Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework
Abstract
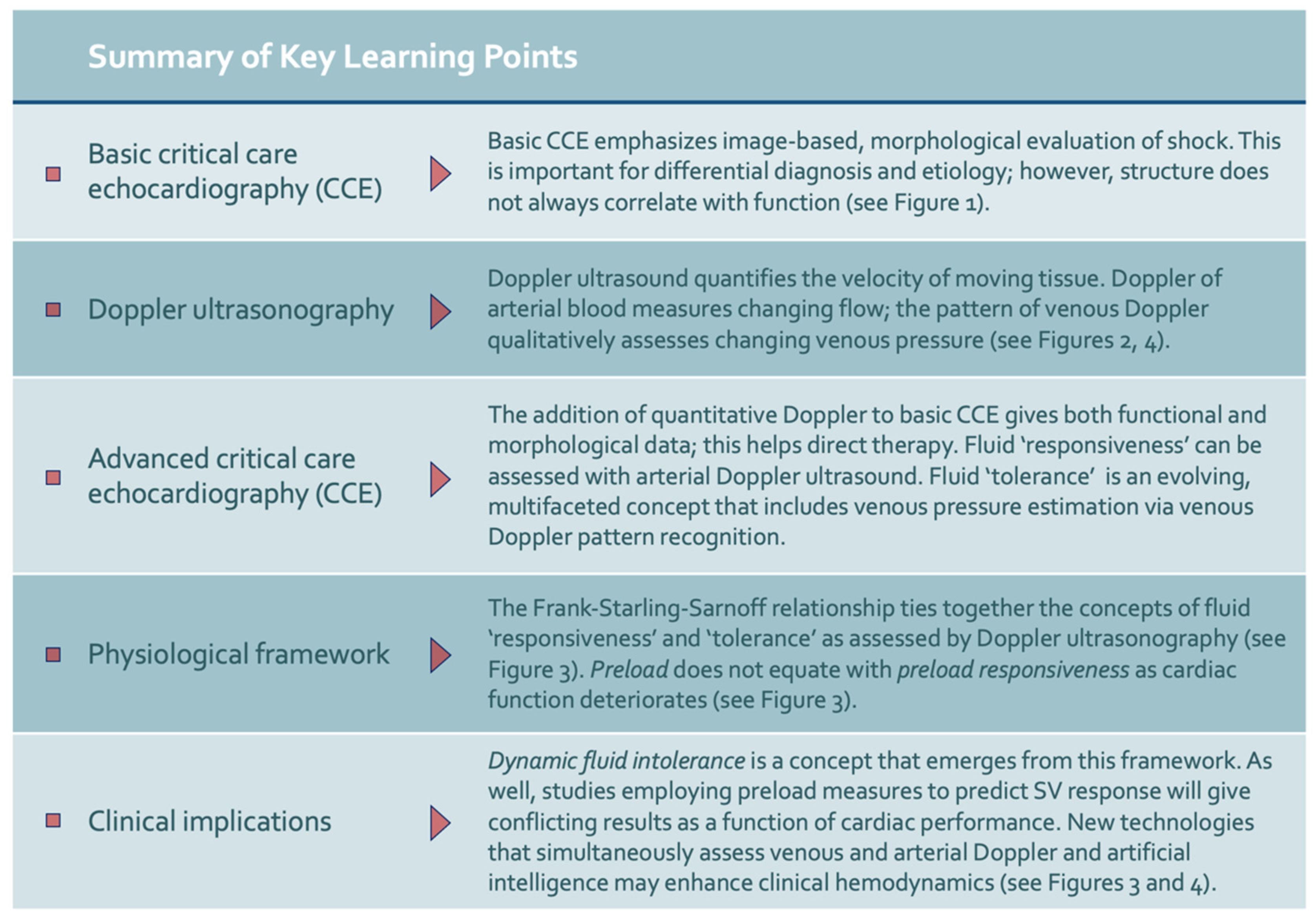
:1. Introduction
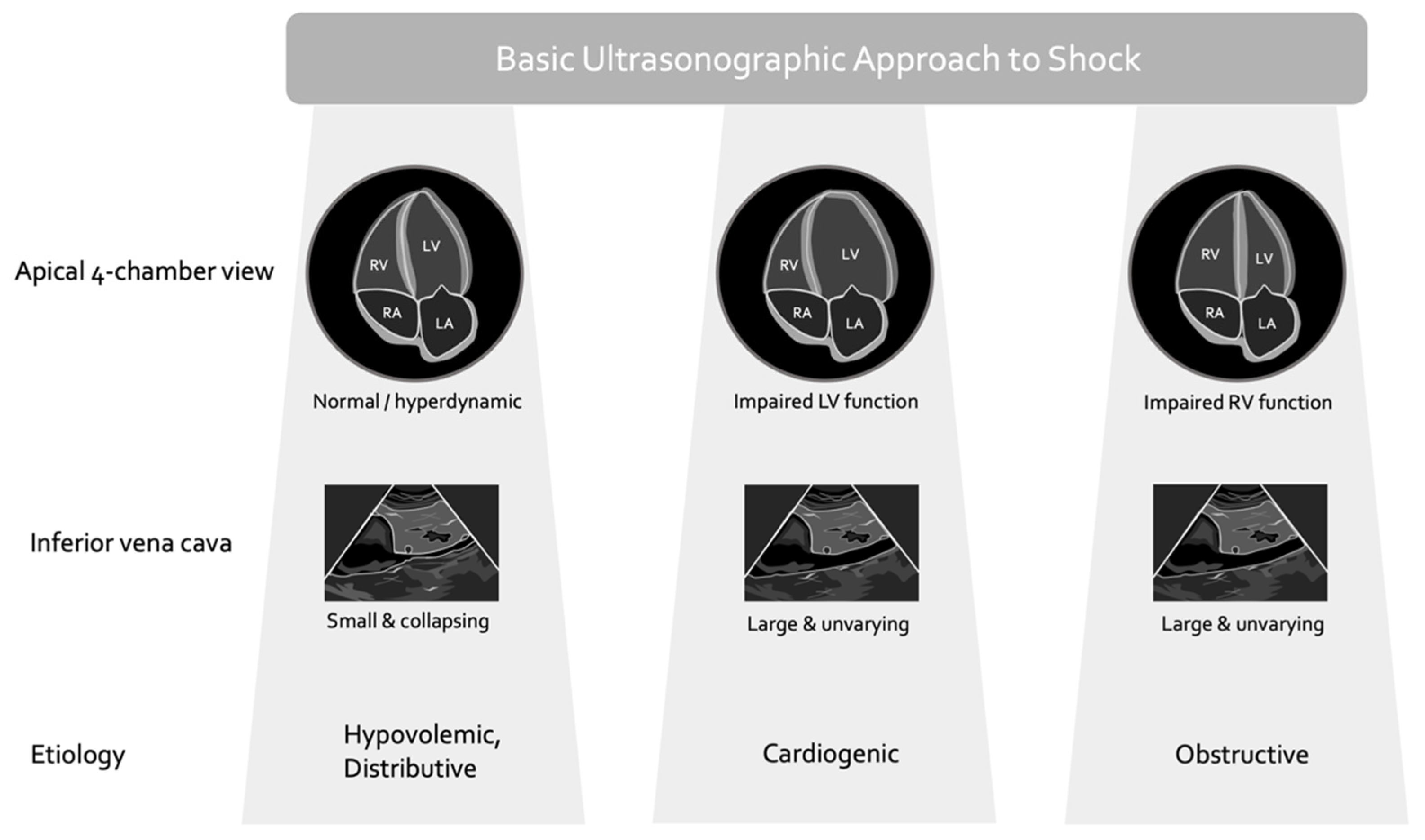
2. Basic Critical Care Echocardiography and Its Caveats
- (1.)
