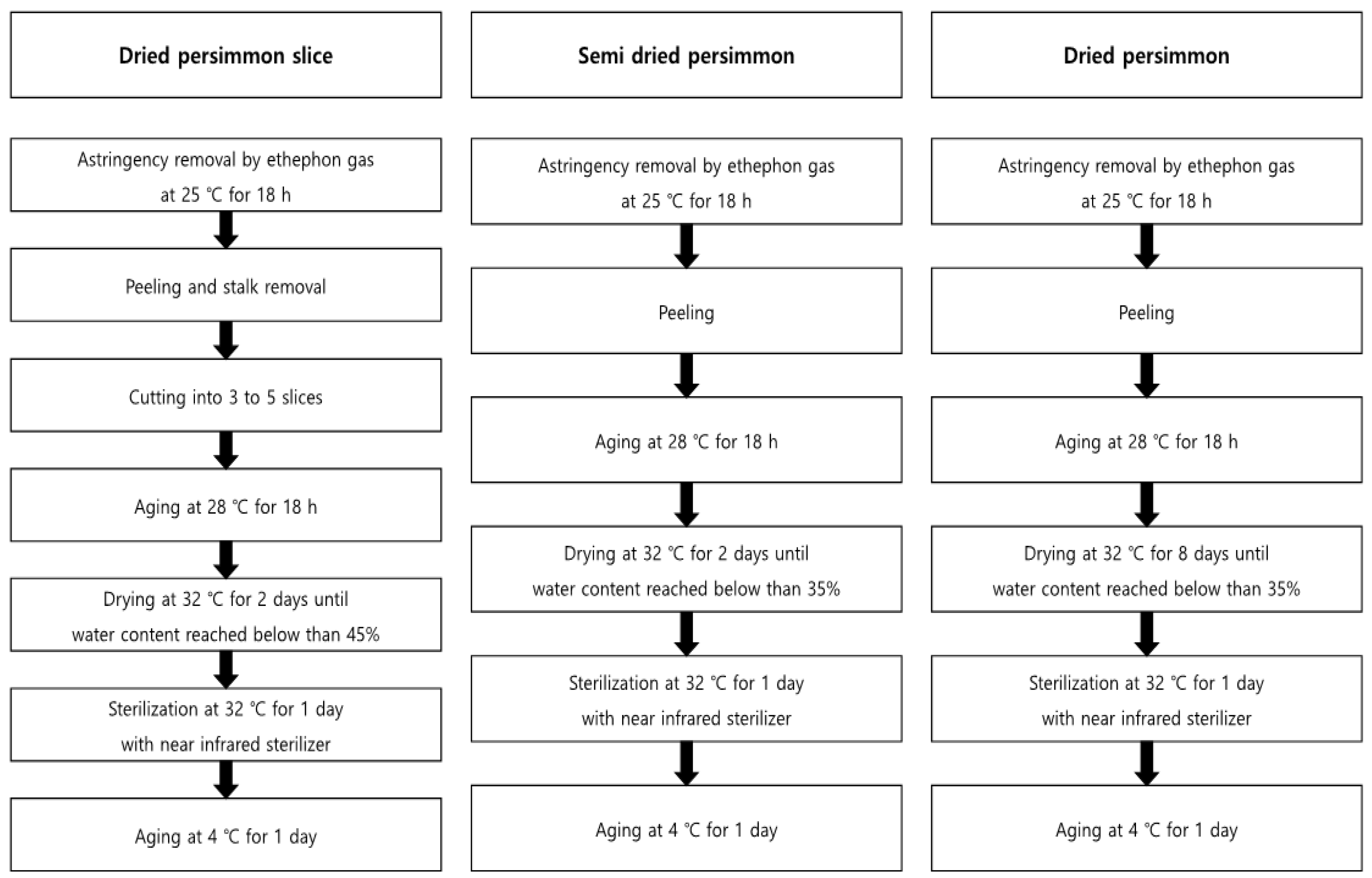
2.3. Sample Preparation
2.3.1. Dinotefuran and Its Metabolites (DN, MNG and UF)
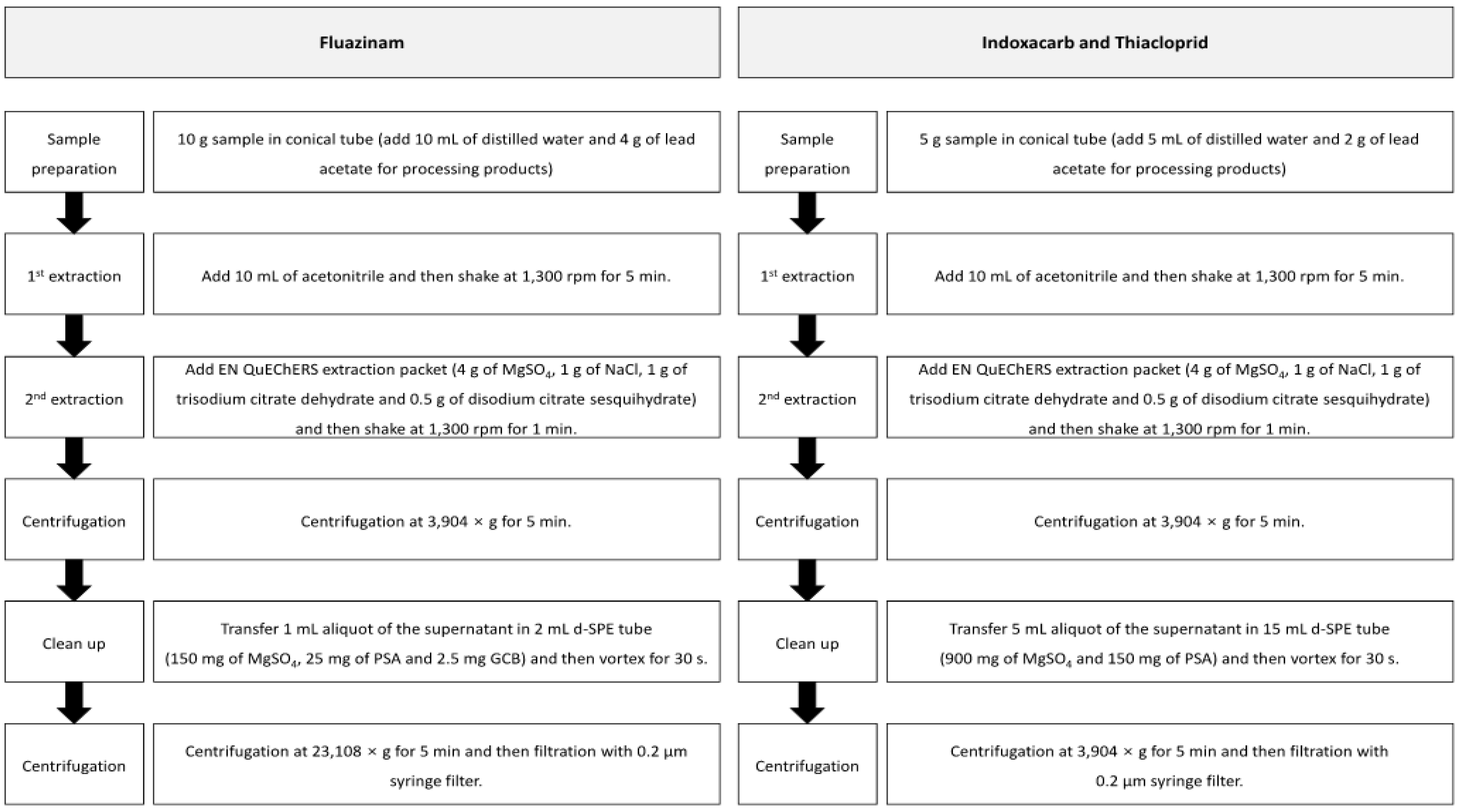
2.3.2. Fluazinam, Indoxacarb, and Thiacloprid
2.4. Instrumental Analysis
2.5. Method Validation
2.6. Residue Definition of Test Pesticides
2.7. Half-Lives of Test Pesticide in Fresh Persimmon
2.8. Processing and Reduction Factors
2.9. Statistical Analysis
3. Results and Discussion
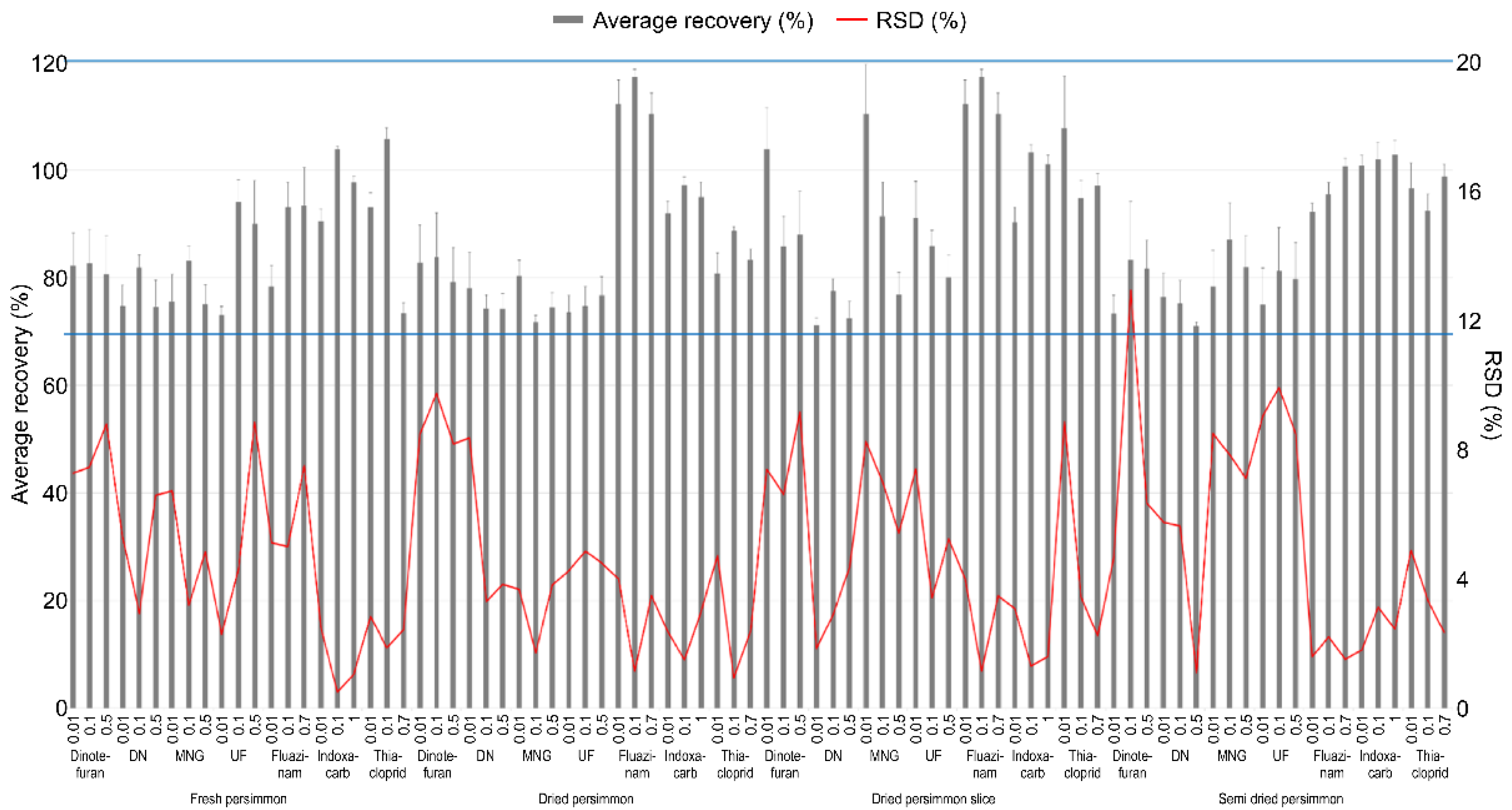
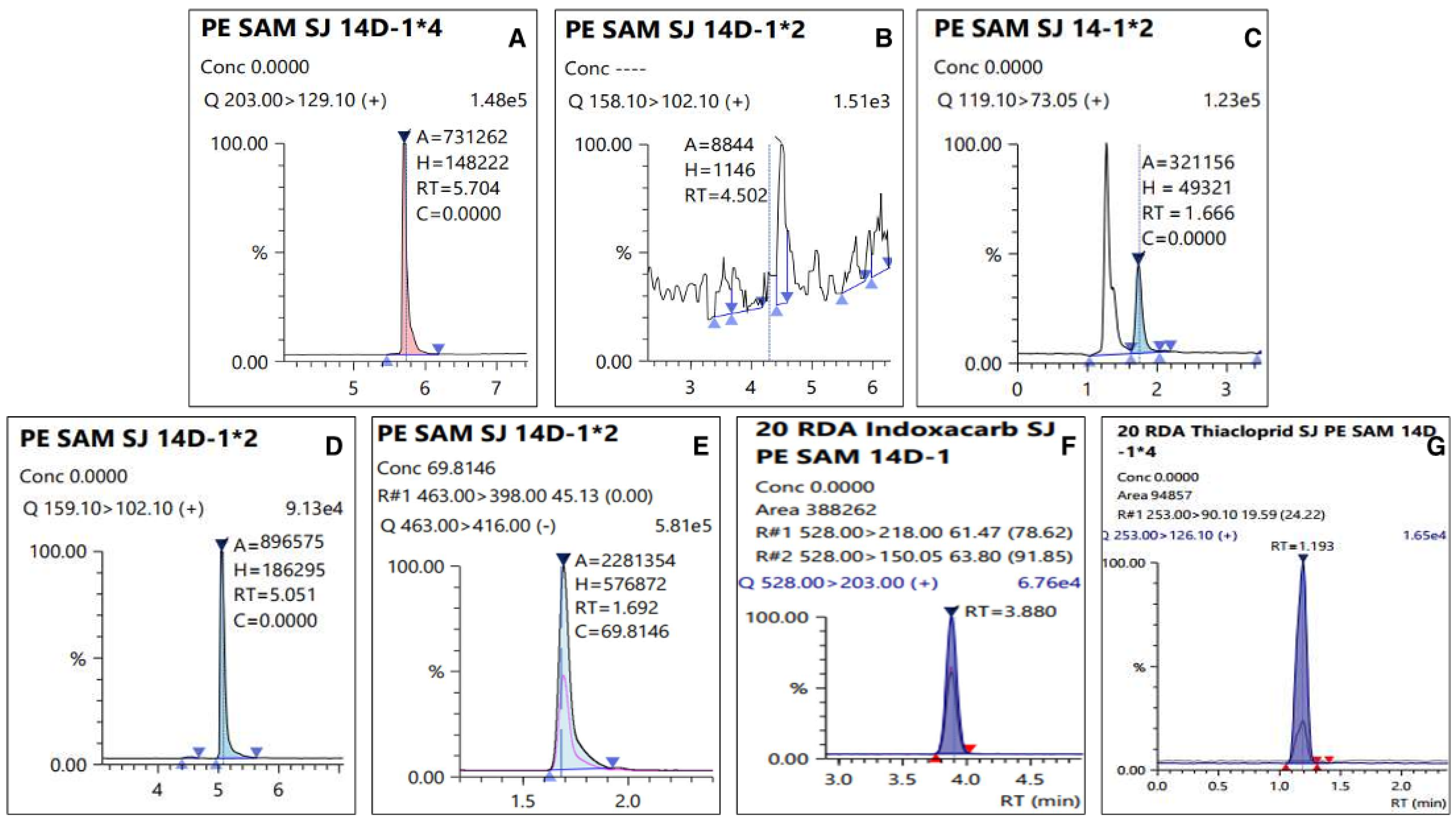
3.1. Validation of the Analytical Method
3.2. Results of Pesticide Residue Analysis
3.2.1. Dissipation Pattern of Test Pesticide in Fresh Persimmon
3.2.2. Residual Concentration of Pesticide on Harvest Day
3.2.3. Processing Factor and Reduction Factor
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loewy, R.M.; Monza, L.B.; Kirs, V.E.; Savini, M.C. Pesticide distribution in an agricultural environment in Argentina. J. Environ. Sci. Health Part B 2011, 46, 662–670. [Google Scholar]
- Tabasum, A.; Alghuthaymi, M.; Qazi, U.Y.; Shahid, I.; Abbas, Q.; Javaid, R.; Nadeem, N.; Zahid, M. UV-Accelerated photocatalytic degradation of pesticide over magnetite and cobalt ferrite decorated graphene oxide composite. Plants 2020, 10, 6. [Google Scholar] [CrossRef]
