New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols
Abstract
:1. Introduction
2. The Role of Immunity in Combating SARS-CoV-2
2.1. Mechanisms of the Immune Response against SARS-CoV-2
2.1.1. The Identification of the Spike Glycoprotein of SARS-CoV-2
2.1.2. Elimination of the Virus, and the Infected Cell
2.1.3. Immunological Memory
3. Reasons for System Immune Failure
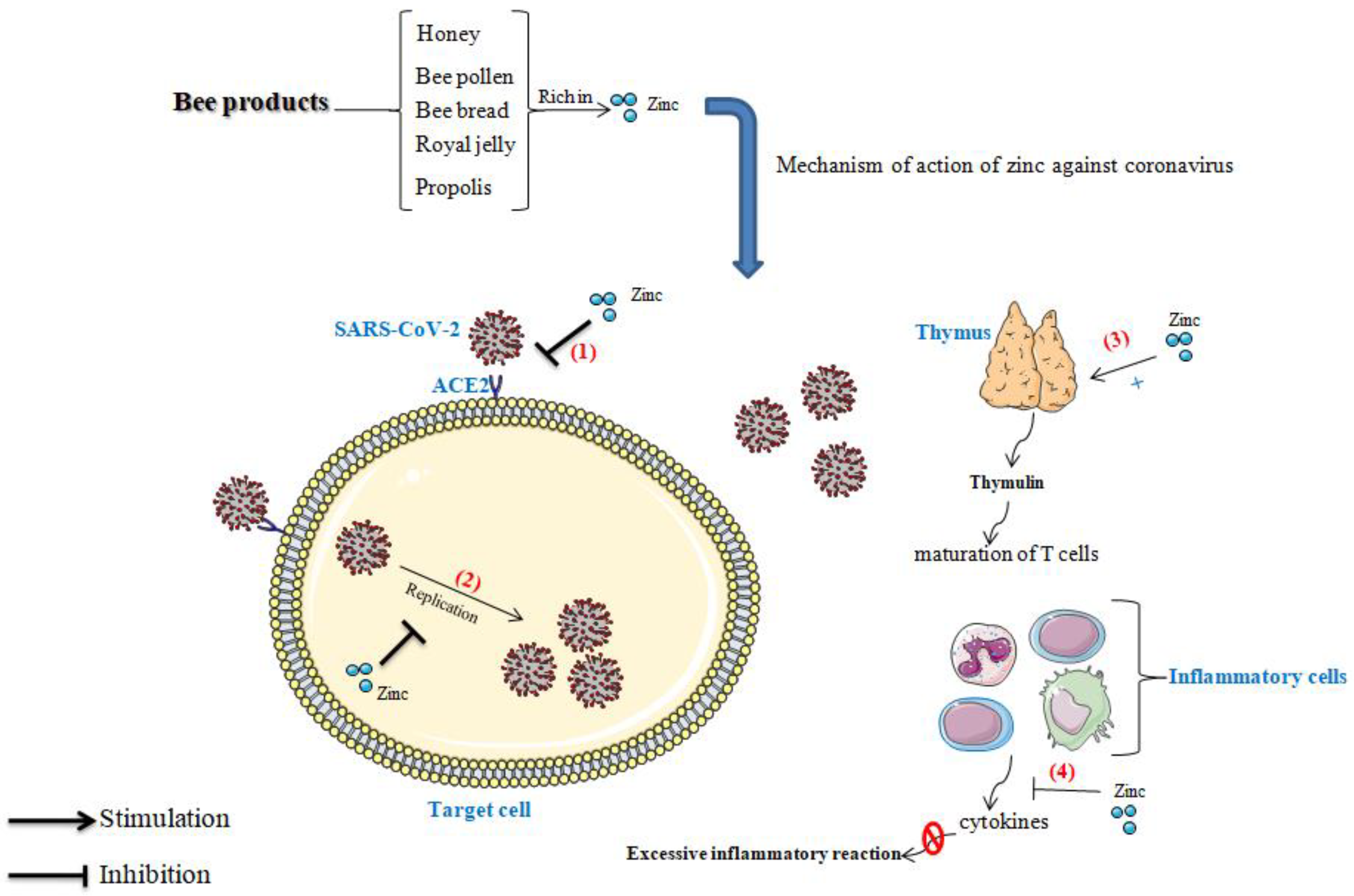
4. The Influence of Zinc on Immunity
4.1. Importance of Zinc in Human Health
4.2. Zinc, Immune System, and Coronavirus
4.3. Effect of Zinc in Thymulin
4.4. Role of Zinc in the Blockage of Viral Replication
4.5. Preventive Effect of Zinc against the Excessive Inflammatory Reaction
5. The Antiviral Effect of Bee Products
5.1. Propolis
5.2. Royal Jelly
5.3. Honey
5.4. Bee Pollen, Bee Bread, and Bee Venom
Concern of Safety of Bee Products
6. Role of Bioactive Compounds in Bee Products in the Management of COVID-19
6.1. Zinc
6.1.1. The Content of Zinc in Bee Products
| Bee Products | Country | Collection Period | Content in Zinc | Reference |
|---|---|---|---|---|
| Bee bread | Lithuania | 2018 | From 11.5 to 42.7 mg/kg | [120] |
| Morocco | - | 3.31 ± 0.4 mg/kg | [117] | |
| Romania | - | From 29.54 to 31.85 mg/kg | [119] | |
| Malaysia | - | 60.61 ± 11.81 mg/kg | [118] | |
| Turkey | April to September 2018 | From 52.55 to 73.96 mg/kg | [87] | |
| Bee pollen | Italy | 2018 | 25.5 mg/kg | [120] |
| Denmark | 2018 | 22.3 mg/kg | [120] | |
| Sweden | 2018 | 23.3 mg/kg | [120] | |
| Slovakia | 2018 | 28.7 mg/kg | [120] | |
| Poland | 2018 | 31.7 mg/kg | [120] | |
| Lithuania | 2018 | From 20.3 to 27.8 mg/kg | [120] | |
| Ukraine | 2018 | From 20.8 to 22.1 mg/kg | [120] | |
| Latvia | 2018 | From 23.5 to 24.7 mg/kg | [120] | |
| Turkey | (April to September 2018) | From 39.37 to 68 mg/kg | [87] | |
| Serbia | (spring and summer of 2011) | From 28.76 to 75.92 mg/kg | [159] | |
| Turkey | - | From 25.94 to 49.74 mg/kg | [121] | |
| Brazil | August 2005 to April 2006 | From 45.07 to 55.22 mg/kg | [160] | |
| Spain | 1993 | From 18.8 to 81.1 mg/kg | [129] | |
| Jordan | March to October 2017 | From 25.24 to 77.02 mg/kg | [124] | |
| Turkey | - | From 14.832 to 39.037 mg/kg | [122] | |
| Slovakia | Spring season 2007 | From 31.9 to 39.9 mg/kg | [128] | |
| Poland | June or August 2009. | From 75.2 to 159.3 mg/kg | [125] | |
| China | Flower season of 2010 | From 28.25 to 65.30 mg/kg | [130] | |
| Turkey | - | From 20.21 to 59.57 mg/kg | [123] | |
| Brazil | - | From 39 to 76 mg/kg | [161] | |
| Greece | March to October 2018 | From 24 to 90 mg/kg | [127] | |
| Propolis | Poland | 2018 | 52.4 mg/kg | [120] |
| Lithuania | 2018 | From 31.9 to 102.1 mg/kg | [120] | |
| Argentina | - | From 53 to 68 mg/kg | [136] | |
| Turkey | - | From 17.60 to 67.60 mg/kg | [133] | |
| Poland | - | From 16.88 to 99.68 mg/kg | [138] | |
| Spain | - | From 163 to 279 mg/kg | [131] | |
| Brazil | - | 113.5 ± 16.9 mg/kg | [139] | |
| Argentina | - | From 34.0 to 105.0 mg/kg | [132] | |
| Chile | - | From 5.5 to 105.0 mg/kg | [135] | |
| Spain | - | From 17.4 to 460.7 mg/kg | [135] | |
| Poland | from May to September 2018 | 40.1 ± 2.7 mg/kg | [120] | |
| Lithuania | from May to September 2018 | From 31.9 to 102.1 mg/kg | [120] | |
| Brazil | from January to March 2011 | From 10 to 50 mg/kg | [134] | |
| Serbia | 2013 | From 19.2 to 241 mg/kg | [140] | |
| Greece | Between spring 2013 and August 2014 | From 30.7 to 383.8 mg/kg | [137] | |
| Honey | Morocco | Summer of 2015 and 2016 | From 1.09 to 4.02 mg/kg | [145] |
| Lithuania | 2018 | From 1.08 to 5.15 mg/kg | [120] | |
| Italy | 2018 | 2.03 mg/kg | [120] | |
| Greece | 2018 | 2.18 mg/kg | [120] | |
| Serbia | 2016 | From 0.78 to 1.84 mg/kg | [153] | |
| New Zealand | Autumn 2007 | From 0.2 to 2.46 mg/kg | [155] | |
| Malaysia | From January 2013 to March 2014 | From 1.25 to 4.56 mg/kg | [144] | |
| Brazil | From July 2007 to March 2009 | From 0.07 to 1.85 mg/kg | [146] | |
| Palestine | Between April and August 2009 | From 2.06 to 8.36 mg/kg | [154] | |
| Malaysia | between July 2010 and August 2011 | From 4.70 to 173.77 mg/kg | [162] | |
| China | - | From 0.59 to 22.85 mg/kg | [152] | |
| Poland | 2004–2005 | From 0.30 to 8.40 mg/kg | [143] | |