- the relationship between cardiac filling and stroke volume (i.e., the Frank–Starling mechanism) varies significantly, especially in the critically ill;
- (2.)
- the relationship between cardiac filling and total vascular volume (i.e., volume status) is poor;
- (3.)
- the LVEF is not purely a gauge of cardiac function but rather a measure of energetic coupling between the ventricle and the arterial load.
3. Doppler Ultrasound
4. Advanced Critical Care Echocardiography with Spectral Doppler Ultrasound
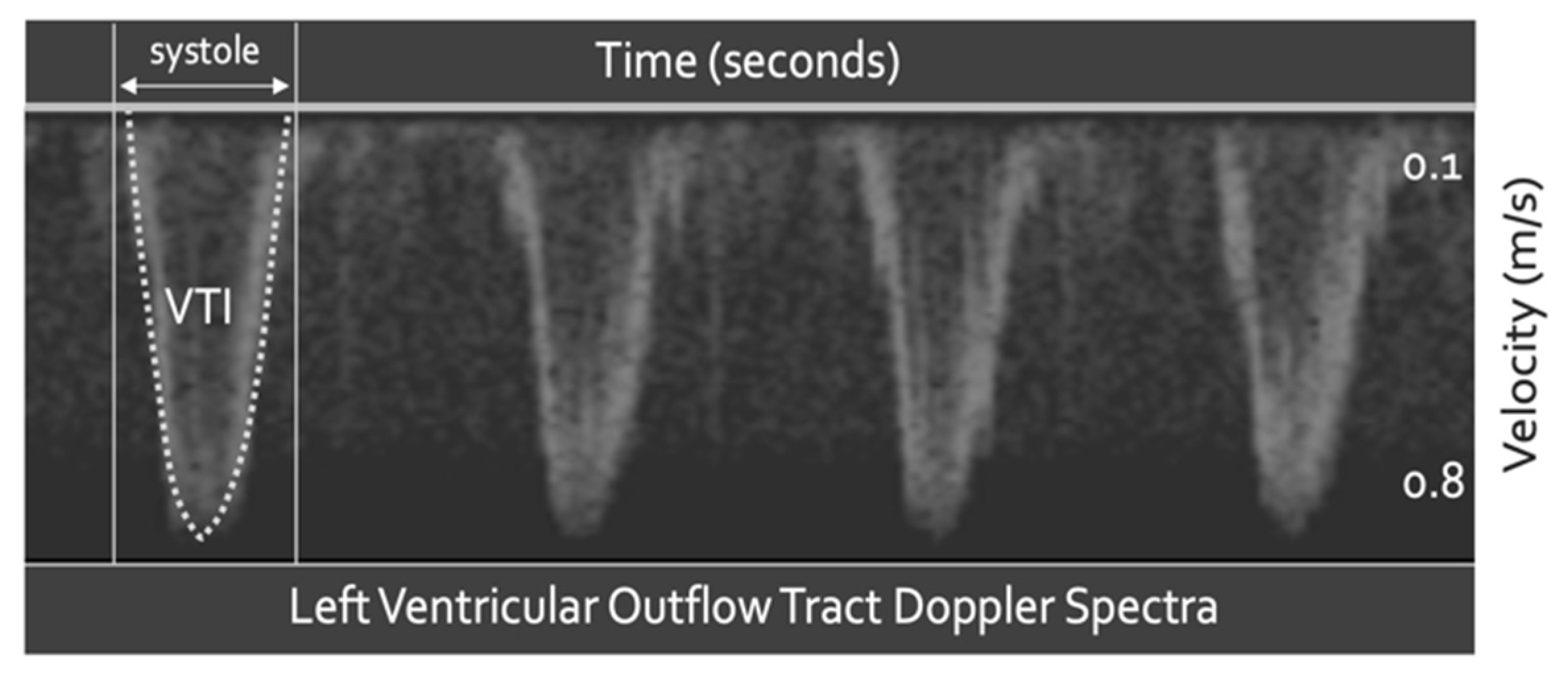
4.1. Fluid Responsiveness
4.2. Fluid Tolerance
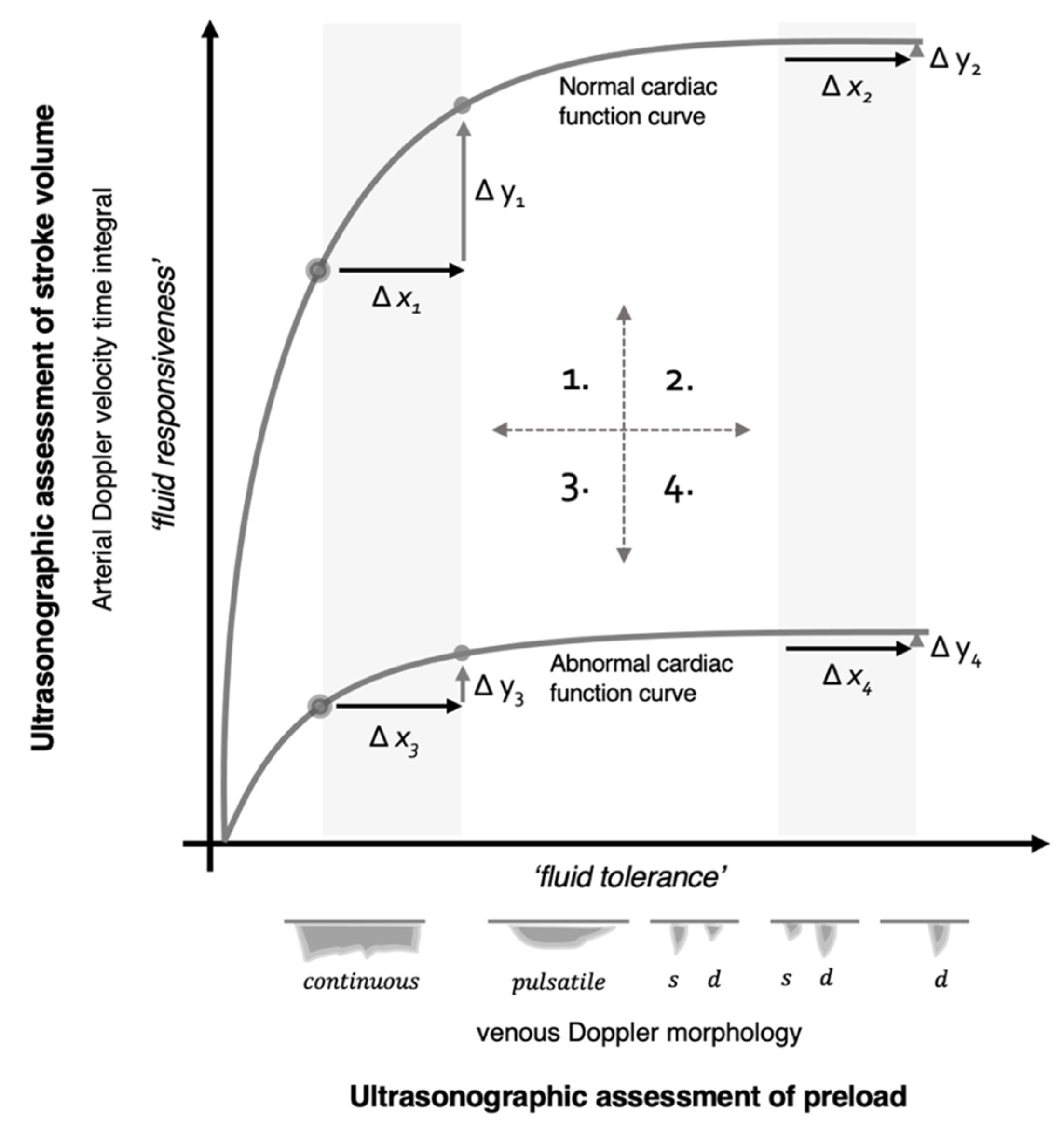
5. Physiological Framework
5.1. Quadrant 1
5.2. Quadrant 2
5.3. Quadrant 3
5.4. Quadrant 4
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vincent, J.L.; De Backer, D. Circulatory shock. N. Engl. J. Med. 2013, 369, 1726–1734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angus, D.C.; Van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, M.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). Jama 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [Green Version]
- Kahn, J.M.; Davis, B.S.; Yabes, J.G.; Chang CC, H.; Chong, D.H.; Hershey, T.B.; Martsolf, G.R.; Angus, D.C. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. Jama 2019, 322, 240–250. [Google Scholar] [CrossRef]
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; Mcintyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021, 47, 1181–1247. [Google Scholar] [CrossRef]
- Klompas, M.; Rhee, C. Has the Medicare sepsis performance measure (SEP-1) catalyzed better outcomes for patients with sepsis? Ann. Intern. Med. 2021, 174, 1010–1011. [Google Scholar] [CrossRef]
- Rhee, C.; Yu, T.; Wang, R.; Kadri, S.S.; Fram, D.; Chen, H.-C.; Klompas, M.; for the CDC Prevention Epicenters Program. Association between implementation of the severe sepsis and septic shock early management bundle performance measure and outcomes in patients with suspected sepsis in US hospitals. JAMA Netw. Open 2021, 4, e2138596. [Google Scholar] [CrossRef]
- Spiegel, R.; Farkas, J.D.; Rola, P.; Kenny, J.-E.; Olusanya, S.; Marik, P.E.; Weingart, S.D. The 2018 surviving sepsis campaign’s treatment bundle: When guidelines outpace the evidence supporting their use. Ann. Emerg. Med. 2019, 73, 356–358. [Google Scholar] [CrossRef] [Green Version]
- Seymour, C.W.; Gesten, F.; Prescott, H.C.; Friedrich, M.E.; Iwashyna, T.J.; Phillips, G.S.; Lemeshow, S.; Osborn, T.; Terry, K.M.; Levy, M.M. Time to treatment and mortality during mandated emergency care for sepsis. N. Engl. J. Med. 2017, 376, 2235–2244. [Google Scholar] [CrossRef] [PubMed]
- Berlin, D.A.; Bakker, J. Starling curves and central venous pressure. Crit. Care 2015, 19, 55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magder, S. The meaning of blood pressure. Crit. Care 2018, 22, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kato, R.; Pinsky, M.R. Personalizing blood pressure management in septic shock. Ann. Intensive Care 2015, 5, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyd, J.H.; Forbes, J.; Nakada, T.-A.; Walley, K.R.; Russell, J.A. Fluid resuscitation in septic shock: A positive fluid balance and elevated central venous pressure are associated with increased mortality. Crit. Care Med. 2011, 39, 259–265. [Google Scholar] [CrossRef]
- Geri, G.; Vignon, P.; Aubry, A.; Fedou, A.-L.; Charron, C.; Silva, S.; Repessé, X.; Vieillard-Baron, A. Cardiovascular clusters in septic shock combining clinical and echocardiographic parameters: A post hoc analysis. Intensive Care Med. 2019, 45, 657–667. [Google Scholar] [CrossRef]