- Shefali, R.; Kumar; Sankhla, M.S.; Kumar, R.; Sonone, S.S. Impact of pesticide toxicity in aquatic environment. Biointerface Res. Appl. Chem. 2021, 11, 10131–10140. [Google Scholar]
- Mehmood, Y.; Arshard, M.; Kaechele, H.; Mahmood, N.; Kong, R. Pesticide residues, health risks, and vegetable farmers’ risk perceptions in Punjab, Pakistan. Hum. Ecol. Risk Assess. Int. J. 2020, 27, 846–864. [Google Scholar] [CrossRef]
- Liang, C.P.; Sack, C.; McGrath, S.; Cao, Y.; Thompson, C.J.; Robin, L.P. US food and drug administration regulatory pesticide residue monitoring of human foods: 2009–2017. Food Addit. Contam. Part A 2021, 38, 1520–1538. [Google Scholar] [CrossRef]
- Cabrera, L.C.; Pastor, P.M. The 2019 European Union report on pesticide residues in food. EFSA J. 2021, 19, e06491. [Google Scholar]
- Khalil, Y.; Flower, K.; Siddique, K.H.M.; Ward, P. Rainfall affects leaching of pre-emergent herbicide from wheat residue into the soil. PLoS ONE 2019, 14, e0210219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acoglu, B.; Omeroglu, P.Y. Effectiveness of different type of washing agents on reduction of pesticide residues in orange (Ctrus sinensis). LWT-Food Sci. Technol. 2021, 147, 111690. [Google Scholar] [CrossRef]