| Egypt | Cotton season 2011 | From 1.63 to 2.57 mg/kg | [142] | |
| Ethiopia | - | From 0.370 to 1.124 mg/kg | [150] | |
| Kenya | - | From 0.05 to 0.35 mg/kg | [147,151] | |
| Cuba | - | From 0.20 to 1.71 mg/kg | [141] | |
| Mexico | -- | From 1.51 to 6.80 mg/kg | [148] | |
| Royal jelly | Germany, | 2017 | From 18.1 to 19.7 mg/kg | [120] |
| Lithuania | 2018 | 24.1 mg/kg | [120] | |
| France and china | May, June, and July 2001 | From 19.4 to 24.8 mg/kg | [156] | |
| China | Between September and October 2014 | From 17.56 to 24.91 mg/kg | [157] | |
| China | - | From 20 to 26 mg/kg | [158] |
6.1.2. Role of Zinc as Adjuvant Therapy in the Management of COVID-19: A Database of Clinical Trials
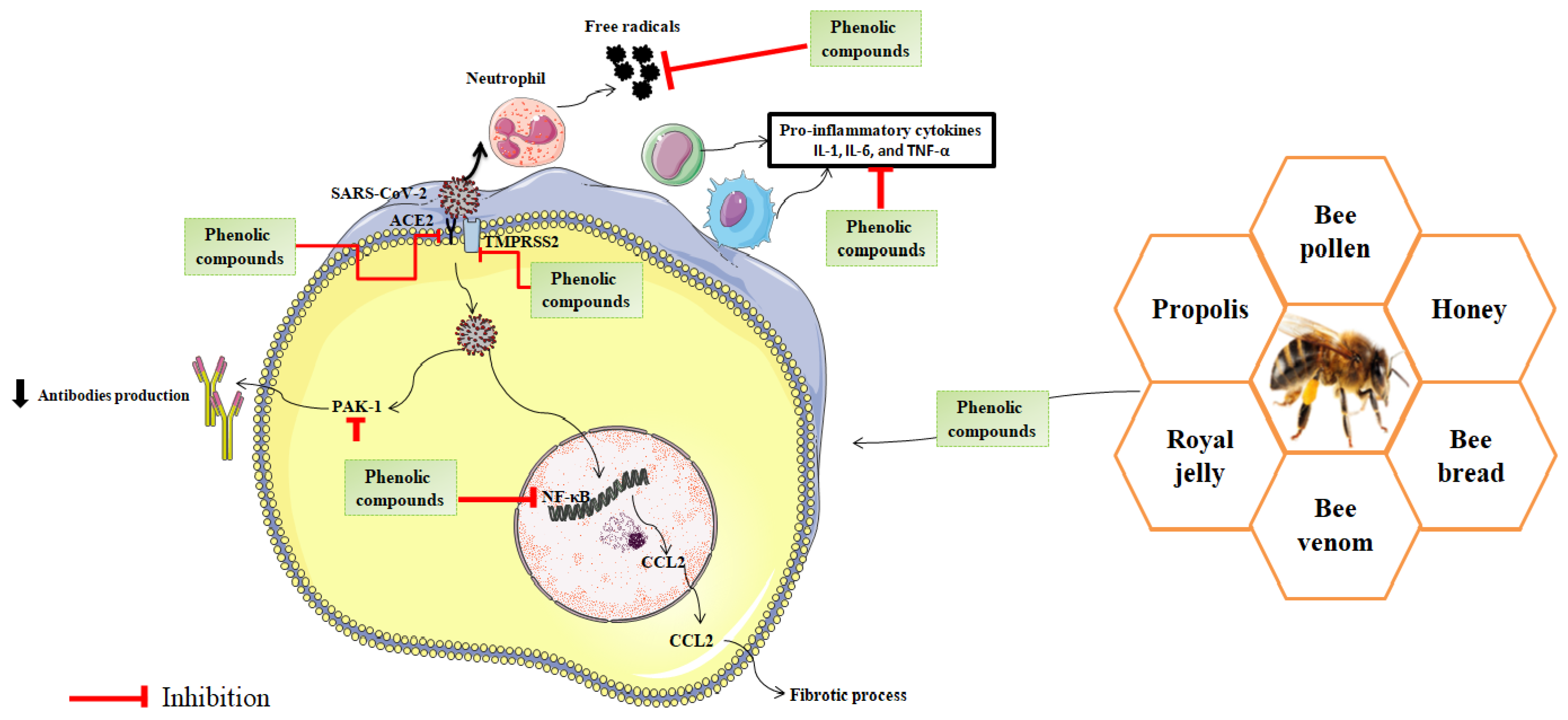
6.2. Polyphenols
6.2.1. Phenolic Compounds in Bee Products
| Bee Products | Country | Collection Period | Content in Individual Polyphenols (mg/kg) | Reference |
|---|---|---|---|---|
| Propolis | Morocco | From the first of May to mid-June 2018 | Catechin (9.6 ± 1.17); vanilic acid (5.6 ± 0.90); p-coumaric acid + epicatechin (ranged from 5.4 ± 0.21 to 190.5 ± 42.00); o-coumaric acid (ranged from 4.1 ± 0.10 to 180.2 ± 0.54); ferulic acid (ranged from 8.3 ± 0.02 to 31.0 ± 4.05); ellagic acid (ranged from 6.5 ± 0.19 to 134.8 ± 19.35); naringin (ranged between 7.6 ± 0.89 and 68.10 ± 7.81); hesperidin (ranged between 7.2 ± 1.28 and 67.2 ± 6.26); apigenin (ranged between 7.1 ± 0.12 and 262.8 ± 40.03); cinnamic acid (ranged between 0.9 ± 0.07 and 34.2 ± 3.54); resveratrol (ranged between 9.9 ± 0.04 and 38.7 ± 9.24); rosmarinic acid (ranged between 13.0 ± 0.12 and 65.6 ± 14.72); rutin (ranged between 3.7 ± 0.88 and 160.6 ± 03.85); chlorogenic acid (ranged between 8.8 ± 0.01 and 32.0 ± 7.23); quercetin (ranged between 7.1 ± 0.19 and 26.7 ± 0.05); kaempferol (ranged between 5.1 ± 0.94 and 303.2 ± 21.15) | [55] |
| Italy | Not mentioned | Chrysin (781.5 ± 80.1); Apigenin (132.1 ± 21.6); Acacetin (1133.3 ± 256.2); Tectochrysin (130.9 ± 21.6); Pinocembrin (769.4 ± 77.9); Pinostrobin (575.6 ± 48.6); Sakuranetin (152.9 ± 20.6); Galangin (70.4 ± 11.8); Kaempferide (39.8 ± 3.6); Quercetin (153.5 ± 18.6); Prenyl caffeate (33.3 ± 2.6); Benzyl caffeate (527.1 ± 61.8); caffeic acid phenylethyl ester (1745.2 ± 245.2) | [187] | |
| China | Not mentioned | Chrysin (2333.0 ± 350.0); Apigenin (178.6 ± 34.8); Acacetin (1578.0 ± 141.9); Tectochrysin (238.3 ± 24.6); Pinocembrin (2087.5 ± 347.0); Sakuranetin (146.0 ± 18.4); Galangin (1400.3 ± 126.6); Kaempferide (38.8 ± 5.0); Quercetin (70.9 ± 9.7); Prenyl caffeate (56.2 ± 6.0); Benzyl caffeate (1477.0 ± 116.6); caffeic acid phenylethyl ester (2525.0 ± 199.3) | [187] | |
| Argentina | Not mentioned | Chrysin (2347.6 ± 215.7); Apigenin (336.9 ± 41.7); Acacetin (831.5 ± 16.0); Tectochrysin (224.7 ± 29.0); Pinocembrin (3362.5 ± 418.8); Sakuranetin (27.5 ± 4.1); Galangin (2253.7 ± 294.4); Kaempferide (38.1 ± 4.6); Prenyl caffeate (29.7 ± 4.2); Benzyl caffeate (1180.1 ± 200.8); caffeic acid phenylethyl ester (1111.6 ± 125.6) | [187] | |
| Ukraine | Not mentioned | Chrysin (922.0 ± 111.2); Apigenin (177.4 ± 15.2); Acacetin (658.9 ± 67.0); Tectochrysin (153.6 ± 18.7); Pinocembrin (1196.5 ± 91.9); Pinostrobin (1479.3 ± 303.5); Sakuranetin (2184.0 ± 196.1); Galangin (952.9 ± 106.8); Kaempferide (91.9 ± 13.7); Quercetin (28.9 ± 4.1); Prenyl caffeate (53.9 ± 6.1); Benzyl caffeate (465.1 ± 31.9); caffeic acid phenylethyl ester (1145.9 ± 98.6) | [187] | |
| Macedonia | Not mentioned | Chrysin (1649.8 ± 177.5); Apigenin (236.4 ± 34.1); Acacetin (1343.0 ± 200.2); Tectochrysin (987.1 ± 104.0); Pinocembrin (2112.0 ± 184.5); Pinostrobin (3816.0 ± 397.1); Sakuranetin (2203.4 ± 269.0); Galangin (903.9 ± 88.2); Kaempferide (43.2 ± 7.0); Quercetin (94.8 ± 10.3); Benzyl caffeate (400.6 ± 62.6); caffeic acid phenylethyl ester (1263.1 ± 212.6) | [187] | |
| Morocco | May 2018 | Ferrulic acid (40.60 ± 0.6); o-Coumaric acid (35.47 ± 0.2); Chlorogenic acid (25.31 ± 0.0); Rosmarinic acid (222.02 ± 6.2); Vanilic acid (10.58 ± 0.1); Ellagic acid (37.94 ± 0.1); Catechin (18.83 ± 0.1); Naringin (290.19 ± 0.2); Hesperidin (271.77 ± 0.0); Quercetin (14.78 ± 0.2); Apigenin (50.37 ± 0.8); Kaempferol (26.48 ± 1.2); Rutin (34.37 ± 1.3); Resveratrol (86.25 ± 0.2) | [54] | |
