Stand Biomass at Treeline Ecotone in Russian Subarctic Mountains Is Primarily Related to Species Composition but Its Dynamics Driven by Improvement of Climatic Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Trees and Stands Data Sampling and Calculation
3. Results
3.1. Current Stand Structure and Biomass in Forest-Tundra Transition of Three Subarctic Regions
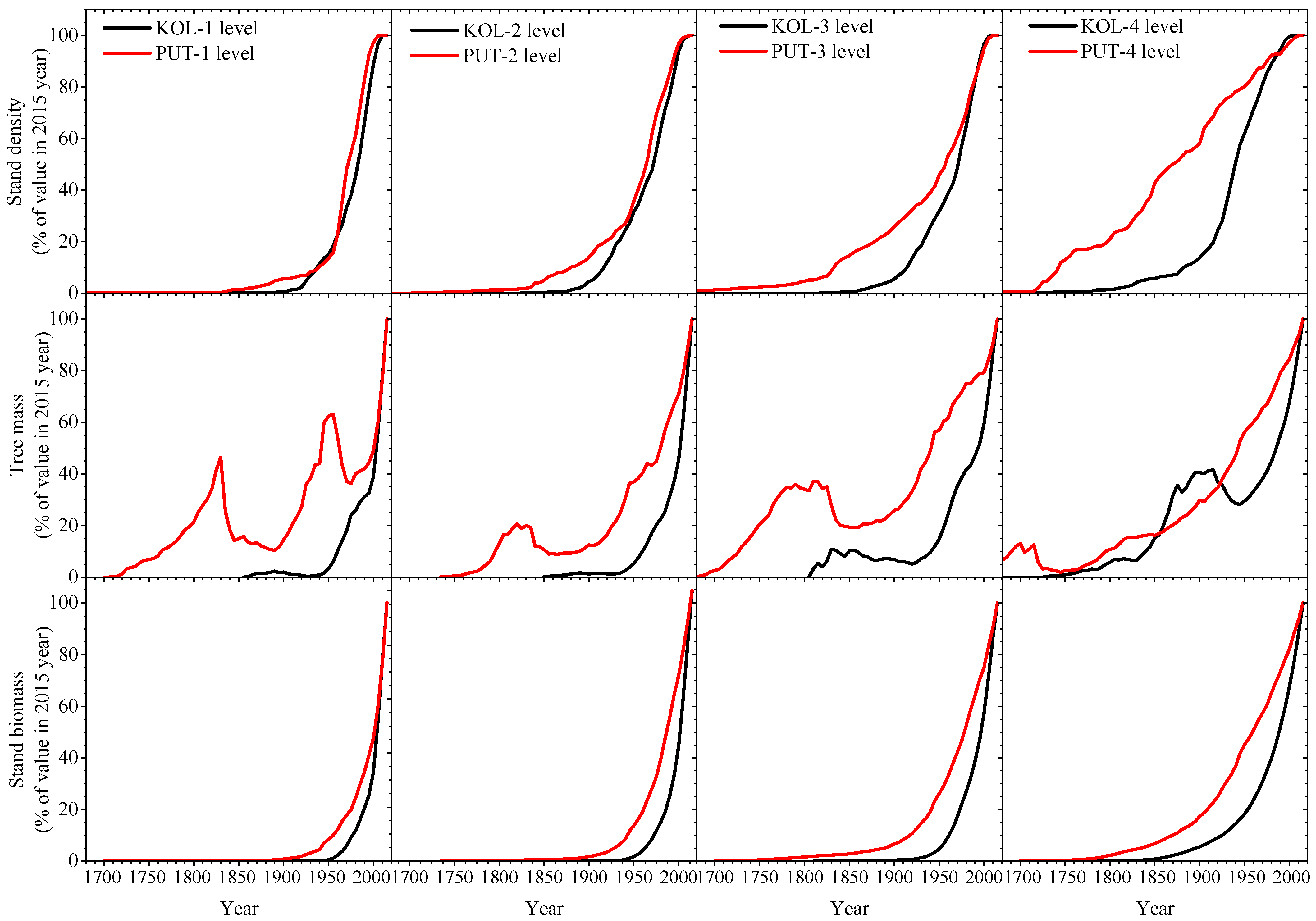
3.2. Changes of Tree Stand Density and Productivity over Last Centuries at Climatically Contrast Regions
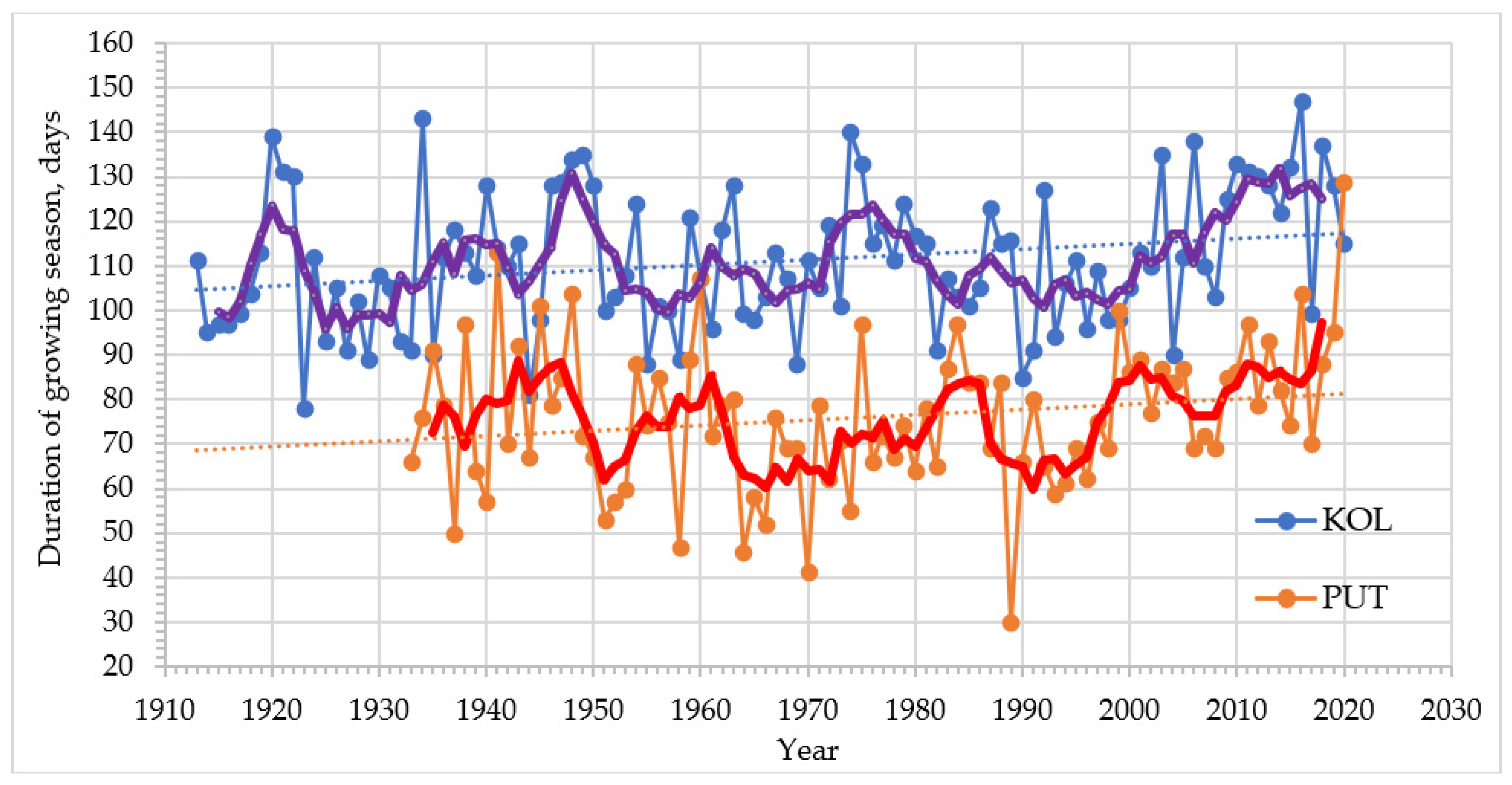
3.3. Climatic Conditions on Treeline Ecotone Sites in Three Subarctic Regions
4. Discussion
4.1. Climatic, Site Conditions, and Dominant Species at Treeline
4.2. Little Longitudinal Patterns in Stand Biomass
4.3. Altitudinal Changes of Microclimate and Stand Biomass
4.4. Climate and Stands Changes
4.5. Consequences for C Sequestration
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IPCC. 2021: Summary for Policymakers. In Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Masson-Delmotte, V., Zhai, P., Pirani, A., Connors, S.L., Péan, C., Berger, S., Caud, N., Chen, Y., Goldfarb, L., Gomis, M.I., et al., Eds.; Cambridge University Press: Cambridge, UK, 2021; ISBN 978-92-9169-158-6. [Google Scholar]
- Pepin, N.; Bradley, R.S.; Diaz, H.F.; Baraer, M.; Caceres, E.B.; Forsythe, N.; Fowler, H.; Greenwood, G.; Hashmi, M.Z.; Liu, X.D.; et al. Elevation-dependent warming in mountain regions of the world. Nat. Clim. Chang. 2015, 5, 424–430. [Google Scholar] [CrossRef] [Green Version]
- Hudson, J.M.G.; Henry, G.H.R. Increased plant biomass in a high arctic heath community from 1981 to 2008. Ecology 2009, 90, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Alpine Treelines: Functional Ecology of the Global High Elevation Tree Limits; Springe: Basel, Switzerland, 2012; ISBN 978-3034-80395-3. [Google Scholar]
- Dirnböck, T.; Essl, F.; Rabitsch, W. Disproportional risk for habitat loss of high-altitude endemic species under climate change. Glob. Chang. Biol. 2011, 17, 990–996. [Google Scholar] [CrossRef]
- Pauli, H.; Gottfried, M.; Dullinger, S.; Abdaladze, O.; Akhalkatsi, M.; Alonso, J.L.B.; Coldea, G.; Dick, J.; Erschbamer, B.; Calzado, R.F.; et al. Recent plant diversity changes on Europe’s mountain summits. Science 2012, 336, 353–355. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, S.; Jump, A.S. Consequences of Treeline Shifts for the Diversity and Function of High Altitude Ecosystems. Arctic. Antarct. Alp. Res. 2014, 46, 829–840. [Google Scholar] [CrossRef] [Green Version]
- Moiseev, P.A.; Bubnov, M.O.; Devi, N.M.; Nagimov, Z.Y. Changes in the structure and phytomass of tree stands at the upper limit of their growth in the Southern Urals. Russ. J. Ecol. 2016, 47, 219–227. [Google Scholar] [CrossRef]