- Elkins, E.R. Effect of commercial processing on pesticide residues in selected fruits and vegetables. J. Assoc. Off. Agric. Chem. 1989, 72, 533–535. [Google Scholar] [CrossRef]
- Kaushik, G.S.; Satya, S.; Naik, N. Food processing a tool to pesticide residue dissipation—A review. Food Res. Int. 2009, 42, 26–40. [Google Scholar] [CrossRef]
- Noh, H.H.; Shin, H.W.; Kim, D.J.; Lee, J.W.; Jo, S.H.; Kim, D.; Kyung, K.S. Effect of processing on residual buprofezin levels in ginseng products. Int. J. Environ. Res. Public Health 2021, 18, 471. [Google Scholar] [CrossRef]
- Yakushiji, H.; Nakatsuka, A. Recent persimmon research in Japan. Jpn. J. Plant Sci. 2007, 1, 42–62. [Google Scholar]
- Adams, R.E.; Brichel, J.A.; Bhat, V.S. Chemical-specific maximum allowable levels for pesticide residues in dietary supplements. Food Chem. Toxicol. 2019, 123, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Henson, S.; Loader, R. Barriers to agricultural exports from developing countries: The role of sanitary and phytosanitary requirements. World Dev. 2001, 29, 85–102. [Google Scholar] [CrossRef]
- Monterio, C.A.; Moubarac, J.C.; Cannon, G.; Ng, S.W.; Popkin, B. Ultra-processed products are becoming dominant in the global food system. Obes. Rev. 2013, 14, 21–28. [Google Scholar] [CrossRef] [PubMed]