| Morocco | July 2018 | Vanillic acid (8.61 ± 0.30); o-Coumaric acid (11.44 ± 4.63); Ferulic acid (18.84 ± 0.21); Ellagic acid (28.55 ± 1.99); Naringin (35.78 ± 4.10); Hesperidin (417.18 ± 50.0); Apigenin (38.39 ± 2.60); Resveratrol (116.89 ± 12.7); Rosmarinic acid (470.35 ± 52.00); Rutin (12.40 ± 0.42); Chlorogenic acid (16.11 ± 0.12); Quercetin (12.02 ± 0.13); Kaempferol (21.90 ± 1.60) | [56] | |
| Bee bread | Morocco | Not mentioned | Kaempferol- O-hexosyl-O-rutinoside (570 ± 10); Quercetin-O-hexosyl-O-hexoside (950 ± 30); Methylherbacetrin-O-dihexoside (545 ± 4); isorhamnetin-O-hexosyl-O-rutinoside (1480 ± 50); Quercetin-O-pentosyl-hexoside (580 ± 10); Quercetin 3-O-rutinoside (530 ± 10); methylherbacetrin-3-O-rutinoside (510 ± 10); isorhamnetin-O-pentosyl-hexoside (930 ± 10); kaempferol-3-O-rutinoside (510 ± 10); isorhamnetin-O-rhamnoside-hexoside (560 ± 10) | [117] |
| Romania | spring of 2020 | kaempferol (31.25), myricetin (3.15), luteolin (1.17), rosmarinic acid (0.23), Caffeic acid (0.10), p-Coumaric acid (0.11), Quercetin (0.06) | [188] | |
| North-East European countries | 2015 | Gallic Acid (300); Caffeic Acid (between 700 to 6400); Catechin (between 900 and 52,100); Clorogenic acid (between 800 to 1400); p-Coumaric acid (between 2300 and 11,400); Ferulic acid (between 400 and 1100); Naringenin +Quercetin (between 400 and 3200): Apigenin +Kaempferol (between 600 and 15,800); Pinocembrin (between 400 and 56,600); CAPE +Galangin (between 2400 and 71,900) | [189] | |
| South-West European countries | 2015 | Caffeic acid (between 4600 to 6600); Catechin (between 1700 and 6300); Clorogenic acid (between 400 to 7300); p-Coumaric acid (between 2000 and 12,200); Ferulic acid (between 800 and 2900); Naringenin +Quercetin (between 300 and 1000); Apigenin +Kaempferol (between 4000 and 32,200); Pinocembrin (between 13700 and 33,900); CAPE +Galangin (between 1300 and 110,600) | [189] | |
| South American tropical zone | 2015 | Gallic acid (300); Catechin (between 20800 to 34,100); Clorogenic acid (200); p-Coumaric acid (between 1200 and 2800); Ferulic acid (between 300 and 800); Naringenin +Quercetin (between 500 and 700); Apigenin +Kaempferol (between 1500 and 6500); Pinocembrin (33,300); CAPE +Galangin (between 7600 and 9000) | [189] | |
| Bee pollen | Morocco | May 2018 | Ferrulic acid (17.17 ± 0.4); cinnamic acid (46.01 ± 7.8); o-Coumaric acid (27.10 ± 1.9); Rosmarinic acid (127.30 ± 6.2); Gallic acid (32.54 ± 2.2); Vanilic acid (6.13 ± 0.1); Ellagic acid (13.02 ± 0.0); Naringin (113.71 ± 6.8); Hesperidin (15.63 ± 6.8); Quercetin (48.12 ± 2.8); Apigenin (162.85 ± 17.7); Rutin (95.36 ± 3.7); Resveratrol (44.00 ± 0.4) | [54] |
| Turkey | 2007–2008 | Gallic acid (from 9.46 to 18.59); Protocatechuic acid (from 4.73 to 19.77); p-OH benzoic acid (from 2.74 to 122.68); Chlorogenic acid (from 14.64 to 75.08); Vanillic acid (from 22.96 to 87.02); Caffeic acid (from 10.88 to 98.03); Syringic acid (from 10.55 to 259.53); Epicatechin (from 39.15 to 520.02); p-Coumaric acid (from 34.16 to 127.85); Ferulic acid (from 36.83 to 230.55); Benzoic acid (from 46.87 to 1077.64); Rutin (from 25.59 to 692.85); o-Coumaric acid (from 2.63 to 42.23); Abscisic acid (from 21.04 to 288.70); tert-Cinnamic acid (from 6.82 to 56.38); Quercetin (from 61.40 to 499.20) | [190] | |
| Chile | Not mentioned | Feluric acid (7.75 ± 0.39); Syringic acid (7.92 ± 0.40); p-coumaric acid (52.17 ± 2.60); Rutin (86.60 ± 4.30); Luteolin (28.37 ± 1.42); Cinnamic acid (5.77 ± 0.29); Quercetin (32.72 ± 1.64); Miricetin (6.76 ± 0.34) | [191] | |
| Honey | Morocco | July 2018 | Vanillic acid (2.90 ± 0.01); Ferulic acid (8.35 ± 0.01); Ellagic acid (5.09 ± 0.02); Cinnamic acid (4.25 ± 0.01); Chlorogenic acid (7.06 ± 0.11); Gallic acid (30.06 ± 0.23) | [56] |
| Chile | Not mentioned | Chlorogenic acid+caffeic acid (19.67 ± 0.98); Sinapic acid (from 17.58 to 23.86); Feluric acid (from 4.21 to 16.29); Syringic acid (4.73 ± 0.24); Luteolin (from 8.16 to 11.55); Cinnamic acid (3.00 ± 0.15); Quercetin (5.88 ± 0.29); Kaempherol (from 17.00 to 37.00) | [191] | |