- Devi, N.M.; Kukarskih, V.V.; Galimova, A.A.; Mazepa, V.S.; Grigoriev, A.A. Climate change evidence in tree growth and stand productivity at the upper treeline ecotone in the Polar Ural Mountains. For. Ecosyst. 2020, 7, 2–16. [Google Scholar] [CrossRef] [Green Version]
- Shevtsova, I.; Herzschuh, U.; Heim, B.; Schulte, L.; Stünzi, S.; Pestryakova, L.; Zakharov, E.; Kruse, S. Recent above-ground biomass changes in central Chukotka (Russian Far East) using field sampling and Landsat satellite data. Biogeosciences 2021, 18, 3343–3366. [Google Scholar] [CrossRef]
- Holtmeier, F.-K. Mountain Timberlines: Ecology, Patchiness, and Dynamics; Springer: Berlin/Heidelberg, Germany, 2009; ISBN 1402097042. [Google Scholar]
- Payette, S. Contrasted dynamics of Northern Labrador tree lines caused by climate change and migrational lag. Ecology 2007, 88, 770–780. [Google Scholar] [CrossRef]
- Holtmeier, F.K.; Broll, G. Altitudinal and polar treelines in the northern hemisphere causes and response to climate change. Polarforschung 2009, 79, 139–153. [Google Scholar] [CrossRef]
- Wieser, G. Alpine and Polar Treelines in A Changing Environment. Forest 2020, 11, 254. [Google Scholar] [CrossRef] [Green Version]
- Harsch, M.A.; Hulme, P.E.; McGlone, M.S.; Duncan, R.P. Are treelines advancing? A global meta-analysis of treeline response to climate warming. Ecol. Lett. 2009, 12, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Hagedorn, F.; Shiyatov, S.G.; Mazepa, V.S.; Devi, N.M.; Grigor’ev, A.A.; Bartysh, A.A.; Fomin, V.V.; Kapralov, D.S.; Terent’ev, M.; Bugman, H.; et al. Treeline advances along the Ural mountain range – driven by improved winter conditions? Glob. Chang. Biol. 2014, 20, 3530–3543. [Google Scholar] [CrossRef] [PubMed]
- Davis, E.L.; Brown, R.; Daniels, L.; Kavanagh, T.; Gedalof, Z. Regional variability in the response of alpine treelines to climate change. Clim. Change 2020, 62, 1365–1384. [Google Scholar] [CrossRef]
- Bernoulli, M.; Körner, C. Dry matter allocation in treeline trees. Phyt.-Ann. Rei Bot. 1999, 39, 7–12. [Google Scholar]
- Grafius, D.R.; Malanson, G.P. Biomass distributions in dwarf tree, krummholz, and tundra vegetation in the alpine treeline ecotone. Phys. Geogr. 2015, 36, 337–352. [Google Scholar] [CrossRef]
- Xin, L.; Yu, N.; Gao-qiang, K.; Tia, L. Contrasting changes in above- and below ground biomass allocation across treeline ecotones in southeast Tibet. J. Mt. Sci. 2016, 13, 2036–2045. [Google Scholar] [CrossRef]
- Rai, I.D.; Padalia, H.; Singh, G.; Adhikari, B.S.; Rawat, G.S. Vegetation dry matter dynamics along treeline ecotone in Western Himalaya, India. Trop. Ecol. 2020, 61, 116–127. [Google Scholar] [CrossRef]
- Speed, J.D.M.; Martinsen, V.; Hester, A.J.; Holand Mulder, J.; Mysterud, A.; Austrheim, G. Continuous and discontinuous variation in ecosystem carbon stocks with elevation across a treeline ecotone. Biogeosciences 2015, 12, 1615–1627. [Google Scholar] [CrossRef] [Green Version]