- KOSIS (Korean Statistical Information Service). Domestic Statistics. Available online: http://kosis.kr/statisticsList/statisticsListIndex.do?menuId=M_01_01&vwcd=MT_ZTITLE&parmTabId=M_01_01&outLink=Y&entrType=#content-group (accessed on 15 December 2021).
- European Commission (EC). Analytical Quality Control and Method Validation Procedures for Pesticide Residues Analysis in Food and Feed (SANTE/12682/2019). Available online: https://www.eurl-pesticides.eu/userfiles/file/EurlALL/AqcGuidance_SANTE_2019_12682.pdf (accessed on 26 September 2021).
- Food and Agriculture Organization of the United Nations (FAO). Submission and evaluation of pesticide residues data for the estimation of maximum residue levels in food and feed. In Pesticide Residue; FAO: Rome, Italy, 2016. [Google Scholar]
- Li, W.; Shen, S.; Chen, H.; Guo, Q. Dissipation study and dietary risk assessment of dinotefuran, DN, and UF in wolfberry. Int. J. Environ. Anal. Chem. 2020, 100, 1524–1535. [Google Scholar] [CrossRef]
- Codex Alimentarius. Codex Pesticide Residues in Food Online Database. 2021. Available online: http://www.fao.org/fao-who-codexalimentarius/codex-texts/dbs/pestres/pesticides/en/ (accessed on 15 December 2021).