| Hong Kong, Spain, Italy, Korea, China, Canada, Brazil, New Zealand, and Germany | Not mentioned | gallic acid (from 20 to 66); protocatechuic acid (from 7.1 to 63); 2,3,4-trihydroxybenzoic acid (from 11 to 28); protocatechualdehyde (from 7 to 63); p-hydroxybenzoic acid (from 8.1 to 21.7); vanillic acid (5.1); vanillin (from 4.1 to 22.7); syringic acid (from 1.1 to 87.7); caffeic acid (from 6.61 to 23.6); chlorogenic acid (from 11.4 to 21.2); p-coumaric acid (from 8.7 to 13.6); syringaldehyde (from 1.7 to 87.7); genistic acid (from 8.2 to 39.6) | [192] | |
| Morocco | Not mentioned | Methyl syringate (from 277.93 to 443.89); Epicatechin (from 30.67 to 179.29); Syringic acid (from 25.16 to 105.86); Catechin (from 14.56 to 66.13); 4-coumaric acid (from 5.13 to 49.43); Gallic acid (from 0.00 to 34.40); Quercetin (from 7.53 to 42.77); Apigenin (from 1.25 to 33.38); Luteolin (from 0.00 to 22.08); Kaempferol (from 5.24 to 19.64); Naringenin (from 0.00 to 42.41); Formononetin (from 0.87 to 17.88); Genistein (from 0.00 to 24.00); 3-coumaric acid (from 2.40 to 8.66); Daidzein + Pelargonidin (from 1.84 to 8.16); 2-coumaric acid (from 0.45 to 8.17); Biochanin A (from 0.86 to 4.10); Cyanidin (from 0.00 to 2.30) | [193] |
6.2.2. Role of Polyphenols in the Management of COVID-19
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mütze, T.; Friede, T. Data Monitoring Committees for Clinical Trials Evaluating Treatments of COVID-19. Contemp. Clin. Trials 2020, 98, 106154. [Google Scholar] [CrossRef] [PubMed]
- Ather, A.; Patel, B.; Ruparel, N.B.; Diogenes, A.; Hargreaves, K.M. Coronavirus Disease 19 (COVID-19): Implications for Clinical Dental Care. J. Endod. 2020, 46, 584–595. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Rational Use of Personal Protective Equipment (PPE) for Coronavirus Disease (COVID-19): Interim Guidance, 19 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Mullol, J.; Alobid, I.; Mariño-Sánchez, F.; Izquierdo-Domínguez, A.; Marin, C.; Klimek, L.; Wang, D.-Y.; Liu, Z. The Loss of Smell and Taste in the COVID-19 Outbreak: A Tale of Many Countries. Curr. Allergy Asthma Rep. 2020, 20, 61. [Google Scholar] [CrossRef]
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The Emergence of COVID-19 as a Global Pandemic: Understanding the Epidemiology, Immune Response and Potential Therapeutic Targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Samaee, H.; Mohsenzadegan, M.; Ala, S.; Maroufi, S.S.; Moradimajd, P. Tocilizumab for Treatment Patients with COVID-19: Recommended Medication for Novel Disease. Int. Immunopharmacol. 2020, 89, 107018. [Google Scholar] [CrossRef]
- Yavuz, S.; Çelikyurt, F.I.K. An Update of Anti-Viral Treatment of COVID-19. Turk. J. Med. Sci. 2021, 51, 3372–3390. [Google Scholar] [CrossRef]
- Drożdżal, S.; Rosik, J.; Lechowicz, K.; Machaj, F.; Szostak, B.; Przybyciński, J.; Lorzadeh, S.; Kotfis, K.; Ghavami, S.; Łos, M.J. An Update on Drugs with Therapeutic Potential for SARS-CoV-2 (COVID-19) Treatment. Drug Resist. Updat. 2021, 59, 100794. [Google Scholar] [CrossRef]
- Bolarin, J.A.; Oluwatoyosi, M.A.; Orege, J.I.; Ayeni, E.A.; Ibrahim, Y.A.; Adeyemi, S.B.; Tiamiyu, B.B.; Gbadegesin, L.A.; Akinyemi, T.O.; Odoh, C.K. Therapeutic Drugs for SARS-CoV-2 Treatment: Current State and Perspective. Int. Immunopharmacol. 2021, 90, 107228. [Google Scholar] [CrossRef]
- Heskin, J.; Pallett, S.J.; Mughal, N.; Davies, G.W.; Moore, L.S.; Rayment, M.; Jones, R. Caution Required with Use of Ritonavir-Boosted PF-07321332 in COVID-19 Management. Lancet 2022, 399, 21–22. [Google Scholar] [CrossRef]
- Parums, D.V. Editorial: Current Status of Oral Antiviral Drug Treatments for SARS-CoV-2 Infection in Non-Hospitalized Patients. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2022, 28, e935952. [Google Scholar] [CrossRef]
- Lima, W.G.; Brito, J.C.; da Cruz Nizer, W.S. Bee Products as a Source of Promising Therapeutic and Chemoprophylaxis Strategies against COVID-19 (SARS-CoV-2). Phytother. Res. 2021, 35, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Elmahallawy, E.K.; Mohamed, Y.; Abdo, W.; El-Gohary, F.A.; Ahmed Awad Ali, S.; Yanai, T. New Insights into Potential Benefits of Bioactive Compounds of Bee Products on COVID-19: A Review and Assessment of Recent Research. Front. Mol. Biosci. 2021, 7, 513. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, M. Efficacy of Natural Honey Treatment in Patients with Novel Coronavirus. Clinicaltrials.Gov. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT04323345 (accessed on 11 February 2022).