- Hagedorn, F.; Dawes, M.A.; Bubnov, M.O.; Devi, N.M.; Grigoriev, A.A.; Mazepa, V.S.; Nagimov, Z.Y.; Shiyatov, S.G.; Moiseev, P.A. Latitudinal decline in stand biomass and productivity at the elevational treeline in the Ural mountains despite a common thermal growth limit. J. Biogeogr. 2020, 47, 1–16. [Google Scholar] [CrossRef]
- Rees, W.G.; Tomaney, J.; Tutubalina, O.; Zharko, V.; Bartalev, S. Estimation of boreal forest growing stock volume in russia from sentinel-2 msi and land cover classification. Remote Sens. 2021, 13, 4483. [Google Scholar] [CrossRef]
- Coops, N.C.; Morsdorf, F.; Schaepman, M.E.; Zimmermann, N.E. Characterization of an alpine tree line using airborne LiDAR data and physiological modeling. Glob. Chang. Biol. 2013, 19, 3808–3821. [Google Scholar] [CrossRef] [PubMed]
- Montesano, P.M.; Nelson, R.F.; Dubayah, R.O.; Sun, G.; Cook, B.D.; Ranson, K.J.R.; Næsset, E.; Kharuk, V. The uncertainty of biomass estimates from LiDAR and SAR across a boreal forest structure gradient. Remote Sens. Environ. 2014, 154, 398–407. [Google Scholar] [CrossRef]
- Bolton, D.K.; Coops, N.C.; Hermosilla, T.; Wulder, M.A.; White, J.C. Evidence of vegetation greening at alpine treeline ecotones: Three decades of Landsat spectral trends informed by lidar-derived vertical structure. Environ. Res. Lett. 2018, 13, 084022. [Google Scholar] [CrossRef]
- Shiyatov, S.G.; Terent’ev, M.M.; Fomin, V.V.; Zimmermann, N.E. Altitudinal and horizontal shifts of the upper boundaries of open and closed forests in the Polar Urals in the 20th century. Russ. J. Ecol. 2007, 38, 223–227. [Google Scholar] [CrossRef]
- Rinn, F. TSAP V 3.5: Computer Program for Tree-Ring Analysis and Presentation; RinnTech: Heidelberg, Germany, 1998. [Google Scholar]
- Holmes, R.L. Dendrochronological Program Library (Computer Program); University of Arizona, Laboratory of Tree Ring Research: Tuscon, AZ, USA, 1995. [Google Scholar]
- Braeker, O.U. Der Alterstrend bei Jahrringdichten und Jahrringbreiten von Nadelhölzern und sein Ausgleich. Mitt. Forstl. Bundes-Vers. Wien 1981, 142, 75–102. [Google Scholar]
- Moiseev, P.A.; Bartysh, A.A.; Nagimov, Z.Y. Climate changes and tree stand dynamics at the upper limit of their growth in the North Ural mountains. Russ. J. Ecol. 2010, 41, 486–497. [Google Scholar] [CrossRef]
- Körner, C.; Paulsen, J. A world-wide study of high altitude treeline temperatures. J. Biogeogr. 2004, 31, 713–732. [Google Scholar] [CrossRef]
- Hoch, G. Reciprocal root-shoot cooling and soil fertilization effects on the seasonal growth of two treeline conifer species. Plant Ecol. Divers. 2013, 6, 21–30. [Google Scholar] [CrossRef]
- Hoch, G.; Körner, C. Global patterns of mobile carbon stores in trees at the high-elevation tree line. Glob. Ecol. Biogeogr. 2012, 21, 861–871. [Google Scholar] [CrossRef]