- Douglas, I.S.; Alapat, P.M.; Corl, K.A.; Exline, M.C.; Forni, L.G.; Holder, A.L.; Kaufman, D.A.; Khan, A.; Levy, M.M.; Martin, G.S.; et al. Fluid response evaluation in sepsis hypotension and shock: A randomized clinical trial. Chest 2020, 158, 1431–1445. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Herritt, B.; Lewis, K.; Diaz-Gomez, J.L.; Fox-Robichaud, A.; Ball, I.; Granton, J.; Rochwerg, B. Dosing fluids in early septic shock. Chest 2021, 159, 1493–1502. [Google Scholar] [CrossRef]
- Dubin, A.; Loudet, C.; Kanoore Edul, V.S.; Osatnik, J.; Ríos, F.; Vásquez, D.; Pozo, M.; Lattanzio, B.; Palizas, F.; Klein, F.; et al. Characteristics of resuscitation, and association between use of dynamic tests of fluid responsiveness and outcomes in septic patients: Results of a multicenter prospective cohort study in Argentina. Ann. Intensive Care 2020, 10, 40. [Google Scholar] [CrossRef] [Green Version]
- Cecconi, M.; De Backer, D.; Antonelli, M.; Beale, R.; Bakker, J.; Hofer, C.; Jaeschke, R.; Mebazaa, A.; Pinsky, M.R.; Teboul, J.-L.; et al. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014, 40, 1795–1815. [Google Scholar] [CrossRef]
- Repessé, X.; Bodson, L.; Vieillard-Baron, A. Doppler echocardiography in shocked patients. Curr. Opin. Crit. Care 2013, 19, 221–227. [Google Scholar] [CrossRef]
- Vieillard-Baron, A.; Prin, S.; Chergui, K.; Dubourg, O.; Jardin, F. Hemodynamic instability in sepsis: Bedside assessment by Doppler echocardiography. Am. J. Respir. Crit. Care Med. 2003, 168, 1270–1276. [Google Scholar] [CrossRef] [PubMed]
- Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. The RUSH exam: Rapid Ultrasound in SHock in the evaluation of the critically lll. Emerg. Med. Clin. 2010, 28, 29–56. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.; Stawicki, S.; Evans, D.C.; Bahner, D.; Sparks, S.; Sharpe, R.; Cipolla, J. From FAST to E-FAST: An overview of the evolution of ultrasound-based traumatic injury assessment. Eur. J. Trauma Emerg. Surg. 2016, 42, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, P.; McAuley, D.; Kendall, R.; Abeyakoon, O.; Reid, C.; Connolly, J.; Lewis, D. Abdominal and Cardiac Evaluation with Sonography in Shock (ACES): An approach by emergency physicians for the use of ultrasound in patients with undifferentiated hypotension. Emerg. Med. J. 2009, 26, 87–91. [Google Scholar] [CrossRef] [Green Version]
- Bughrara, N.; Diaz-Gomez, J.L.; Pustavoitau, A. Perioperative management of patients with sepsis and septic shock, Part II: Ultrasound support for resuscitation. Anesthesiol. Clin. 2020, 38, 123–134. [Google Scholar] [CrossRef]
- Nikravan, S.; Song, P.; Bughrara, N.; Díaz-Gómez, J.L. Focused ultrasonography for septic shock resuscitation. Curr. Opin. Crit. Care 2020, 26, 296–302. [Google Scholar] [CrossRef]
- Stickles, S.P.; Carpenter, C.R.; Gekle, R.; Kraus, C.K.; Scoville, C.; Theodoro, D.; Tran, V.H.; Ubiñas, G.; Raio, C. The diagnostic accuracy of a point-of-care ultrasound protocol for shock etiology: A systematic review and meta-analysis. Cjem 2019, 21, 406–417. [Google Scholar] [CrossRef]
- Atkinson, P.R.; Milne, J.; Diegelmann, L.; Lamprecht, H.; Stander, M.; Lussier, D.; Pham, C.; Henneberry, R.; Fraser, J.M.; Howlett, M.K.; et al. Does point-of-care ultrasonography improve clinical outcomes in emergency department patients with undifferentiated hypotension? An international randomized controlled trial from the SHoC-ED investigators. Ann. Emerg. Med. 2018, 72, 478–489. [Google Scholar] [CrossRef]
- Millington, S.J.; Koenig, S. Ultrasound Assessment of the Inferior Vena Cava for Fluid Responsiveness: Making the Case for Skepticism. J. Intensive Care Med. 2021, 36, 1223–1227. [Google Scholar] [CrossRef]