- Silveira, M.A.D.; De Jong, D.; Berretta, A.A.; Galvão, E.B.D.S.; Ribeiro, J.C.; Cerqueira-Silva, T.; Amorim, T.C.; Conceição, L.F.M.R.D.; Gomes, M.M.D.; Teixeira, M.B.; et al. Efficacy of Brazilian Green Propolis (EPP-AF®) as an Adjunct Treatment for Hospitalized COVID-19 Patients: A Randomized, Controlled Clinical Trial. Biomed. Pharmacother. 2021, 138, 111526. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, K.; Janssen, E.; Beutler, B. The Interface between Innate and Adaptive Immunity. Nat. Immunol. 2004, 5, 971–974. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.A.; Hossain, N.; Kashem, M.A.; Shahid, M.A.; Alam, A. Immune Response in COVID-19: A Review. J. Infect. Public Health 2020, 13, 1619–1629. [Google Scholar] [CrossRef] [PubMed]
- Dosch, S.F.; Mahajan, S.D.; Collins, A.R. SARS Coronavirus Spike Protein-Induced Innate Immune Response Occurs via Activation of the NF-KappaB Pathway in Human Monocyte Macrophages in Vitro. Virus Res. 2009, 142, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Yen, Y.-T.; Singh, S.; Kao, C.-L.; Wu-Hsieh, B.A. SARS-CoV Regulates Immune Function-Related Gene Expression in Human Monocytic Cells. Viral Immunol. 2012, 25, 277–288. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-M.A.; Liang, S.-Y.; Shih, Y.-P.; Chen, C.-Y.; Lee, Y.-M.; Chang, L.; Jung, S.-Y.; Ho, M.-S.; Liang, K.-Y.; Chen, H.-Y.; et al. Epidemiological and Genetic Correlates of Severe Acute Respiratory Syndrome Coronavirus Infection in the Hospital with the Highest Nosocomial Infection Rate in Taiwan in 2003. J. Clin. Microbiol. 2006, 44, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Kaiko, G.E.; Horvat, J.C.; Beagley, K.W.; Hansbro, P.M. Immunological Decision-Making: How Does the Immune System Decide to Mount a Helper T-Cell Response? Immunology 2008, 123, 326–338. [Google Scholar] [CrossRef]
- Anaya, J.-M.; Shoenfeld, Y.; Rojas-Villarraga, A.; Levy, R.A.; Cervera, R. Autoimmunity: From Bench to Bedside; El Rosario University Press: Bogota, Colombia, 2013; ISBN 958-738-366-4. [Google Scholar]
- Paul, W.E.; Zhu, J. How Are T(H)2-Type Immune Responses Initiated and Amplified? Nat. Rev. Immunol. 2010, 10, 225–235. [Google Scholar] [CrossRef]
- Stockinger, B.; Bourgeois, C.; Kassiotis, G. CD4+ Memory T Cells: Functional Differentiation and Homeostasis. Immunol. Rev. 2006, 211, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.-Y.; Huang, Z.-T.; Li, L.; Wu, M.-H.; Yu, T.; Koup, R.A.; Bailer, R.T.; Wu, C.-Y. Characterization of SARS-CoV-Specific Memory T Cells from Recovered Individuals 4 Years after Infection. Arch. Virol. 2009, 154, 1093–1099. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Dela Cruz, C.S.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020, 71, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Röltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-CoV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response during SARS-CoV-2 Infection: Lessons from the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045. [Google Scholar] [CrossRef]
- Chiappelli, F.; Khakshooy, A.; Greenberg, G. COVID-19 Immunopathology and Immunotherapy. Bioinformation 2020, 16, 219. [Google Scholar] [CrossRef]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A Multipurpose Trace Element. Arch. Toxicol. 2005, 80, 1. [Google Scholar] [CrossRef]
- Ishida, T. Anti-Viral Vaccine Activity of Zinc (II) for Viral Prevention, Entry, Replication, and Spreading during Pathogenesis Process. Curr. Trends Biomed. Eng. Biosci. 2019, 19, 6. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Feng, H.-L.; Jeng, S.-S. Zinc Supplementation Stimulates Red Blood Cell Formation in Rats. Int. J. Mol. Sci. 2018, 19, 2824. [Google Scholar] [CrossRef] [Green Version]
- Haboubi, N.Y.; Baker, M.A.; Gyde, O.H.; Small, N.A.; Haboubi, N. Zinc Supplementation and Erythropoiesis in the Elderly. J. Clin. Pathol. 1988, 41, 706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhardwaj, P.; Dhawan, D.K. Zinc Treatment Modulates Hematological and Morphological Changes in Rat Erythrocytes Following Arsenic Exposure. Toxicol. Ind. Health 2019, 35, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Barman, S.; Srinivasan, K. Zinc Supplementation Alleviates Hyperglycemia and Associated Metabolic Abnormalities in Streptozotocin-Induced Diabetic Rats. Can. J. Physiol. Pharmacol. 2016, 94, 1356–1365. [Google Scholar] [CrossRef]
- Baltaci, A.K.; Mogulkoc, R.; Baltaci, S.B. The Role of Zinc in the Endocrine System. Pak. J. Pharm. Sci. 2019, 32, 231–239. [Google Scholar]
- Ko, W.-S.; Guo, C.-H.; Hsu, G.-S.W.; Chiou, Y.-L.; Yeh, M.-S.; Yaun, S.-R. The Effect of Zinc Supplementation on the Treatment of Chronic Hepatitis C Patients with Interferon and Ribavirin. Clin. Biochem. 2005, 38, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Maares, M.; Haase, H. Zinc and Immunity: An Essential Interrelation. Arch. Biochem. Biophys. 2016, 611, 58–65. [Google Scholar] [CrossRef]
- McPherson, S.W.; Keunen, J.E.; Bird, A.C.; Chew, E.Y.; van Kuijk, F.J. Investigate Oral Zinc as a Prophylactic Treatment for Those at Risk for COVID-19. Am. J. Ophthalmol. 2020, 216, A5–A6. [Google Scholar] [CrossRef] [PubMed]
- DiSilvestro, R.A.; Dardenne, M.; Joseph, E. Comparison of Thymulin Activity with Other Measures of Marginal Zinc Deficiency. Biol. Trace Elem. Res. 2020, 199, 585–587. [Google Scholar] [CrossRef] [PubMed]
- Haddad, J.J. Thymulin and Zinc (Zn2+)-Mediated Inhibition of Endotoxin-Induced Production of Proinflammatory Cytokines and NF-ΚB Nuclear Translocation and Activation in the Alveolar Epithelium: Unraveling the Molecular Immunomodulatory, Anti-Inflammatory Effect of Thymulin/Zn2+ in Vitro. Mol. Immunol. 2009, 47, 205–214. [Google Scholar] [CrossRef]
- Nasseri, B.; Zaringhalam, J.; Daniali, S.; Manaheji, H.; Abbasnejad, Z.; Nazemian, V. Thymulin Treatment Attenuates Inflammatory Pain by Modulating Spinal Cellular and Molecular Signaling Pathways. Int. Immunopharmacol. 2019, 70, 225–234. [Google Scholar] [CrossRef]
- Prasad, A.S. Lessons Learned from Experimental Human Model of Zinc Deficiency. J. Immunol. Res. 2020, 2020, 9207279. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.K.; Shor-Posner, G.; Campa, A. Zinc Status in Human Immunodeficiency Virus Infection. J. Nutr. 2000, 130, 1421S–1423S. [Google Scholar] [CrossRef] [PubMed]