- Rossi, S.; Deslauriers, A.; Anfodillo, T.; Carraro, V. Evidence of threshold temperatures for xylogenesis in conifers at high altitudes. Oecologia 2007, 152, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gorchakovsky, P.L.; Shiaytov, S.G. The Upper Forest Limit in the Mountains of the Boreal Zone of the USSR. Arct. Alp. Res. 1978, 10, 349–363. [Google Scholar] [CrossRef]
- Juntunen, V.; Neuvonen, S.; Norokorpi, Y.; Tasanen, T. Potential for timberline advance in northern Finland, as revealed by monitoring during 1983–99. Arctic 2002, 55, 348–361. [Google Scholar] [CrossRef] [Green Version]
- Kullman, L.; Öberg, L. Post-Little Ice Age tree line rise and climate warming in the Swedish Scandes: A landscape ecological perspective. J. Ecol. 2009, 97, 415–429. [Google Scholar] [CrossRef]
- Kammer, A.; Hagedorn, F.; Shevchenko, I.; Leifeld, J.; Guggenberger, G.; Goryacheva, T.; Rigling, A.; Moiseev, P. Treeline shifts in the Ural mountains affect soil organic matter dynamics. Glob. Chang. Biol. 2009, 15, 1570–1583. [Google Scholar] [CrossRef]
- Hagedorn, F.; Gavazov, K.; Alexander, J.M. Above- and belowground linkages shape responses ofmountain vegetation to climate change. Science 2019, 365, 1119–1123. [Google Scholar] [CrossRef]
- Moiseev, P.A.; Galimova, A.A.; Bubnov, M.O.; Devi, N.M.; Fomin, V.V. Tree Stands and Their Productivity Dynamics at the Upper Growing Limit in Khibiny on the Background of Modern Climate Changes. Russ. J. Ecol. 2019, 50, 431–444. [Google Scholar] [CrossRef]
- Grigor’ev, A.A.; Devi, N.M.; Kukarskikh, V.V.; V’yukhin, S.O.; Galimova, A.A.; Moiseev, P.A.; Fomin, V.V. Structure and Dynamics of Tree Stands at the Upper Timberline in the Western Part of the Putorana Plateau. Russ. J. Ecol. 2019, 50, 311–322. [Google Scholar] [CrossRef]
- Grigoriev Andrey, A.; Shalaumova Yulia, V.; Vyukhin Sergey, O.; Balakin Dmitriy, S.; Kukarskikh Vladimir, V.; Vyukhina Arina, A.; Camarero, J.; Julio, M.P.A. Upward treeline shifts in two regions of subarctic Russia are governed by summer thermal and winter snow conditions. Forests 2022, 13, 174. [Google Scholar] [CrossRef]
- Bunn, A.G.; Goetz, S.J.; Kimball, J.S.; Zhang, K. Northern high-latitude ecosystems respond to climate change. Eos 2007, 88, 333–335. [Google Scholar] [CrossRef]
- Kirdyanov, A.V.; Hagedorn, F.; Knorre, A.A.; Fedotova, E.V.; Vaganov, E.A.; Naurzbaev, M.M.; Moiseev, P.A.; Rigling, A. 20th century tree-line advance and vegetation changes along an altitudinal transect in the Putorana Mountains, northern Siberia. Boreas 2012, 41, 56–67. [Google Scholar] [CrossRef]
- Tømmervik, H.; Johansen, B.; Riseth, J.Å.; Karlsen, S.R.; Solberg, B.; Høgda, K.A. Above ground biomass changes in the mountain birch forests and mountain heaths of Finnmarksvidda, northern Norway, in the period 1957-2006. For. Ecol. Manag. 2009, 257, 244–257. [Google Scholar] [CrossRef]