- Bodson, L.; Vieillard-Baron, A. Respiratory variation in inferior vena cava diameter: Surrogate of central venous pressure or parameter of fluid responsiveness? Let the physiology reply. Crit. Care 2012, 16, 181. [Google Scholar] [CrossRef] [Green Version]
- Via, G.; Tavazzi, G.; Price, S. Ten situations where inferior vena cava ultrasound may fail to accurately predict fluid responsiveness: A physiologically based point of view. Intensive Care Med. 2016, 42, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- Millington, S.J. Ultrasound assessment of the inferior vena cava for fluid responsiveness: Easy, fun, but unlikely to be helpful. Can. J. Anesth. /J. Can. D’anesthésie 2019, 66, 633–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kircher, B.J.; Himelman, R.B.; Schiller, N.B. Noninvasive estimation of right atrial pressure from the inspiratory collapse of the inferior vena cava. Am. J. Cardiol. 1990, 66, 493–496. [Google Scholar] [CrossRef]
- Eskesen, T.; Wetterslev, M.; Perner, A. Systematic review including re-analyses of 1148 individual data sets of central venous pressure as a predictor of fluid responsiveness. Intensive Care Med. 2016, 42, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Shippy, C.R.; Appel, P.L.; Shoemaker, W.C. Reliability of clinical monitoring to assess blood volume in critically ill patients. Crit. Care Med. 1984, 12, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Orso, D.; Paoli, I.; Piani, T.; Cilenti, F.L.; Cristiani, L.; Guglielmo, N. Accuracy of ultrasonographic measurements of inferior vena cava to determine fluid responsiveness: A systematic review and meta-analysis. J. Intensive Care Med. 2020, 35, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Robotham, J.L.; Takata, M.; Berman, M.; Harasawa, Y. Ejection fraction revisited. Anesthesiology 1991, 74, 172–183. [Google Scholar] [CrossRef]
- García, M.I.M.; Jian, Z.; Settels, J.J.; Hunley, C.; Cecconi, M.; Hatib, F.; Pinsky, M.R. Determinants of left ventricular ejection fraction and a novel method to improve its assessment of myocardial contractility. Ann. Intensive Care 2019, 9, 48. [Google Scholar] [CrossRef]
- Monge García, M.I.; Cecconi, M.; Pinsky, M.R. Assessing left ventricular systolic function with ejection fraction: Using a double-edged knife as a hammer. Ann. Intensive Care 2019, 9, 111. [Google Scholar] [CrossRef] [Green Version]
- Sanfilippo, F.; Corredor, C.; Arcadipane, A.; Landesberg, G.; Vieillard-Baron, A.; Cecconi, M.; Fletcher, N. Tissue Doppler assessment of diastolic function and relationship with mortality in critically ill septic patients: A systematic review and meta-analysis. BJA Br. J. Anaesth. 2017, 119, 583–594. [Google Scholar] [CrossRef] [Green Version]
- Landesberg, G.; Levin, P.D.; Gilon, D.; Goodman, S.; Georgieva, M.; Weissman, C.; Jaffe, A.S.; Sprung, C.L.; Barak, V. Myocardial dysfunction in severe sepsis and septic shock. Chest 2015, 148, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Aurigemma, G.P.; Gaasch, W.H. Diastolic Heart Failure. N. Engl. J. Med. 2004, 351, 1097–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, P.; Bellomo, R. A rational approach to fluid therapy in sepsis. BJA Br. J. Anaesth. 2015, 116, 339–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McNaughton, D.A.; Abu-Yousef, M.M. Doppler US of the liver made simple. Radiographics 2011, 31, 161–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, P. Rationale for using the velocity–time integral and the minute distance for assessing the stroke volume and cardiac output in point-of-care settings. Ultrasound J. 2020, 12, 21. [Google Scholar] [CrossRef] [Green Version]