- European Food Safety Authority (EFSA). Reasoned opinion on the setting of a new MRL for fluazinam in tomatoes. EFSA J. 2015, 13, 4154. [Google Scholar]
- Wang, H.; Zheng, L.; Yu, W.; Cao, X.; Yang, R. Dissipation behavior of chlorpyrifos residues and risk assessment in sugarcane fields. Biomed. Chromatogr. 2018, 33, e4424. [Google Scholar] [CrossRef]
- Kumar, S.V.; Subhashchandran, K.P.; George, T.; Paul, A.; Xavier, G.; Vijayasree, V.; Suryamol, S. Dinotefuran residues and their dissipation in chilli pepper (Capasicum annuum L.) and soil and their risk assessment. Pestic. Res. J. 2019, 31, 211–219. [Google Scholar] [CrossRef]
- Mach, B.M.; Bondarenko, S.; Potter, D.A. Uptake and dissipation of neonicotinoid residues in nectar and foliage of systemically treated woody landscape plants. Environ. Toxicol. Chem. 2018, 37, 860–870. [Google Scholar] [CrossRef]
- Wang, Q.S.; Wei, P.; Cao, M.C.; Liu, Y.N.; Wang, M.C.; Guo, Y.R. Residual behavior and risk assessment of the mixed formulation of benzene kresoxim-methyl and fluazinam in cucumber filed application. Environ. Monit. Assess. 2016, 188, 341. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Z.; Wen, Y.; Fan, S.; Liu, C. Dissipation of fluazinam in citrus groves and a risk assessment for its dietary intake. J. Sci. Food Agric. 2020, 100, 2052–2056. [Google Scholar] [CrossRef]
- Cabras, P.; Angioni, A.; Garau, V.L.; Piris, F.M.; Esponoza, J.; Mendoza, A.; Cabitza, F.; Pala, M.; Brandolini, V. Fate of azoxystrobin, fluazinam, kresoxim-methyl, mepanipyrim, and tetraconazole from vine to wine. J. Agric. Food Chem. 1998, 46, 3249–3251. [Google Scholar] [CrossRef]
- Feng, X.; Wang, K.; Mu, Z.; Zhao, Y.; Zhang, H. Fluazinam residue and dissipation in potato tubers and vines, and in field soil. Am. J. Potato Res. 2015, 92, 567–572. [Google Scholar] [CrossRef]
- Sun, D.; Zhu, Y.; Pang, J.; Zhou, Z.; Jiao, B. Residue level, persistence and safety of spirodiclofen-pyridaben mixture in citrus fruits. Food Chem. 2016, 194, 805–810. [Google Scholar] [CrossRef]
- Zhang, P.W.; Wang, S.Y.; Huang, C.L.; Fu, J.T.; Huang, R.L.; Li, Z.H.; Zhang, Z.X. Dissipation and residue of clothianidin in granules and pesticide fertilizers used in cabbage and soil under field conditions. Environ. Sci. Pollut. Res. 2018, 25, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jyot, G.; Sahoo, S.K.; Kaur, S.; Battu, R.S.; Singh, B. Estimation of indoxacarb residues by QuEChERS technique and its degradation pattern in cabbage. Bull. Environ. Contam. Toxicol. 2012, 88, 372–376. [Google Scholar]
- Sakthiselvi, T.; Paramasivam, M.; Vasanthi, D.; Bhuvaneswari, K. Persistence, dietary and ecological risk assessment of indoxacarb residue in/on tomato and soil using GC-MS. Food Chem. 2020, 328, 127134. [Google Scholar] [CrossRef] [PubMed]
- Hulbert, D.; Isaacs, R.; Vandervoot, C.; Wise, J.C. Rainfastness and residual activity of insecticides to control Japanese beetle (Coleoptera: Scarabaeidae) in grape. J. Econ. Entomol. 2011, 104, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, S.; Siddamallaiah, L.; Matadha, N.Y.; Udupi, V.R.; Raj, D.P.; Gadigeppa, S. Dissipation of neonicotinoid insecticides imidacloprid, indoxacarb and thiamethoxam on pomegranate (Punica granatum L.). Exotoxicology Environ. Saf. 2019, 171, 130–137. [Google Scholar] [CrossRef]
- Li, R.; Chen, Z.; Dong, F.; Xu, J.; Liu, X.; Wu, X.; Pan, X.; Tao, Y.; Zheng, Y. Supercritical fluid chromatographic-tandem mass spectrometry method for monitoring dissipation of thiacloprid in greenhouse vegetables and soil under different application modes. J. Chromatogr. B 2018, 1081–1082, 25–32. [Google Scholar] [CrossRef]