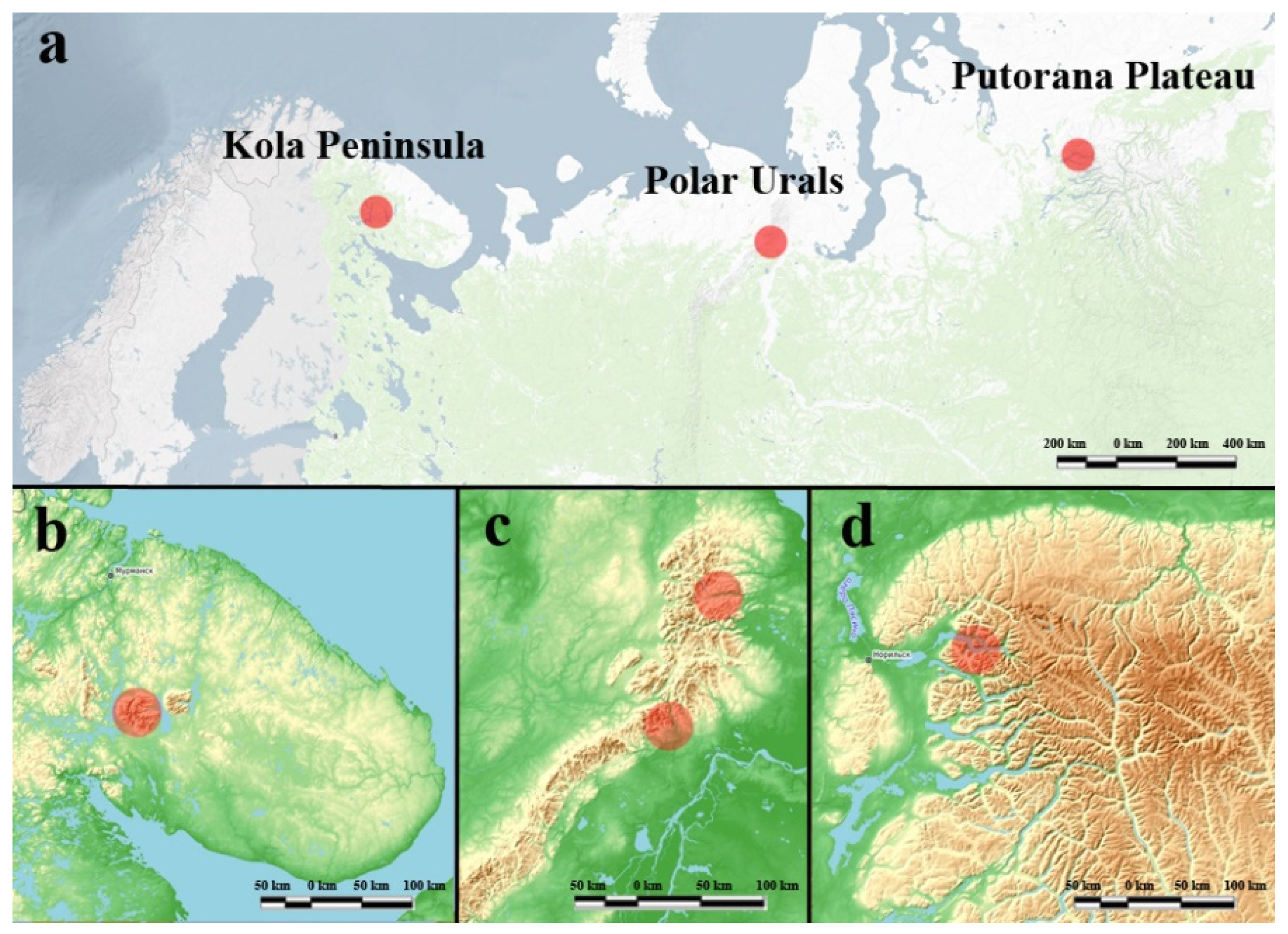
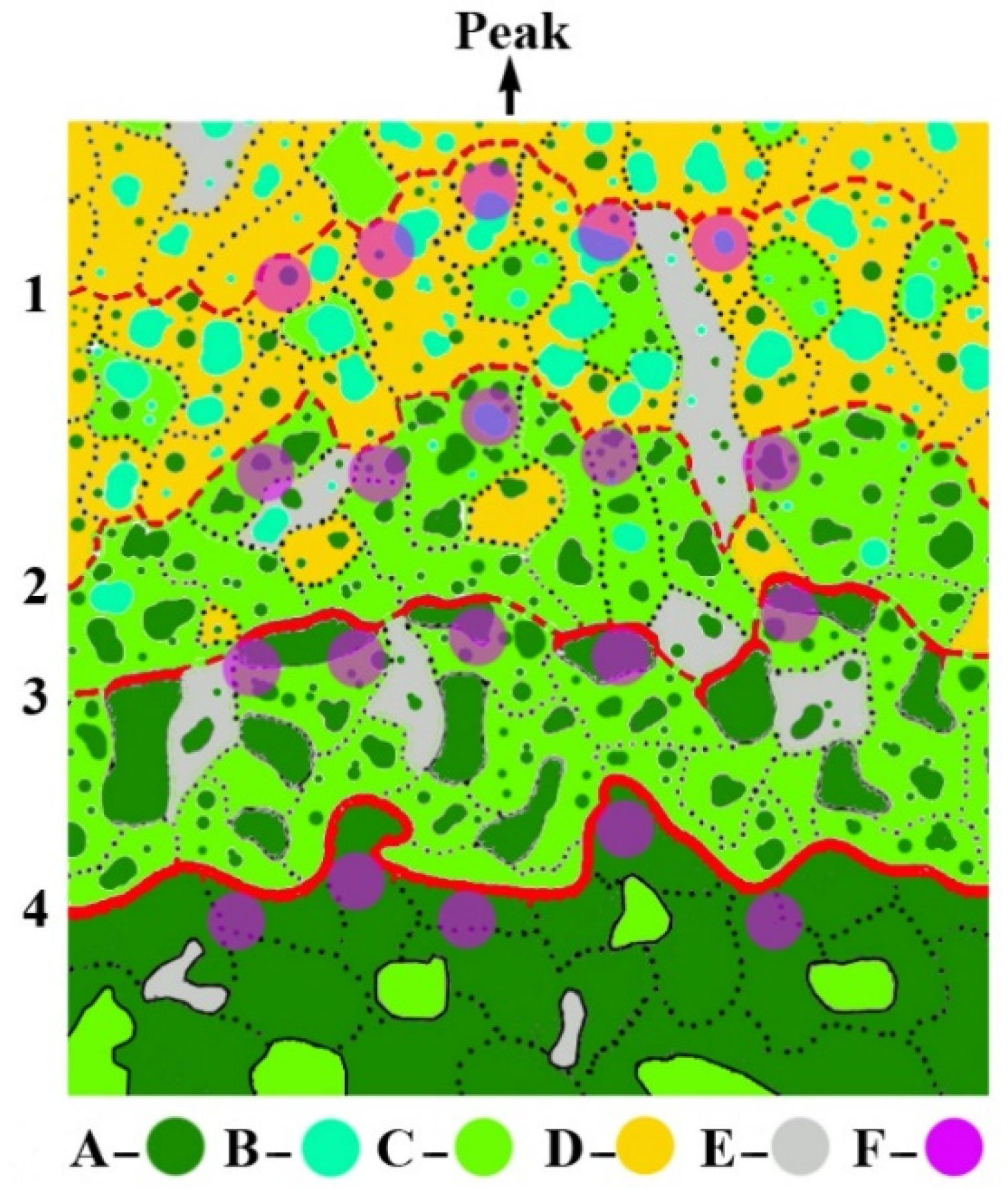

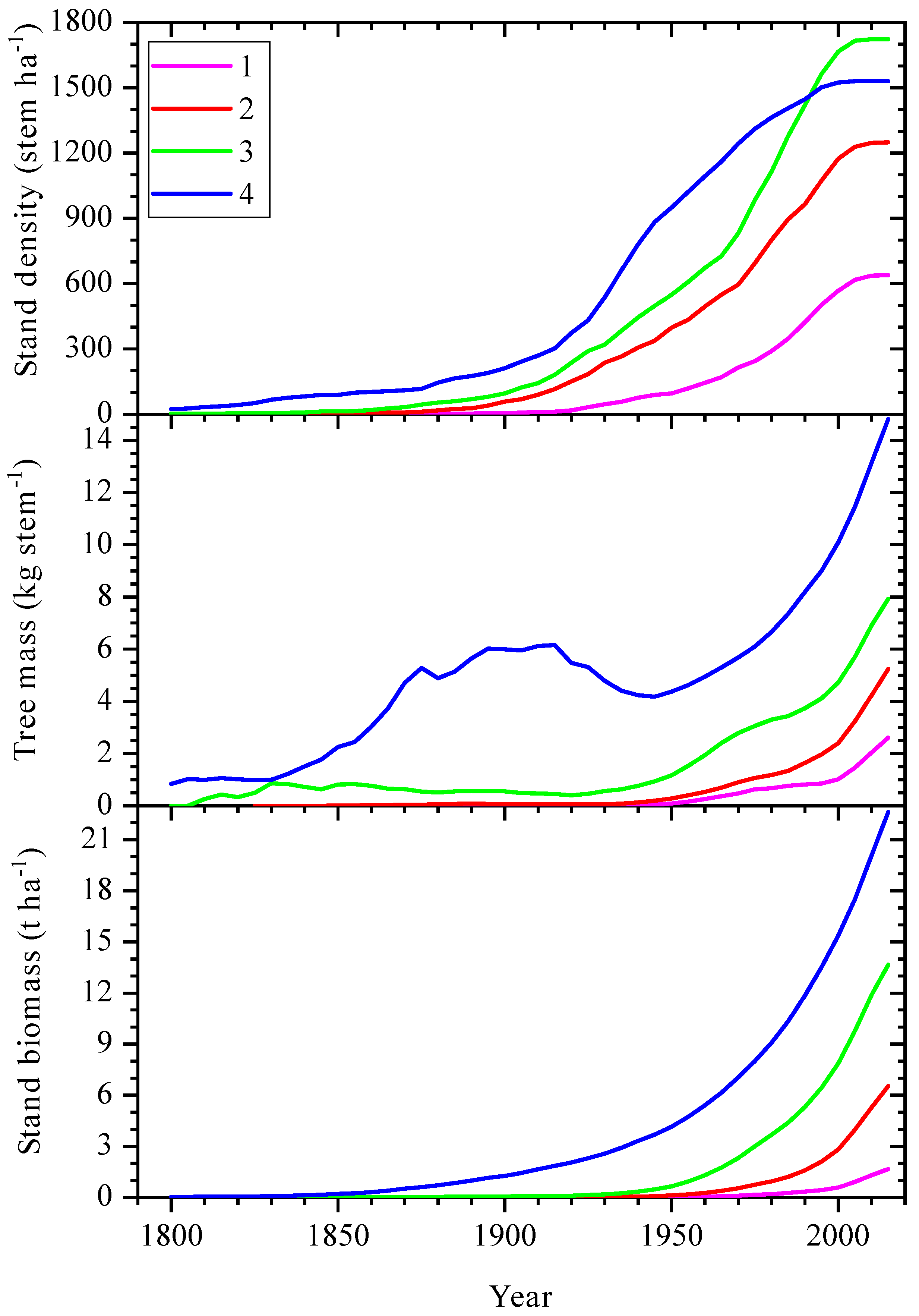
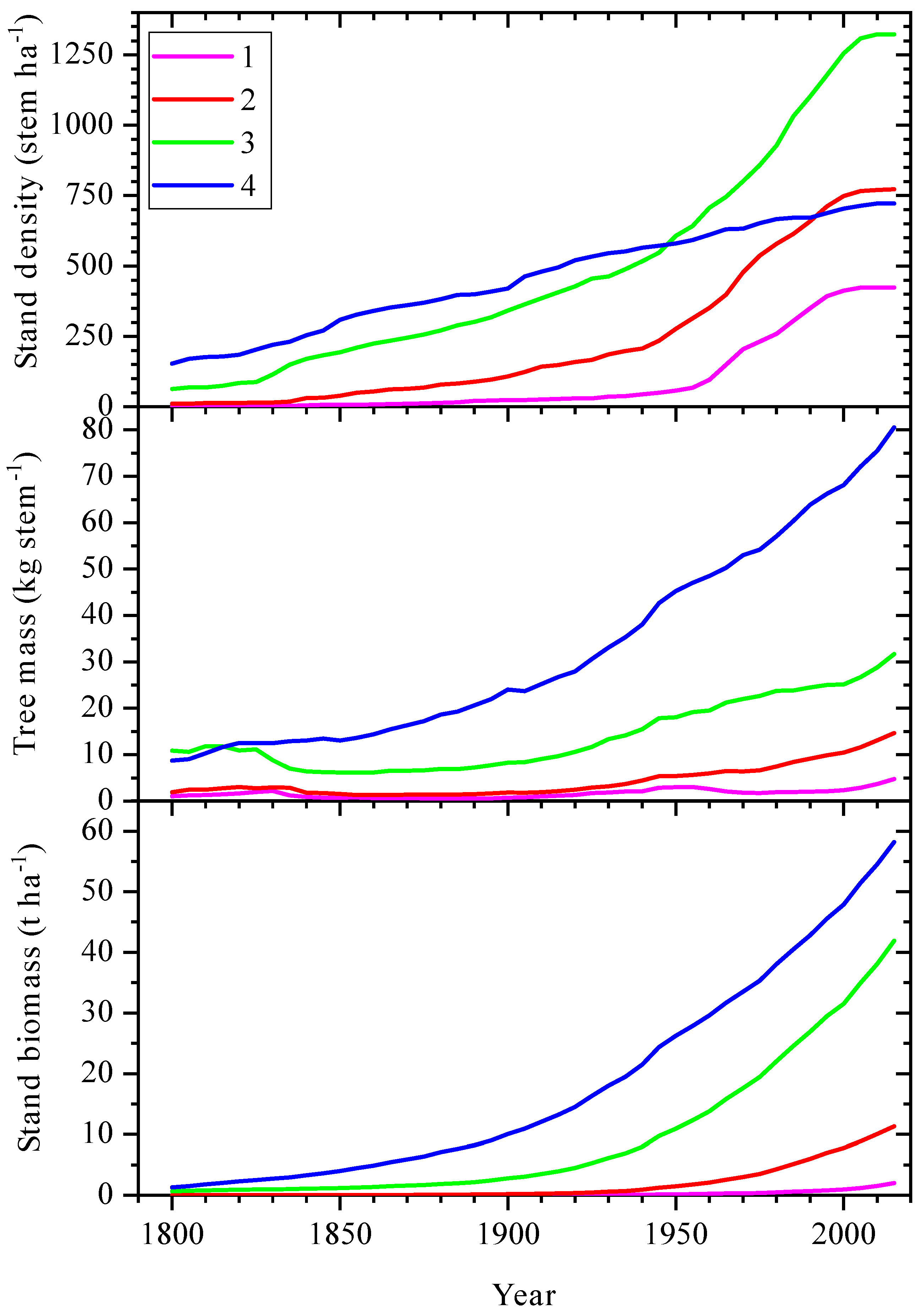
| Region | Kola Peninsula | Polar Urals | Putorana Plateau |
|---|---|---|---|
| Local name of studied mountains | Khibiny massif | Tchernaya, Slantzevay, Malikpe | Sukhie Gory massif |
| Geographical coordinates N E | 67.55°–67.95° 33.25°–34.40° | 66.75°–67.95° 65.50°–66.95° | 69.34°–69.45° 90.50°–90.95° |
| Geology | Plutonic rock | Gabbro, shale phyllitic; quartzite; sandstone arkosic | Crystalline basalt |
| Mean July air temperature, °C 1 | +13.5 (+8.8) | +14.2 (+8.5) | +12.8 (+8.5) |
| Mean January air temperature, °C 1 | −12.6 (−12.1) | −22.1 (−17.9) | −28.0 (−26.7) |
| Annual precipitation, mm 1 | 928 (1342) | 460 (881) | 579 (699) |
| Snowpack depth, cm | 1.0–1.5 | 0.8–1.0 | 0.6–0.8 |
| Snow cover duration, days | 220–250 | 230–250 | 240–270 |
| Prevailing winds in summer | W, SW | W, SW | W, NW |
| Prevailing winds in winter | W, NW, | W, NW, | S, SE |
| Mean altitude and range of treeline, m a.s.l. | 397 (320–660) | 242 (180–350) | 409 (250–710) |
| Dominant tree species within treeline ecotone 2 | BP, PO, PSy | LS, BP | LG |
| Region | Transect Name | Latitude (°N) | Longi-tude (°E) | Altitudinal Range (m a.s.l.) 1 | Horizontal Distance (m) | Slope (%) | Aspect (°) | Prevail Species 4 | Instal-Led Plots | Studied Area, m2 | Sampled Trees | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low Part 2 | Upper Part 3 | Low Part 2 | Upper Part 3 | ||||||||||
| Kola Penin-sula | IMA-I | 67.83 | 33.34 | 365–391–410 | 99 | 116 | 15.3 | 9.4 | 180 | PSy | 12 | 2400 | 857 |
| IMA-II | 67.84 | 33.34 | 345–370–414 | 103 | 240 | 14.0 | 10.6 | 0 | BP | 12 | 2400 | 323 | |
| KOA-I | 67.59 | 34.08 | 265–323–409 | 474 | 409 | 15.3 | 9.4 | 112 | BP | 10 | 3200 | 968 | |
| KOA-II | 67.59 | 34.07 | 281–334–434 | 226 | 307 | 11.5 | 19.0 | 123 | BP | 10 | 3200 | 577 | |
| KOA-III | 67.58 | 34.07 | 306–343–447 | 135 | 433 | 16.0 | 13.8 | 152 | BP | 8 | 2800 | 696 | |
| VUD-I | 67.66 | 33.58 | 479–497–602 | 221 | 434 | 4.5 | 14.0 | 85 | BP | 12 | 2400 | 285 | |
| VUD-II | 67.67 | 33.58 | 446–481–552 | 292 | 397 | 6.8 | 10.2 | 107 | BP | 12 | 2400 | 225 | |
| VUD-III | 67.67 | 33.57 | 490–542–630 | 135 | 158 | 22.6 | 34.2 | 169 | BP | 12 | 2400 | 342 | |
| VUD-IV | 67.68 | 33.62 | 514–569–663 | 105 | 217 | 31.2 | 25.7 | 257 | BP | 12 | 2400 | 560 | |
| Polar Urals | PUR1 | 66.81 | 65.58 | 189–310 | – | 1879 | – | 3.7 | 127 | LS | 54 | 21,600 | 699 |
| PUR2 | 66.79 | 65.52 | 230–267 | – | 535 | – | 4.0 | 55 | LS | 9 | 3600 | 217 | |
| PUR3 | 66.77 | 65.50 | 241–292 | – | 1003 | – | 2.9 | 175 | LS | 9 | 3600 | 145 | |
| SLA | 66.91 | 65.75 | 230–260–310 | 160 | 220 | 10.6 | 12.8 | 270 | LS | 34 | 13,300 | 546 | |