- Sattin, M.; Burhani, Z.; Jaidka, A.; Millington, S.; Arntfield, R. How I do it: Stroke volume determination by echocardiography. Chest 2022, in press. [Google Scholar] [CrossRef]
- Millington, S.J.; Wiskar, K.; Hobbs, H.; Koenig, S. Risks and benefits of fluid administration as assessed by ultrasound. Chest 2021, 160, 2196–2208. [Google Scholar] [CrossRef]
- Phillips, R.; Smith, B.; Madigan, V. Stroke volume monitoring: Novel continuous wave Doppler parameters, algorithms and advanced noninvasive haemodynamic concepts. Curr. Anesthesiol. Rep. 2017, 7, 387–398. [Google Scholar] [CrossRef] [Green Version]
- Pinsky, M.R. Functional hemodynamic monitoring: Current concepts in critical care. Curr. Opin. Crit. Care 2014, 20, 288. [Google Scholar] [CrossRef]
- Monnet, X.; Marik, P.E.; Teboul, J.-L. Prediction of fluid responsiveness: An update. Ann. Intensive Care 2016, 6, 111. [Google Scholar] [CrossRef] [Green Version]
- Monnet, X.; Marik, P.; Teboul, J.-L. Passive leg raising for predicting fluid responsiveness: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Marik, P.E. Fluid responsiveness and the six guiding principles of fluid resuscitation. Crit. Care Med. 2016, 44, 1920–1922. [Google Scholar] [CrossRef]
- Marik, P.E.; Cavallazzi, R. Does the central venous pressure predict fluid responsiveness? An updated meta-analysis and a plea for some common sense. Crit. Care Med. 2013, 41, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Michard, F.; Teboul, J.-L. Using heart-lung interactions to assess fluid responsiveness during mechanical ventilation. Crit. Care 2000, 4, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magder, S. Clinical usefulness of respiratory variations in arterial pressure. Am. J. Respir. Crit. Care Med. 2004, 169, 151–155. [Google Scholar] [CrossRef]
- Feissel, M.; Mangin, I.; Ruyer, O.; Faller, J.-P.; Michard, F.; Teboul, J.-L. Respiratory changes in aortic blood velocity as an indicator of fluid responsiveness in ventilated patients with septic shock. Chest 2001, 119, 867–873. [Google Scholar] [CrossRef]
- Slama, M.; Masson, H.; Teboul, J.-L.; Arnout, M.-L.; Susic, D.; Frohlich, E.; Andrejak, M. Respiratory variations of aortic VTI: A new index of hypovolemia and fluid responsiveness. Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1729–H1733. [Google Scholar] [CrossRef]
- Gavelli, F.; Teboul, J.-L.; Monnet, X. The end-expiratory occlusion test: Please, let me hold your breath! Crit. Care 2019, 23, 274. [Google Scholar] [CrossRef] [Green Version]
- Jozwiak, M.; Depret, F.; Teboul, J.-L.; Alphonsine, J.-E.; Lai, C.; Richard, C.; Monnet, X. Predicting fluid responsiveness in critically ill patients by using combined end-expiratory and end-inspiratory occlusions with echocardiography. Crit. Care Med. 2017, 45, e1131–e1138. [Google Scholar] [CrossRef]
- Dépret, F.; Jozwiak, M.; Teboul, J.-L.; Alphonsine, J.-E.; Richard, C.; Monnet, X. Esophageal Doppler can predict fluid responsiveness through end-expiratory and end-inspiratory occlusion tests. Crit. Care Med. 2019, 47, e96–e102. [Google Scholar] [CrossRef]
- Muller, L.; Toumi, M.; Bousquet, P.J.; Riu-Poulenc, B.; Louart, G.; Candela, D.; Zoric, L.; Suehs, C.; De La Coussaye, J.-E.; Molinari, N.; et al. An increase in aortic blood flow after an infusion of 100 ml colloid over 1 minute can predict fluid responsiveness: The mini-fluid challenge study. Anesthesiology 2011, 115, 541–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barthélémy, R.; Kindermans, M.; Delval, P.; Collet, M.; Gaugain, S.; Cecconi, M.; Mebazaa, A.; Chousterman, B.G. Accuracy of cumulative volumes of fluid challenge to assess fluid responsiveness in critically ill patients with acute circulatory failure: A pharmacodynamic approach. Br. J. Anaesth. 2021 128, 236–243. [CrossRef]