- Suara, R.O.; Crowe, J.E. Effect of Zinc Salts on Respiratory Syncytial Virus Replication. Antimicrob. Agents Chemother. 2004, 48, 783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumura, H.; Nirei, K.; Nakamura, H.; Arakawa, Y.; Higuchi, T.; Hayashi, J.; Yamagami, H.; Matsuoka, S.; Ogawa, M.; Nakajima, N.; et al. Zinc Supplementation Therapy Improves the Outcome of Patients with Chronic Hepatitis C. J. Clin. Biochem. Nutr. 2012, 51, 178–184. [Google Scholar] [CrossRef] [Green Version]
- Yuasa, K.; Naganuma, A.; Sato, K.; Ikeda, M.; Kato, N.; Takagi, H.; Mori, M. Zinc Is a Negative Regulator of Hepatitis C Virus RNA Replication. Liver Int. 2006, 26, 1111–1118. [Google Scholar] [CrossRef]
- Kaushik, N.; Anang, S.; Ganti, K.P.; Surjit, M. Zinc: A Potential Antiviral against Hepatitis E Virus Infection? DNA Cell Biol. 2018, 37, 593–599. [Google Scholar] [CrossRef]
- Haraguchi, Y.; Sakurai, H.; Hussain, S.; Anner, B.M.; Hoshino, H. Inhibition of HIV-1 Infection by Zinc Group Metal Compounds. Antiviral Res. 1999, 43, 123–133. [Google Scholar] [CrossRef]
- Ragab, D.; Salah Eldin, H.; Taeimah, M.; Khattab, R.; Salem, R. The COVID-19 Cytokine Storm; What We Know So Far. Front. Immunol. 2020, 11, 1446. [Google Scholar] [CrossRef]
- Bao, B.; Prasad, A.S.; Beck, F.W.J.; Fitzgerald, J.T.; Snell, D.; Bao, G.W.; Singh, T.; Cardozo, L.J. Zinc Decreases C-Reactive Protein, Lipid Peroxidation, and Inflammatory Cytokines in Elderly Subjects: A Potential Implication of Zinc as an Atheroprotective Agent. Am. J. Clin. Nutr. 2010, 91, 1634–1641. [Google Scholar] [CrossRef] [Green Version]
- Haase, H.; Rink, L. Functional Significance of Zinc-Related Signaling Pathways in Immune Cells. Annu. Rev. Nutr. 2009, 29, 133–152. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Aboulghazi, A.; Ferreira-Santos, P.; Genisheva, Z.; Antonio Teixeira, J.; Lyoussi, B. Effect of Antioxidant-Rich Propolis and Bee Pollen Extracts against D-Glucose Induced Type 2 Diabetes in Rats. Food Res. Int. 2020, 138, 109802. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ferreira-Santos, P.; Genisheva, Z.; Bakour, M.; Ousaaid, D.; Teixeira, J.A.; Lyoussi, B. Unraveling the Chemical Composition, Antioxidant, α-Amylase and α-Glucosidase Inhibition of Moroccan Propolis. Food Biosci. 2021, 42, 101160. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bakour, M.; Ousaaid, D.; Ferreira-Santos, P.; Genisheva, Z.; El Ghouizi, A.; Aboulghazi, A.; Teixeira, J.A.; Lyoussi, B. Protective Effect of Honey and Propolis against Gentamicin-Induced Oxidative Stress and Hepatorenal Damages. Oxid. Med. Cell. Longev. 2021, 2021, 9719906. [Google Scholar] [CrossRef] [PubMed]
- Gekker, G.; Hu, S.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 Activity of Propolis in CD4+ Lymphocyte and Microglial Cell Cultures. J. Ethnopharmacol. 2005, 102, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Governa, P.; Cusi, M.G.; Borgonetti, V.; Sforcin, J.M.; Terrosi, C.; Baini, G.; Miraldi, E.; Biagi, M. Beyond the Biological Effect of a Chemically Characterized Poplar Propolis: Antibacterial and Antiviral Activity and Comparison with Flurbiprofen in Cytokines Release by LPS-Stimulated Human Mononuclear Cells. Biomedicines 2019, 7, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amoros, M.; Sauvager, F.; Girre, L.; Cormier, M. In Vitro Antiviral Activity of Propolis. Apidologie 1992, 23, 231–240. [Google Scholar] [CrossRef] [Green Version]
- Búfalo, M.; Figueiredo, A.; De Sousa, J.; Candeias, J.; Bastos, J.; Sforcin, J. Anti-poliovirus Activity of Baccharis Dracunculifolia and Propolis by Cell Viability Determination and Real-time PCR. J. Appl. Microbiol. 2009, 107, 1669–1680. [Google Scholar] [CrossRef]
- Bankova, V.; Galabov, A.; Antonova, D.; Vilhelmova, N.; Di Perri, B. Chemical Composition of Propolis Extract ACF® and Activity against Herpes Simplex Virus. Phytomedicine 2014, 21, 1432–1438. [Google Scholar] [CrossRef]
- Schnitzler, P.; Neuner, A.; Nolkemper, S.; Zundel, C.; Nowack, H.; Sensch, K.H.; Reichling, J. Antiviral Activity and Mode of Action of Propolis Extracts and Selected Compounds. Phytother. Res. 2010, 24, S20–S28. [Google Scholar] [CrossRef]
- Sartori, G.; Pesarico, A.P.; Pinton, S.; Dobrachinski, F.; Roman, S.S.; Pauletto, F.; Rodrigues, L.C.; Prigol, M. Protective Effect of Brown Brazilian Propolis against Acute Vaginal Lesions Caused by Herpes Simplex Virus Type 2 in Mice: Involvement of Antioxidant and Anti-inflammatory Mechanisms. Cell Biochem. Funct. 2012, 30, 1–10. [Google Scholar] [CrossRef]
- Mazia, R.S.; de Araújo Pereira, R.R.; de Francisco, L.M.B.; Natali, M.R.M.; Dias Filho, B.P.; Nakamura, C.V.; Bruschi, M.L.; Ueda-Nakamura, T. Formulation and Evaluation of a Mucoadhesive Thermoresponsive System Containing Brazilian Green Propolis for the Treatment of Lesions Caused by Herpes Simplex Type I. J. Pharm. Sci. 2016, 105, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Miryan, M.; Soleimani, D.; Dehghani, L.; Sohrabi, K.; Khorvash, F.; Bagherniya, M.; Sayedi, S.M.; Askari, G. The Effect of Propolis Supplementation on Clinical Symptoms in Patients with Coronavirus (COVID-19): A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2020, 21, 996. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Garzarella, E.U.; Bocchino, B.; D’Avino, M.; Caruso, G.; Buonomo, A.R.; Sacchi, R.; Galeotti, F.; Tenore, G.C.; Zaccaria, V. A Standardized Polyphenol Mixture Extracted from Poplar-Type Propolis for Remission of Symptoms of Uncomplicated Upper Respiratory Tract Infection (URTI): A Monocentric, Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytomedicine 2021, 80, 153368. [Google Scholar] [CrossRef]
- Kosari, M.; Noureddini, M.; Khamechi, S.P.; Najafi, A.; Ghaderi, A.; Sehat, M.; Banafshe, H.R. The Effect of Propolis plus Hyoscyamus Niger L. Methanolic Extract on Clinical Symptoms in Patients with Acute Respiratory Syndrome Suspected to COVID-19: A Clinical Trial. Phytother. Res. PTR 2021, 35, 4000–4006. [Google Scholar] [CrossRef] [PubMed]