| SHU1 | 67.85 | 66.87 | 259–297 | – | 228 | – | 9.6 | 193 | LS | 12 | 4800 | 294 | |
| SHU2 | 67.85 | 66.84 | 237–285 | – | 217 | – | 12.9 | 180 | LS | 5 | 2000 | 188 | |
| Putorana Plateau | LAM-I | 69.40 | 90.75 | 515–563–604 | 89 | 95 | 32.4 | 26.1 | 210 | LG | 12 | 4800 | 450 |
| LAM-II | 69.41 | 90.69 | 358–413–550 | 97 | 260 | 34.5 | 30.6 | 110 | LG | 15 | 6000 | 464 | |
| LAM-III | 69.36 | 90.73 | 570–614–708 | 100 | 189 | 25.9 | 30.1 | 197 | LG | 9 | 3600 | 192 | |
| LAM-IV | 69.41 | 90.88 | 458–530–630 | 177 | 206 | 24.2 | 29.1 | 270 | LG | 12 | 4800 | 547 | |
| LAM-V | 69.42 | 90.89 | 230–258–400 | 56 | 230 | 30.3 | 30.5 | 335 | LG | 11 | 4500 | 320 | |
| Region | Species | Growth Form | a | b | n | R2 |
|---|---|---|---|---|---|---|
| Kola Peninsula | PO | single | 0.0365 | 2.221 | 11 | 0.99 |
| BP | multi | 0.0145 | 2.509 | 53 | 0.98 | |
| PSy | single | 0.0139 | 2.685 | 16 | 0.98 | |
| Polar Urals | LS | single | 0.0141 | 2.587 | 21 | 0.99 |
| Putorana Plateau | LG | single | 0.0116 | 2.725 | 38 | 0.99 |
| Levels | Kola Peninsula | Polar Urals | Putorana Plateau |
|---|---|---|---|
| Sum of crown projections, m2 ha−1 | |||
| 1 | 1303 ± 879 (2860) | 900 ± 861 (2583) | 1013 ± 696 (1570) |
| 2 | 4656 ± 1351 (6759) | 4526 ± 2442 (7669) | 3416 ± 2847 (4702) |
| 3 | 6581 ± 1564 (10,244) | 7483 ± 2986 (11,743) | 8461 ± 3120 (13,096) |
| 4 | 10,743 ± 1524 (13,301) | – | 6137 ± 1251 (7065) |
| Stand density, trees ha−1 | |||
| 1 | 356 ± 188 (733) | 303 ± 318 (838) | 481 ± 303 (727) |
| 2 | 1099 ± 450 (1526) | 835 ± 379 (1363) | 684 ± 322 (1217) |
| 3 | 1366 ± 686 (2525) | 840 ± 335 (1475) | 1149 ± 420 (1475) |
| 4 | 1580 ± 341(2500) | – | 898 ± 363 (1467) |
| Stem basal diameter, cm | |||
| 1 | 4.1 ± 1.2 (6.2) | 6.0 ± 3.1 (10.0) | 7.4 ± 1.9 (9.0) |
| 2 | 6.0 ± 1.4 (8.5) | 12.2 ± 5.3 (20.2) | 11.0 ± 3.0 (15.3) |
| 3 | 7.0 ± 2.0 (9.1) | 19.2 ± 4.4 (25.1) | 14.3 ± 7.2 (17.6) |
| 4 | 8.9 ± 1.5 (11.5) | – | 23.6 ± 1.8 (29.9) |
| Tree height, m | |||
| 1 | 1.4 ± 0.5 (2.4) | 2.5 ± 0.8 (3.7) | 3.1 ± 0.6 (3.8) |
| 2 | 2.2 ± 0.4 (2.8) | 5.7 ± 1.9 (9.0) | 5.0 ± 1.0 (6.6) |
| 3 | 2.9 ± 0.9 (3.7) | 7.5 ± 2.2 (11.0) | 6.2 ± 2.5 (7.4) |
| 4 | 5.6 ± 1.0 (7.4) | – | 9.8 ± 3.4 (12.7) |
| Tree age, years | |||
| 1 | 45 ± 13 (72) | 38 ± 13 (66) | 51 ± 15 (56) |
| 2 | 58 ± 19 (92) | 94 ± 38 (156) | 78 ± 14 (91) |
| 3 | 62 ± 16 (91) | 134 ± 34 (189) | 100 ± 58 (151) |
| 4 | 85 ± 10 (107) | – | 187 ± 67 (252) |
| Aboveground stand biomass, t ha−1 | |||
| 1 | 1.6 ± 1.6 (5.5) | 2.1 ± 2.1 (5.7) | 2.2 ± 2.1 (3.9) |
| 2 | 7.6 ± 3.0 (8.8) | 16.1 ± 8.4 (45.4) | 12.3 ± 8.1 (24.3) |
| 3 | 15.3 ± 7.4 (20.7) | 40.6 ± 12.1 (99.6) | 38.2 ± 18.3 (56.4) |
| 4 | 27.5 ± 10.6 (37.7) | – | 61.5 ± 12.5 (76.1) |
| Aboveground tree mass, kg stem−1 | |||
| 1 | 3.7 ± 1.9 (7.4) | 5.2 ± 3.9 (11.3) | 5.4 ± 3.7 (9.7) |
| 2 | 7.8 ± 3.9 (17.9) | 22.0 ± 12.3 (40.8) | 21.0± 10.7 (47.0) |