- Lamia, B.; Ochagavia, A.; Monnet, X.; Chemla, D.; Richard, C.; Teboul, J.-L. Echocardiographic prediction of volume responsiveness in critically ill patients with spontaneously breathing activity. Intensive Care Med. 2007, 33, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Monnet, X.; Rienzo, M.; Osman, D.; Anguel, N.; Richard, C.; Pinsky, M.R.; Teboul, J.L. Passive leg raising predicts fluid responsiveness in the critically ill. Crit. Care Med. 2006, 34, 1402–1407. [Google Scholar] [CrossRef] [PubMed]
- Maizel, J.; Airapetian, N.; Lorne, E.; Tribouilloy, C.; Massy, Z.; Slama, M. Diagnosis of central hypovolemia by using passive leg raising. Intensive Care Med. 2007, 33, 1133–1138. [Google Scholar] [CrossRef]
- Marik, P.E.; Levitov, A.; Young, A.; Andrews, L. The use of bioreactance and carotid Doppler to determine volume responsiveness and blood flow redistribution following passive leg raising in hemodynamically unstable patients. Chest 2013, 143, 364–370. [Google Scholar] [CrossRef]
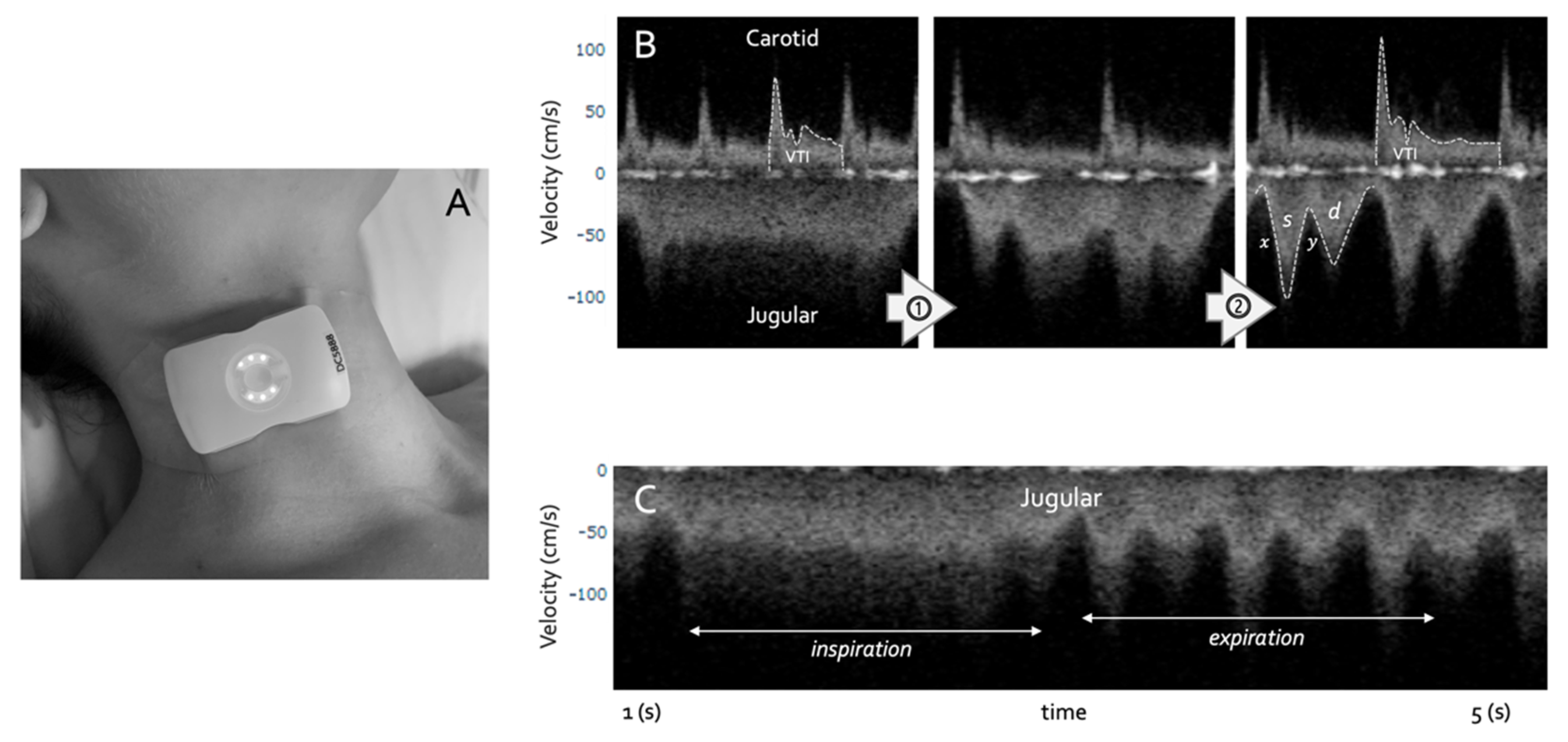
- Barjaktarevic, I.; Toppen, W.E.; Hu, S.; Montoya, E.A.; Ong, S.; Buhr, R.; David, I.J.; Wang, T.; Rezayat, T.; Chang, S.Y.; et al. Ultrasound Assessment of the Change in Carotid Corrected Flow Time in Fluid Responsiveness in Undifferentiated Shock. Crit. Care Med. 2018, 11, 1040–1046. [Google Scholar] [CrossRef]
- Beier, L.; Davis, J.; Esener, D.; Grant, C.; Fields, J.M. Carotid ultrasound to predict fluid responsiveness: A systematic review. J. Ultrasound Med. 2020, 39, 1965–1976. [Google Scholar] [CrossRef]
- Kenny, J.-E.S.; Barjaktarevic, I. Letter to the Editor: Stroke volume is the key measure of fluid responsiveness. Crit. Care 2021, 25, 104. [Google Scholar] [CrossRef]
- Denault, A.; Canty, D.; Azzam, M.; Amir, A.; Gebhard, C.E. Whole body ultrasound in the operating room and intensive care unit. Korean J. Anesthesiol. 2019, 72, 413. [Google Scholar] [CrossRef]
- Scheinfeld, M.H.; Bilali, A.; Koenigsberg, M. Understanding the spectral Doppler waveform of the hepatic veins in health and disease. Radiographics 2009, 29, 2081–2098. [Google Scholar] [CrossRef]
- Appleton, C.P.; Hatle, L.K.; Popp, R.L. Superior vena cava and hepatic vein Doppler echocardiography in healthy adults. J. Am. Coll. Cardiol. 1987, 10, 1032–1039. [Google Scholar] [CrossRef] [Green Version]
- Sivaciyan, V.; Ranganathan, N. Transcutaneous doppler jugular venous flow velocity recording. Circulation 1978, 57, 930–939. [Google Scholar] [CrossRef] [Green Version]
- Ghio, S.; Recusani, F.; Sebastiani, R.; Klersy, C.; Raineri, C.; Campana, C.; Lanzarini, L.; Gavazzi, A.; Tavazzi, L. Doppler velocimetry in superior vena cava provides useful information on the right circulatory function in patients with congestive heart failure. Echocardiography 2001, 18, 469–477. [Google Scholar] [CrossRef]
- Reynolds, T.; Appleton, C.P. Doppler flow velocity patterns of the superior vena cava, inferior vena cava, hepatic vein, coronary sinus, and atrial septal defect: A guide for the echocardiographer. J. Am. Soc. Echocardiogr. 1991, 4, 503–512. [Google Scholar] [CrossRef]
- Hou, Y.; Sun, D.-D.; Yuan, L.-J.; Zhu, X.-Y.; Shang, F.-J.; Hou, C.-J.; Duan, Y.Y. Clinical application of superior vena cava spectra in evaluation of pulmonary hypertension: A comparative echocardiography and catheterization study. Ultrasound Med. Biol. 2016, 42, 110–117. [Google Scholar] [CrossRef]
- Abu-Yousef, M.M. Normal and respiratory variations of the hepatic and portal venous duplex Doppler waveforms with simultaneous electrocardiographic correlation. J. Ultrasound Med. 1992, 11, 263–268. [Google Scholar] [CrossRef]