- Saimandir, J.; Gopal, M. Evaluation of synthetic and natural insecticides for the management of insect pest control of eggplant (Solanum melongena L.) and pesticide residue dissipation pattern. Sci. Res. 2012, 3, 214–227. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Zhong, Z.; Han, Y.; Zheng, Q.; Qin, Y.; Wu, Q.; He, X.; Pan, C. Dissipation of sixteen pesticide residues from various application of commercial formulations on strawberry and their risk assessment under greenhouse conditions. Ecotoxicol. Environ. Saf. 2020, 188, 109842. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Liu, B.; Zheng, Q.; Qin, D.; Luo, P.; Zhao, W.; Ye, C.; Huang, S.; Cheng, D.; Zhang, Z. Residue and dissipation of two formulations of emamectin benzoate in tender cowpea and old cowpea and a risk assessment of dietary intake. Food Chem. 2021, 361, 130043. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Zhou, Y.; Wu, J.; Zhang, Z.; Cheng, D. Dissipation and residue of fosthiazate in tomato and cherry tomato and a risk assessment of dietary intake. Environ. Sci. Pollut. Res. 2021, 29, 9248–9256. [Google Scholar] [CrossRef]
| Pesticide | Site | Application Rate per Treatment | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Active Ingredient (kg ha−1) | Water (L ha−1) | Active Ingredient (kg hL−1) | |||||||||||
| 1st Treatment | 2nd Treatment | 3rd Treatment | 4th Treatment | 1st Treatment | 2nd Treatment | 3rd Treatment | 4th Treatment | 1st Treatment | 2nd Treatment | 3rd Treatment | 4th Treatment | ||
| Dinotefuran | 1 | 0.418 | 0.469 | 0.475 | - | 4185 | 4689 | 4751 | - | 0.00930 | 0.01042 | 0.01056 | - |
| 2 | 0.449 | 0.536 | 0.584 | - | 4489 | 5360 | 5841 | - | 0.00998 | 0.01191 | 0.01298 | - | |
| 3 | 0.443 | 0.439 | 0.451 | - | 4433 | 4386 | 4513 | - | 0.00985 | 0.00975 | 0.01003 | - | |
| Fluazinam | 1 | 0.918 | 0.933 | 1.046 | 1.060 | 4589 | 4667 | 5229 | 5298 | 0.02040 | 0.02074 | 0.02324 | 0.02355 |
| 2 | 1.059 | 0.882 | 1.054 | 1.148 | 5296 | 4412 | 5268 | 5740 | 0.02354 | 0.01961 | 0.02341 | 0.02551 | |
| 3 | 0.915 | 0.927 | 0.917 | 0.944 | 4577 | 4637 | 4587 | 4721 | 0.02034 | 0.02061 | 0.02039 | 0.02098 | |
| Indoxacarb | 1 | 0.259 | 0.269 | 0.277 | - | 5181 | 5381 | 5537 | - | 0.00576 | 0.00598 | 0.00615 | - |
| 2 | 0.278 | 0.237 | 0.223 | - | 5562 | 4736 | 4452 | - | 0.00618 | 0.00526 | 0.00495 | - | |
| 3 | 0.227 | 0.212 | 0.213 | - | 4536 | 4239 | 4250 | - | 0.00504 | 0.00471 | 0.00472 | - | |
| Thiacloprid | 1 | 0.251 | 0.237 | 0.236 | - | 5018 | 4743 | 4723 | - | 0.00558 | 0.00527 | 0.00525 | - |
| 2 | 0.266 | 0.232 | 0.238 | - | 5327 | 4640 | 4766 | - | 0.00592 | 0.00516 | 0.00530 | - | |
| 3 | 0.222 | 0.179 | 0.193 | - | 4433 | 3583 | 3863 | - | 0.00493 | 0.00398 | 0.00429 | - | |
| Compound | Precursor (m/z) | Quantitation | Confirmation | ||
|---|---|---|---|---|---|
| m/z | CE (eV) | m/z | CE (eV) | ||
| Dinotefuran | 203.00 | 129.10 | 13 | 114.10 | 13 |
| DN | 158.10 | 102.10 | 16 | 57.05 | 24 |
| MNG | 119.10 | 73.05 | 12 | 44.00 | 23 |
| UF | 159.10 | 102.10 | 13 | 67.00 | 20 |
| Fluazinam | 463.00 | 416.00 | 19 | 398.00 | 18 |
| Indoxacarb | 528.00 | 203.00 | −40 | 150.00 | −23 |
| Thiacloprid | 253.00 | 126.10 | −21 | 90.1 | −37 |
| Pesticide | Study | Matrix | Linear Range (µg L−1) | r2 |
|---|---|---|---|---|
| Dinotefuran | Harvest | Fresh persimmon | 1–100 | 0.9965 |
| Dried persimmon | 0.9980 | |||
| Dried persimmon slice | 0.9989 | |||
| Semi dried persimmon | 0.9967 | |||