| 3 | 14.8 ± 11.6 (44.7) | 55.0 ± 26.8 (97.1) | 34.8 ± 19.3 (46.8) |
| 4 | 17.1 ± 4.6 (24.5) | - | 88.2 ± 35.3 (147.1) |
| Annual individual tree productivity, kg tree−1 yr−1 | |||
| 1 | 0.083 (0.175) | 0.138 (0.332) | 0.107 (0.186) |
| 2 | 0.135 (0.498) | 0.234 (0.502) | 0.269 (0.626) |
| 3 | 0.237 (0.553) | 0.411 (0.630) | 0.347 (0.564) |
| 4 | 0.200 (0.255) | – | 0.473 (0.727) |
| Long-term net stand productivity, t ha−1 yr−1 | |||
| 1 | 0.04 ± 0.03 (0.11) | 0.05 ± 0.06 (0.17) | 0.04 ± 0.02 (0.07) |
| 2 | 0.15 ± 0.08 (0.22) | 0.16 ± 0.05 (0.56) | 0.16 ± 0.09 (0.32) |
| 3 | 0.26 ± 0.15 (0.32) | 0.33 ± 0.14 (0.85) | 0.38 ± 0.04 (0.44) |
| 4 | 0.31 ± 0.09 (0.44) | – | 0.34 ± 0.10 (0.47) |
| Massif | Position by Relation to Closed Forest Line | Width of Transect Part | Within General Width of Transect Part | Per 1 m of Transect Part | ||||
|---|---|---|---|---|---|---|---|---|
| 1985–2017 | 1955–2017 | 1910–2017 | 1985–2017 | 1955–2017 | 1910–2017 | |||
| Khibiny | above | 300 ± 121 | 175 ± 84 | 215 ± 97 | 222 ± 97 | 0.6 ± 0.3 | 0.7 ± 0.3 | 0.8 ± 0.3 |
| below | 202 ± 125 | 264 ± 173 | 358 ± 224 | 397 ± 245 | 1.4 ± 0.7 | 1.8 ± 0.8 | 2.1 ± 1.0 | |
| Sukhie gory | above | 239± 117 | 148 ± 55 | 236 ± 78 | 285 ± 95 | 0.7 ± 0.2 | 1.1 ± 0.3 | 1.3 ± 0.3 |
| below | 104 ± 44 | 207 ± 67 | 342 ± 124 | 463 ± 193 | 2.2 ± 0.9 | 3.5 ± 1.1 | 4.6 ± 1.1 | |
| Regional Location | Center of Kola Peninsula | Western Slope of Polar Urals | Eastern Slope of Polar Urals | West of Putorana Plateau |
|---|---|---|---|---|
| Treeline altitudes (mean and range), m a.s.l. | 397 (320–660) | 210 (160–270) | 242 (180–350) | 409 (250–710) |
| June–August air temperature, °C | +11.0 ± 1.2 | +10.4 ± 1.7 | +11.5 ± 2.2 | +8.6 ± 1.4 |
| November–March air temperature, °C | −7.4 ± 1.0 | −16.5 ± 2.1 | −15.9 ± 2.0 | −19.2 ± 1.3 |
| Season with temperatures >5 °C, days | 104 | 101 | 101 | 72 |
| Growing degree days (>5 °C), GDD | 543 | 524 | 552 | 364 |
| June–August precipitation, mm | 276 ± 92 | 214 ± 73 | 212 ± 60 | 168 ± 48 |
| November–March precipitation, mm | 521 ± 125 | 205 ± 46 | 155 ± 31 | 304 ± 92 |
| Sum of sunshine hours during summer | 659 ± 131 | 690 ± 127 | 740 ± 108 | 786 ± 136 |
| Mean July soil temperature, °C (year) | 2018 | 2018 | 2005 | 2018 |
| in tundra | +10.3 ± 1.4 | +11.6 ± 3.0 | +12.1 ± 3.0 | +11.5 ± 3.5 |
| on sparse stand line | +10.8 ± 1.2 | – | +14.5 ± 1.9 | +10.8 ± 3.0 |
| on open forest line | +11.3 ± 1.2 | – | +11.7 ± 1.9 | +10.4 ± 2.0 |
| on closed forest line | +12.4 ± 1.5 | – | +13.6 ± 3.3 | +8.6 ± 1.5 |
| Mean February soil temperature, °C (year) | 2018 | 2018 | 2004 | 2018 |
| in tundra | +0.3 ± 0.2 | −5.8 ± 2,5 | −12.4 ± 2.4 | −19.8 ± 1.9 |
| on sparse stand line | +0.4 ± 0.2 | – | −8.6 ± 1.7 | −16.7 ± 1.0 |
| on open forest line | +0.6 ± 0.2 | – | −1.9 ± 0.7 | −6.2 ± 0.3 |