- Abu-Yousef, M.M.; Kakish, M.; Mufid, M. Pulsatile venous Doppler flow in lower limbs: Highly indicative of elevated right atrium pressure. AJR Am. J. Roentgenol. 1996, 167, 977–980. [Google Scholar] [CrossRef]
- Iida, N.; Seo, Y.; Sai, S.; Machino-Ohtsuka, T.; Yamamoto, M.; Ishizu, T.; Kawakami, Y.; Aonuma, K. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016, 4, 674–682. [Google Scholar] [CrossRef]
- Tang, W.W.; Kitai, T. Intrarenal venous flow: A window into the congestive kidney failure phenotype of heart failure? JACC Heart Fail. 2016, 4, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Beaubien-Souligny, W.; Rola, P.; Haycock, K.; Bouchard, J.; Lamarche, Y.; Spiegel, R.; Denault, A.Y. Quantifying systemic congestion with Point-Of-Care ultrasound: Development of the venous excess ultrasound grading system. Ultrasound J. 2020, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Spiegel, R.; Teeter, W.; Sullivan, S.; Tupchong, K.; Mohammed, N.; Sutherland, M.; Leibner, E.; Rola, P.; Murthi, S.B. The use of venous Doppler to predict adverse kidney events in a general ICU cohort. Crit. Care 2020, 24, 615. [Google Scholar] [CrossRef] [PubMed]
- Mullens, W.; Abrahams, Z.; Francis, G.S.; Sokos, G.; Taylor, D.O.; Starling, R.C.; Young, J.B.; Tang, W.W. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J. Am. Coll. Cardiol. 2009, 53, 589–596. [Google Scholar] [CrossRef] [Green Version]
- Verbrugge, F.H.; Dupont, M.; Steels, P.; Grieten, L.; Malbrain, M.; Tang, W.W.; Mullens, W. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J. Am. Coll. Cardiol. 2013, 62, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Tabata, T.; Thomas, J.D.; Klein, A.L. Pulmonary venous flow by Doppler echocardiography: Revisited 12 years later. J. Am. Coll. Cardiol. 2003, 41, 1243–1250. [Google Scholar] [CrossRef] [Green Version]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F.; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: An update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. J. Echocardiogr. 2016, 17, 1321–1360. [Google Scholar]
- Manecke, G. Volume responsiveness: What it does not tell us. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1307–1309. [Google Scholar] [CrossRef]
- Challand, C.; Struthers, R.; Sneyd, J.; Erasmus, P.; Mellor, N.; Hosie, K.; Minto, G. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br. J. Anaesth. 2012, 108, 53–62. [Google Scholar] [CrossRef] [Green Version]
- Magder, S.; Bafaqeeh, F. The clinical role of central venous pressure measurements. J. Intensive Care Med. 2007, 22, 44–51. [Google Scholar] [CrossRef]
- Corl, K.A.; George, N.R.; Romanoff, J.; Levinson, A.T.; Chheng, D.B.; Merchant, R.C.; Levy, M.M.; Napoli, A.M. Inferior vena cava collapsibility detects fluid responsiveness among spontaneously breathing critically-ill patients. J. Crit. Care 2017, 41, 130–137. [Google Scholar] [CrossRef]
- García, M.I.M.; González, P.G.; Romero, M.G.; Cano, A.G.; Oscier, C.; Rhodes, A.; Grounds, R.M.; Cecconi, M. Effects of fluid administration on arterial load in septic shock patients. Intensive Care Med. 2015, 41, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Guarracino, F.; Bertini, P.; Pinsky, M.R. Cardiovascular determinants of resuscitation from sepsis and septic shock. Crit. Care 2019, 23, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahjoub, Y.; Benoit-Fallet, H.; Airapetian, N.; Lorne, E.; Levrard, M.; Seydi, A.-A.; Amennouche, N.; Slama, M.; Dupont, H. Improvement of left ventricular relaxation as assessed by tissue Doppler imaging in fluid-responsive critically ill septic patients. Intensive Care Med. 2012, 38, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Wang, X.-T.; Long, Y.; Liu, D.-W. Monitoring changes in hepatic venous velocities flow after a fluid challenge can identify shock patients who lack fluid responsiveness. Chin. Med. J. 2017, 130, 1202. [Google Scholar] [CrossRef]