- Bilir, O.; Kocak, A.O.; Atas, I. Evaluation of the Effect of Anatolian Propolis on COVID-19 in Healthcare Professionals: Effect of Anatolian Propolis on COVID-19. Sci. Prepr. 2021. [Google Scholar] [CrossRef]
- Refaat, H.; Mady, F.M.; Sarhan, H.A.; Rateb, H.S.; Alaaeldin, E. Optimization and Evaluation of Propolis Liposomes as a Promising Therapeutic Approach for COVID-19. Int. J. Pharm. 2021, 592, 120028. [Google Scholar] [CrossRef] [PubMed]
- Elwakil, B.H.; Shaaban, M.M.; Bekhit, A.A.; El-Naggar, M.Y.; Olama, Z.A. Potential Anti-COVID-19 Activity of Egyptian Propolis Using Computational Modeling. Future Virol. 2021, 16, 107–116. [Google Scholar] [CrossRef]
- Collazo, N.; Carpena, M.; Nuñez-Estevez, B.; Otero, P.; Simal-Gandara, J.; Prieto, M.A. Health Promoting Properties of Bee Royal Jelly: Food of the Queens. Nutrients 2021, 13, 543. [Google Scholar] [CrossRef]
- Habashy, N.H.; Abu-Serie, M.M. Major Royal-Jelly Protein 2 and Its Isoform X1 Are Two Novel Safe Inhibitors for Hepatitis C and B Viral Entry and Replication. Int. J. Biol. Macromol. 2019, 141, 1072–1087. [Google Scholar] [CrossRef]
- Hashemipour, M.A.; Tavakolineghad, Z.; Arabzadeh, S.A.M.; Iranmanesh, Z.; Nassab, S.A.H.G. Antiviral Activities of Honey, Royal Jelly, and Acyclovir Against HSV-1. Wounds Compend. Clin. Res. Pract. 2014, 26, 47–54. [Google Scholar]
- Habashy, N.H.; Abu-Serie, M.M. The Potential Antiviral Effect of Major Royal Jelly Protein2 and Its Isoform X1 against Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insight on Their Sialidase Activity and Molecular Docking. J. Funct. Foods 2020, 75, 104282. [Google Scholar] [CrossRef]
- Abedini, A.; Shafaghi, S.; Ahmad, Z.A.; Javanmardi, E.; Ghorbani, F.; Sharif-Kashani, B.; Naghashzadeh, F.; Shafaghi, M.; Moshirpour, M.; Noorali, S. N-Chromosome Royal Jelly, Propolis and Bee Pollen Supplementation Improve the Clinical Conditions of COVID-19 Patients: A Randomized Controlled Trial. Tradit. Integr. Med. 2021, 6, 360–369. [Google Scholar] [CrossRef]
- Majtán, J.; Kovácová, E.; Bíliková, K.; Simúth, J. The Immunostimulatory Effect of the Recombinant Apalbumin 1-Major Honeybee Royal Jelly Protein-on TNFalpha Release. Int. Immunopharmacol. 2006, 6, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-C.; Chou, W.-M.; Widowati, D.A.; Lin, I.-P.; Peng, C.-C. 10-Hydroxy-2-Decenoic Acid of Royal Jelly Exhibits Bactericide and Anti-Inflammatory Activity in Human Colon Cancer Cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef] [PubMed]
- Kohno, K.; Okamoto, I.; Sano, O.; Arai, N.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly Inhibits the Production of Proinflammatory Cytokines by Activated Macrophages. Biosci. Biotechnol. Biochem. 2004, 68, 138–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado De-Melo, A.A.; Almeida-Muradian, L.B.d.; Sancho, M.T.; Pascual-Maté, A. Composition and Properties of Apis Mellifera Honey: A Review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Bravo Lamas, L.; Martínez Flórez, S.; Agudo Toyos, P. Phenolic Compounds in Honey and Their Associated Health Benefits: A Review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef] [Green Version]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar] [CrossRef]
- Shahzad, A.; Cohrs, R.J. In Vitro Antiviral Activity of Honey against Varicella Zoster Virus (VZV): A Translational Medicine Study for Potential Remedy for Shingles. Transl. Biomed. 2012, 3. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K.; Rahmasari, R.; Matsunaga, A.; Haruyama, T.; Kobayashi, N. Anti-Influenza Viral Effects of Honey in Vitro: Potent High Activity of Manuka Honey. Arch. Med. Res. 2014, 45, 359–365. [Google Scholar] [CrossRef]
- Ashraf, S.; Ashraf, S.; Akmal, R.; Ashraf, M.; Kalsoom, L.; Maqsood, A.; Imran, M.A.; Farooq, I.; Ashraf, S.; Siddiqui, U.N.; et al. Prophylactic Potential of Honey and Nigella Sativa L. against Hospital and Community-Based SARS-CoV-2 Spread: A Structured Summary of a Study Protocol for a Randomised Controlled Trial. Trials 2021, 22, 618. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, S.M.; Aboonq, M.S.; El Rashedy, A.G.; Aljehani, Y.T.; Abou El-Magd, R.M.; Okashah, A.M.; El-Anzi, M.E.; Alharbi, M.B.; El-Tahlawi, R.; Nabo, M.M.H.; et al. Promising Preventive and Therapeutic Effects of TaibUVID Nutritional Supplements for COVID-19 Pandemic: Towards Better Public Prophylaxis and Treatment (A Retrospective Study). Am. J. Blood Res. 2020, 10, 266–282. [Google Scholar] [PubMed]
- Mustafa, M.Z.; Shamsuddin, S.H.; Sulaiman, S.A.; Abdullah, J.M. Anti-Inflammatory Properties of Stingless Bee Honey May Reduce the Severity of Pulmonary Manifestations in COVID-19 Infections. Malays. J. Med. Sci. 2020, 27, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Mayda, N.; Özkök, A.; Bayram, N.E.; Gerçek, Y.C.; Sorkun, K. Bee Bread and Bee Pollen of Different Plant Sources: Determination of Phenolic Content, Antioxidant Activity, Fatty Acid and Element Profiles. J. Food Meas. Charact. 2020, 14, 1795–1809. [Google Scholar] [CrossRef]
- Didaras, N.A.; Dimitriou, T.; Daskou, M.; Karatasou, K.; Mossialos, D. In Vitro Assessment of the Antiviral Activity of Greek Bee Bread and Bee Collected Pollen against Enterovirus D68. J. Microbiol. Biotechnol. Food Sci. 2021, 11, e4859. [Google Scholar] [CrossRef]
- Lee, I.-K.; Hwang, B.S.; Kim, D.-W.; Kim, J.-Y.; Woo, E.-E.; Lee, Y.-J.; Choi, H.J.; Yun, B.-S. Characterization of Neuraminidase Inhibitors in Korean Papaver Rhoeas Bee Pollen Contributing to Anti-Influenza Activities in Vitro. Planta Med. 2016, 82, 524–529. [Google Scholar] [CrossRef] [Green Version]
- Wehbe, R.; Frangieh, J.; Rima, M.; El Obeid, D.; Sabatier, J.-M.; Fajloun, Z. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019, 24, 2997. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-D.; Park, H.-J.; Chae, Y.; Lim, S. An Overview of Bee Venom Acupuncture in the Treatment of Arthritis. Evid. Based Complement. Alternat. Med. 2005, 2, 79–84. [Google Scholar] [CrossRef]
- Kasozi, K.I.; Niedbała, G.; Alqarni, M.; Zirintunda, G.; Ssempijja, F.; Musinguzi, S.P.; Usman, I.M.; Matama, K.; Hetta, H.F.; Mbiydzenyuy, N.E.; et al. Bee Venom—A Potential Complementary Medicine Candidate for SARS-CoV-2 Infections. Front. Public Health 2020, 8, 755. [Google Scholar] [CrossRef]