| on closed forest line | +0.6 ± 0.3 | – | −1.4 ± 0.3 | −5.3 ± 0.3 |
| Snow depth, cm (year) | 2019 | 2018 | 2006 | 2019 |
| in tundra | 107 ± 35 (29–234) | 76 ± 26 | 19 ± 4 (3–30) | 21 ± 7 (0–105) |
| on sparse stand line | 114 ± 34 (87–160) | – | 33 ± 7 (5–66) | 42 ± 21 (1–136) |
| on open forest line | 168 ± 38 (128–208) | – | 171 ± 18 (100–260) | 114 ± 41 (33–198) |
| on closed forest line | 155 ± 30 (124–185) | – | 140 ± 19 (75–215) | 119 ± 36 (58–211) |
| in tree stands below closed forest line | 137 ± 18 (119–156) | – | 137 ± 18 (89–165) | 132 ± 7 (83–200) |
| Region | Central Part of Kola Peninsula | Western Macroslope of Polar Urals | Eastern Macroslope of Polar Urals | Western Part of Putorana Plateau | ||||
|---|---|---|---|---|---|---|---|---|
| Summer | Winter | Summer | Winter | Summer | Winter | Summer | Winter | |
| Climatic and site conditions | moderately warm | warm | moderately cold | moderately cold | warm | moderately cold | cold | extremely cold |
| cloudy | cloudy | moderately cloudy | moderately cloudy | slightly cloudy | slightly cloudy | little cloudy | little cloudy | |
| rainy | snowy | moderately rainy | slightly snowy | moderately rainy | little snowy | slightly rainy | moderately snowy | |
| Extremely deep snowpack, late snowmelt, unfrozen soils | BP | BP, DF | BP, DF | DF | ||||
| Deep snowpack, normal snowmelt, slightly frozen soils | BP | BP, PO | BP, LS | LG | ||||
| Middle snowpack, early snowmelt, frozen soils in winter, comparably wet summer | BP | PO | LS | LG | ||||
| Shallow snowpack, early snowmelt, frozen soils in winter, dry and warm in summer | PSy | LS | LS | LG | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moiseev, P.A.; Hagedorn, F.; Balakin, D.S.; Bubnov, M.O.; Devi, N.M.; Kukarskih, V.V.; Mazepa, V.S.; Viyukhin, S.O.; Viyukhina, A.A.; Grigoriev, A.A. Stand Biomass at Treeline Ecotone in Russian Subarctic Mountains Is Primarily Related to Species Composition but Its Dynamics Driven by Improvement of Climatic Conditions. Forests 2022, 13, 254. https://doi.org/10.3390/f13020254
Moiseev PA, Hagedorn F, Balakin DS, Bubnov MO, Devi NM, Kukarskih VV, Mazepa VS, Viyukhin SO, Viyukhina AA, Grigoriev AA. Stand Biomass at Treeline Ecotone in Russian Subarctic Mountains Is Primarily Related to Species Composition but Its Dynamics Driven by Improvement of Climatic Conditions. Forests. 2022; 13(2):254. https://doi.org/10.3390/f13020254
Chicago/Turabian StyleMoiseev, Pavel A., Frank Hagedorn, Dmitry S. Balakin, Maxim O. Bubnov, Nadezhda M. Devi, Vladimir V. Kukarskih, Valery S. Mazepa, Sergey O. Viyukhin, Arina A. Viyukhina, and Andrey A. Grigoriev. 2022. "Stand Biomass at Treeline Ecotone in Russian Subarctic Mountains Is Primarily Related to Species Composition but Its Dynamics Driven by Improvement of Climatic Conditions" Forests 13, no. 2: 254. https://doi.org/10.3390/f13020254