- Corl, K.; Napoli, A.M.; Gardiner, F. Bedside sonographic measurement of the inferior vena cava caval index is a poor predictor of fluid responsiveness in emergency department patients. Emerg. Med. Australas. 2012, 24, 534–539. [Google Scholar] [CrossRef]
- Vignon, P.; Repesse, X.; Begot, E.; Leger, J.; Jacob, C.; Bouferrache, K.; Slama, M.; Prat, G.; Vieillard-Baron, A. Comparison of echocardiographic indices used to predict fluid responsiveness in ventilated patients. Am. J. Respir. Crit. Care Med. 2017, 195, 1022–1032. [Google Scholar] [CrossRef]
- Kenny, J.-E.S.; Eibl, J.K.; Mackenzie, D.C.; Barjaktarevic, I. Guidance of intravenous fluid by ultrasound will improve with technology. Chest 2021, 161, 132–133. [Google Scholar] [CrossRef]
- Kenny, J.-É.S.; Munding, C.E.; Eibl, J.K.; Eibl, A.M.; Long, B.F.; Boyes, A.; Yin, J.; Verrecchia, P.; Parrotta, M.; Gatzke, R.; et al. A novel, hands-free ultrasound patch for continuous monitoring of quantitative Doppler in the carotid artery. Sci. Rep. 2021, 11, 7780. [Google Scholar] [CrossRef]
- Kenny, J.-É.S.; Barjaktarevic, I.; Mackenzie, D.C.; Rola, P.; Haycock, K.; Eibl, A.M.; Eibl, J.K. Inferring the Frank–Starling Curve from Simultaneous Venous and Arterial Doppler: Measurements from a wireless, wearable ultrasound patch. Front. Med. Technol. 2021, 3, 16. [Google Scholar] [CrossRef]
- Kenny, J.-É.S. Functional hemodynamic monitoring with a wireless ultrasound patch. J. Cardiothorac. Vasc. Anesth. 2021, 35, 1509–1515. [Google Scholar] [CrossRef]
- Barjaktarevic, I.; Kenny, J.-É.S.; Berlin, D.; Cannesson, M. The evolution of ultrasound in critical care: From procedural guidance to hemodynamic monitor. J. Ultrasound Med. 2021, 40, 401. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.-É.S.; Barjaktarevic, I.; Mackenzie, D.C.; Elfarnawany, M.; Yang, Z.; Eibl, A.M.; Eibl, J.K.; Kim, C.-H.; Johnson, B.D. Carotid Doppler ultrasonography correlates with stroke volume in a human model of hypovolaemia and resuscitation: Analysis of 48,570 cardiac cycles. Br. J. Anaesth. 2021, 127, e60–e63. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.-É.S.; Barjaktarevic, I.; Mackenzie, D.C.; Elfarnawany, M.; Yang, Z.; Eibl, A.M.; Kim, C.-H.; Johnson, B.D. Carotid artery velocity time integral and corrected flow time measured by a wearable Doppler ultrasound detect stroke volume rise from simulated hemorrhage to transfusion. BMC Res. Notes 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Kenny, J.S.; Clarke, G.; Myers, M.; Elfarnawany, M.; Eibl, A.M.; Eibl, J.K.; Nalla, B.; Atoui, R. A wireless wearable doppler ultrasound detects changing stroke volume: Proof-of-Principle comparison with trans-esophageal echocardiography during coronary bypass surgery. Bioengineering 2021, 8, 203. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kenny, J.-E.S. Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework. Med. Sci. 2022, 10, 12. https://doi.org/10.3390/medsci10010012
Kenny J-ES. Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework. Medical Sciences. 2022; 10(1):12. https://doi.org/10.3390/medsci10010012
Chicago/Turabian StyleKenny, Jon-Emile S. 2022. "Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework" Medical Sciences 10, no. 1: 12. https://doi.org/10.3390/medsci10010012
APA StyleKenny, J.-E. S. (2022). Assessing Fluid Intolerance with Doppler Ultrasonography: A Physiological Framework. Medical Sciences, 10(1), 12. https://doi.org/10.3390/medsci10010012