| Decline | Fresh persimmon | 0.9995 | ||
| DN (Dinotefuran metabolite) | Harvest | Fresh persimmon | 1–100 | 0.9994 |
| Dried persimmon | 0.9987 | |||
| Dried persimmon slice | 0.9998 | |||
| Semi dried persimmon | 0.9998 | |||
| Decline | Fresh persimmon | 0.9998 | ||
| MNG (Dinotefuran metabolite) | Harvest | Fresh persimmon | 1–100 | 0.9984 |
| Dried persimmon | 0.9993 | |||
| Dried persimmon slice | 0.9987 | |||
| Semi dried persimmon | 0.9986 | |||
| Decline | Fresh persimmon | 0.9987 | ||
| UF (Dinotefuran metabolite) | Harvest | Fresh persimmon | 1–100 | 0.9972 |
| Dried persimmon | 0.9998 | |||
| Dried persimmon slice | 0.9995 | |||
| Semi dried persimmon | 0.9981 | |||
| Decline | Fresh persimmon | 0.9992 | ||
| Fluazinam | Harvest | Fresh persimmon | 2–100 | 0.9992 |
| Dried persimmon | 0.9996 | |||
| Dried persimmon slice | 0.9997 | |||
| Semi dried persimmon | 0.9996 | |||
| Decline | Fresh persimmon | 0.9993 | ||
| Indoxacarb | Harvest | Fresh persimmon | 1–100 | 1.0000 |
| Dried persimmon | 1.0000 | |||
| Dried persimmon slice | 0.9999 | |||
| Semi dried persimmon | 1.0000 | |||
| Decline | Fresh persimmon | 0.9999 | ||
| Thiacloprid | Harvest | Fresh persimmon | 1–100 | 0.9996 |
| Dried persimmon | 0.9999 | |||
| Dried persimmon slice | 0.9997 | |||
| Semi dried persimmon | 0.9997 | |||
| Decline | Fresh persimmon | 0.9999 |
| Site | DALA 1 | Mean Residue ± SD 2) (n = 3, mg kg−1) | Half-Life (day) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dinotefuran | Fluazinam | Indoxacarb | Thiacloprid | Dinotefuran | Fluazinam | Indoxacarb | Thiacloprid | ||||||
| Parent | DN | MNG | UF | Total | |||||||||
| 1 | 0 | 0.47 ± 0.04 | <0.01 3 | <0.01 | <0.01 | 0.47 ± 0.04 a 4 | 0.33 ± 0.01 a | 0.23 ± 0.01 a | 0.26 ± 0.02 a | 36 | 11 | 20 | 12 |
| 1 | 0.41 ± 0.03 | <0.01 | <0.01 | <0.01 | 0.41 ± 0.03 b | 0.32 ± 0.02 a | 0.22 ± 0.02 ab | 0.26 ± 0.01 a | |||||
| 3 | 0.38 ± 0.02 | <0.01 | <0.01 | <0.01 | 0.38 ± 0.02 bc | 0.28 ± 0.03 b | 0.20 ± 0.01 bc | 0.25 ± 0.04 a | |||||
| 5 | 0.37 ± 0.02 | <0.01 | <0.01 | <0.01 | 0.37 ± 0.02 bc | 0.21 ± 0.01 c | 0.18 ± 0.01 cd | 0.20 ± 0.01 b | |||||
| 7 | 0.34 ± 0.02 | <0.01 | <0.01 | 0.01 ± 0.00 | 0.35 ± 0.02 c | 0.19 ± 0.01 d | 0.17 ± 0.02 d | 0.16 ± 0.03 c | |||||
| 14 | 0.28 ± 0.01 | <0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.34 ± 0.01 c | 0.15 ± 0.01 e | 0.14 ± 0.01 e | 0.12 ± 0.01 c | |||||
| 2 | 0 | 0.53 ± 0.03 | <0.01 | <0.01 | <0.01 | 0.53 ± 0.03 a | 0.41 ± 0.03 a | 0.23 ± 0.01 a | 0.22 ± 0.05 a | 41 | 10 | 16 | 10 |
| 1 | 0.47 ± 0.04 | <0.01 | <0.01 | <0.01 | 0.47 ± 0.04 ab | 0.40 ± 0.01 a | 0.21 ± 0.01 b | 0.22 ± 0.04 a | |||||
| 3 | 0.45 ± 0.04 | <0.01 | <0.01 | <0.01 | 0.45 ± 0.04 b | 0.34 ± 0.00 b | 0.18 ± 0.02 c | 0.20 ± 0.03 ab | |||||
| 5 | 0.42 ± 0.04 | <0.01 | <0.01 | <0.01 | 0.42 ± 0.04 b | 0.31 ± 0.01 c | 0.17 ± 0.01 c | 0.17 ± 0.04 ab | |||||
| 7 | 0.38 ± 0.03 | <0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.41 ± 0.04 b | 0.23 ± 0.02 d | 0.15 ± 0.01 d | 0.15 ± 0.02 b | |||||
| 14 | 0.24 ± 0.02 | <0.01 | 0.07 ± 0.02 | 0.03 ± 0.01 | 0.40 ± 0.03 b | 0.15 ± 0.01 e | 0.12 ± 0.01 e | 0.08 ± 0.02 c | |||||
| 3 | 0 | 0.46 ± 0.01 | <0.01 | <0.01 | <0.01 | 0.46 ± 0.01 a | 0.36 ± 0.03 a | 0.21 ± 0.01 a | 0.18 ± 0.01 a | 32 | 15 | 17 | 9 |
| 1 | 0.37 ± 0.04 | <0.01 | <0.01 | <0.01 | 0.37 ± 0.04 b | 0.35 ± 0.01 a | 0.21 ± 0.01 a | 0.17 ± 0.01 ab | |||||
| 3 | 0.35 ± 0.03 | <0.01 | <0.01 | <0.01 | 0.35 ± 0.03 bc | 0.35 ± 0.01 a | 0.19 ± 0.01 b | 0.16 ± 0.02 b | |||||
| 5 | 0.33 ± 0.02 | <0.01 | <0.01 | <0.01 | 0.33 ± 0.02 bc | 0.34 ± 0.01 a | 0.16 ± 0.01 c | 0.11 ± 0.02 c | |||||
| 7 | 0.29 ± 0.03 | <0.01 | <0.01 | 0.02 ± 0.00 | 0.32 ± 0.03 c | 0.28 ± 0.02 b | 0.15 ± 0.01 c | 0.09 ± 0.01 c | |||||
| 14 | 0.27 ± 0.03 | <0.01 | <0.01 | 0.04 ± 0.01 | 0.31 ± 0.02 c | 0.19 ± 0.01 c | 0.12 ± 0.01 d | 0.06 ± 0.01 e | |||||
| Matrix | Site | Mean Residue ± SD 1 (n = 3, mg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dinotefuran | Fluazinam | Indoxacarb | Thiacloprid | ||||||
| Parent | DN | MNG | UF | Total | |||||
| Fresh persimmon | 1 | 0.30 ± 0.04 | <0.01 2 | 0.02 ± 0.02 | 0.02 ± 0.01 | 0.35 ± 0.02 | 0.16 ± 0.02 | 0.14 ± 0.01 | 0.11 ± 0.01 |
| 2 | 0.25 ± 0.03 | <0.01 | 0.10 ± 0.02 | 0.03 ± 0.01 | 0.46 ± 0.05 | 0.16 ± 0.02 | 0.12 ± 0.01 | 0.08 ± 0.02 | |
| 3 | 0.28 ± 0.03 | <0.01 | <0.01 | 0.04 ± 0.01 | 0.33 ± 0.02 | 0.18 ± 0.02 | 0.12 ± 0.01 | 0.06 ± 0.01 | |
| Dried persimmon | 1 | 0.77 ± 0.07 | <0.01 | 0.05 ± 0.01 | 0.10 ± 0.01 | 0.99 ± 0.09 | 0.06 ± 0.00 | 0.04 ± 0.01 | 0.10 ± 0.01 |
| 2 | 0.72 ± 0.04 | <0.01 | 0.11 ± 0.01 | 0.10 ± 0.01 | 1.03 ± 0.04 | 0.04 ± 0.00 | 0.03 ± 0.01 | 0.08 ± 0.01 | |
| Semi-dried persimmon | 1 | 0.50 ± 0.03 | <0.01 | 0.02 ± 0.01 | 0.07 ± 0.01 | 0.62 ± 0.04 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.08 ± 0.02 |
| 2 | 0.46 ± 0.04 | <0.01 | 0.07 ± 0.01 | 0.06 ± 0.00 | 0.65 ± 0.06 | 0.03 ± 0.01 | 0.02 ± 0.00 | 0.07 ± 0.01 | |
| Dried persimmon slice | 1 | 0.65 ± 0.03 | <0.01 | 0.07 ± 0.01 | 0.09 ± 0.00 | 0.89 ± 0.05 | <0.01 | 0.02 ± 0.01 | 0.09 ± 0.01 |
| 2 | 0.66 ± 0.02 | <0.01 | 0.15 ± 0.02 | 0.1 ± 0.03 | 1.05 ± 0.05 | <0.01 | 0.01 ± 0.00 | 0.06 ± 0.02 | |
| Site | Matrix | Dinotefuran | Fluazinam | Indoxacarb | Thiacloprid | ||||
|---|---|---|---|---|---|---|---|---|---|
| PF 1 | RF 2 | PF | RF | PF | RF | PF | RF | ||
| 1 | Dried persimmon | 2.81 ± 0.30 | 0.80 ± 0.09 | 0.39 ± 0.04 | 0.11 ± 0.01 | 0.32 ± 0.07 | 0.08 ± 0.02 | 0.91 ± 0.08 | 0.24 ± 0.02 |
| Dried persimmon slice | 1.76 ± 0.17 | 0.83 ± 0.07 | - | - | 0.12 ± 0.04 | 0.04 ± 0.01 | 0.79 ± 0.04 | 0.24 ± 0.01 | |
| Semi-dried persimmon | 2.53 ± 0.23 | 0.80 ± 0.08 | 0.23 ± 0.07 | 0.11 ± 0.03 | 0.22 ± 0.08 | 0.10 ± 0.04 | 0.76 ± 0.17 | 0.36 ± 0.08 | |
| 2 | Dried persimmon | 2.28 ± 0.29 | 0.64 ± 0.08 | 0.25 ± 0.02 | 0.07 ± 0.01 | 0.27 ± 0.03 | 0.08 ± 0.01 | 0.99 ± 0.25 | 0.29 ± 0.07 |
| Dried persimmon slice | 2.31 ± 0.26 | 0.75 ± 0.08 | - | - | 0.08 ± 0.00 | 0.03 ± 0.00 | 0.71 ± 0.09 | 0.25 ± 0.03 | |
| Semi-dried persimmon | 1.45 ± 0.27 | 0.64 ± 0.12 | 0.16 ± 0.04 | 0.07 ± 0.02 | 0.16 ± 0.01 | 0.09 ± 0.00 | 0.89 ± 0.10 | 0.24 ± 0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, H.-H.; Jo, S.-H.; Shin, H.-W.; Kim, D.-J.; Ham, Y.-J.; Kim, J.-Y.; Kim, D.-B.; Kwon, H.-Y.; Kyung, K.-S. Dissipation and Residue Pattern of Dinotefuran, Fluazinam, Indoxacarb, and Thiacloprid in Fresh and Processed Persimmon Using LC-MS/MS. Foods 2022, 11, 416. https://doi.org/10.3390/foods11030416
Noh H-H, Jo S-H, Shin H-W, Kim D-J, Ham Y-J, Kim J-Y, Kim D-B, Kwon H-Y, Kyung K-S. Dissipation and Residue Pattern of Dinotefuran, Fluazinam, Indoxacarb, and Thiacloprid in Fresh and Processed Persimmon Using LC-MS/MS. Foods. 2022; 11(3):416. https://doi.org/10.3390/foods11030416
Chicago/Turabian StyleNoh, Hyun-Ho, Seung-Hyeon Jo, Hyeon-Woo Shin, Dong-Ju Kim, Young-Jin Ham, Jun-Young Kim, Dan-Bi Kim, Hye-Young Kwon, and Kee-Sung Kyung. 2022. "Dissipation and Residue Pattern of Dinotefuran, Fluazinam, Indoxacarb, and Thiacloprid in Fresh and Processed Persimmon Using LC-MS/MS" Foods 11, no. 3: 416. https://doi.org/10.3390/foods11030416