- Uddin, M.B.; Lee, B.-H.; Nikapitiya, C.; Kim, J.-H.; Kim, T.-H.; Lee, H.-C.; Kim, C.G.; Lee, J.-S.; Kim, C.-J. Inhibitory Effects of Bee Venom and Its Components against Viruses In Vitro and In Vivo. J. Microbiol. 2016, 54, 853–866. [Google Scholar] [CrossRef]
- Yang, W.; Hu, F.; Xu, X. Bee Venom and SARS-CoV-2. Toxicon 2020, 181, 69. [Google Scholar] [CrossRef] [PubMed]
- Cherniack, E.P.; Govorushko, S. To Bee or Not to Bee: The Potential Efficacy and Safety of Bee Venom Acupuncture in Humans. Toxicon 2018, 154, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Jagdis, A.; Sussman, G. Anaphylaxis from Bee Pollen Supplement. CMAJ 2012, 184, 1167–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhee, D.; Lee, H.; Chung, W.; Chang, S.; Lee, M.; Choi, J.; Moon, K.; Koh, J. Giant Dermatofibroma with Granular Cell Changes: Side-effect of Bee-venom Acupuncture? Clin. Exp. Dermatol. Clin. Dermatol. 2009, 34, e18–e20. [Google Scholar] [CrossRef]
- Padavattan, S.; Schirmer, T.; Schmidt, M.; Akdis, C.; Valenta, R.; Mittermann, I.; Soldatova, L.; Slater, J.; Mueller, U.; Markovic-Housley, Z. Identification of a B-Cell Epitope of Hyaluronidase, a Major Bee Venom Allergen, from Its Crystal Structure in Complex with a Specific Fab. J. Mol. Biol. 2007, 368, 742–752. [Google Scholar] [CrossRef]
- El-Seedi, H.R.; Eid, N.; El-Wahed, A.; Aida, A.; Rateb, M.E.; Afifi, H.S.; Algethami, A.F.; Zhao, C.; Al Naggar, Y.; Alsharif, S.M. Honey Bee Products: Preclinical and Clinical Studies of Their Anti-Inflammatory and Immunomodulatory Properties. Front. Nutr. 2022, 8, 1109. [Google Scholar]
- Aliboni, A.; D’Andrea, A.; Massanisso, P. Treatment of Propolis Specimens from Central Italy to Yield a Product with a Lower Charge of Allergenic Species. Sep. Purif. Technol. 2011, 82, 71–75. [Google Scholar] [CrossRef]
- Hwang, D.-S.; Kim, S.K.; Bae, H. Therapeutic Effects of Bee Venom on Immunological and Neurological Diseases. Toxins 2015, 7, 2413–2421. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.-Y.; Hsieh, C.-L. Clinical Applications of Bee Venom Acupoint Injection. Toxins 2020, 12, 618. [Google Scholar] [CrossRef]
- Khalifa, S.A.; Elashal, M.H.; Yosri, N.; Du, M.; Musharraf, S.G.; Nahar, L.; Sarker, S.D.; Guo, Z.; Cao, W.; Zou, X. Bee Pollen: Current Status and Therapeutic Potential. Nutrients 2021, 13, 1876. [Google Scholar] [CrossRef]
- Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-Safi, I.; Mechchate, H.; Lyoussi, B. Bee Bread as a Promising Source of Bioactive Molecules and Functional Properties: An Up-to-Date Review. Antibiotics 2022, 11, 203. [Google Scholar] [CrossRef]
- Medeiros, K.; Figueiredo, C.; Figueredo, T.; Freire, K.; Santos, F.; Alcântara-Neves, N.M.; Silva, T.; Piuvezam, M. Anti-Allergic Effect of Bee Pollen Phenolic Extract and Myricetin in Ovalbumin-Sensitized Mice. J. Ethnopharmacol. 2008, 119, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.-H.; Kim, Y.-H.; Kim, J.-K.; Park, K.-K. Anti-Allergic Effect of Bee Venom in an Allergic Rhinitis Mouse Model. Biol. Pharm. Bull. 2014, 37, 1295–1300. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.-J.; Lee, J.; Shin, J.-S.; Kim, M.; Koh, W.; Kim, M.-J.; Lee, J.; Kim, E.J.; Lee, I.-H.; Kim, W.K. In Vitro and in Vivo Anti-Allergic and Anti-Inflammatory Effects of EBV, a Newly Developed Derivative of Bee Venom, through Modulation of IRF3 Signaling Pathway in a Carrageenan-Induced Edema Model. PLoS ONE 2016, 11, e0168120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marichal, T.; Starkl, P.; Reber, L.L.; Kalesnikoff, J.; Oettgen, H.C.; Tsai, M.; Metz, M.; Galli, S.J. A Beneficial Role for Immunoglobulin E in Host Defense against Honeybee Venom. Immunity 2013, 39, 963–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, G.; Bae, H. Bee Venom Phospholipase A2: Yesterday’s Enemy Becomes Today’s Friend. Toxins 2016, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Du, Y.; Lin, X.; Qian, Y.; Zhou, T.; Huang, Z. CD4+ CD25+ Regulatory T Cells in Tumor Immunity. Int. Immunopharmacol. 2016, 34, 244–249. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, Y.; Yan, Y.; Mi, J.; Lu, L.; Luo, Q.; Li, X.; Zeng, X.; Cao, Y. Antioxidant and Immunomodulatory Activities in Vitro of Polysaccharides from Bee Collected Pollen of Chinese Wolfberry. Int. J. Biol. Macromol. 2020, 163, 190–199. [Google Scholar] [CrossRef]
- Li, F.; Yuan, Q.; Rashid, F. Isolation, Purification and Immunobiological Activity of a New Water-Soluble Bee Pollen Polysaccharide from Crataegus Pinnatifida Bge. Carbohydr. Polym. 2009, 78, 80–88. [Google Scholar] [CrossRef]
- Shaldoum, F.; El-kott, A.F.; Ouda, M.M.A.; Abd-Ella, E.M. Immunomodulatory Effects of Bee Pollen on Doxorubicin-induced Bone Marrow/Spleen Immunosuppression in Rat. J. Food Biochem. 2021, 45, e13747. [Google Scholar] [CrossRef]
- Pohl, P.; Dzimitrowicz, A.; Greda, K.; Jamroz, P.; Lesniewicz, A.; Szymczycha-Madeja, A.; Welna, M. Element Analysis of Bee-Collected Pollen and Bee Bread by Atomic and Mass Spectrometry—Development in Addition to Environmental and Nutritional Aspects. TrAC Trends Anal. Chem. 2020, 128, 115922. [Google Scholar] [CrossRef]
- Pal, A.; Squitti, R.; Picozza, M.; Pawar, A.; Rongioletti, M.; Dutta, A.K.; Sahoo, S.; Goswami, K.; Sharma, P.; Prasad, R. Zinc and COVID-19: Basis of Current Clinical Trials. Biol. Trace Elem. Res. 2020, 199, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Wessels, I.; Rolles, B.; Rink, L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front. Immunol. 2020, 11, 1712. [Google Scholar] [CrossRef]
- Bakour, M.; Fernandes, Â.; Barros, L.; Sokovic, M.; Ferreira, I.C.F.R. Badiaa lyoussi Bee Bread as a Functional Product: Chemical Composition and Bioactive Properties. LWT Food Sci. Technol. 2019, 109, 276–282. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, S.M.; Mahmud-Ab-Rashid, N.-K.; Zawawi, N. Botanical Origin and Nutritional Values of Bee Bread of Stingless Bee (Heterotrigona Itama) from Malaysia. J. Food Qual. 2020, 2020, 2845757. [Google Scholar] [CrossRef] [Green Version]
- Stanciu, O.G.; Marghitas, L.A.; Dezmirean, D. Macro-and Oligo-Mineral Elements from Honeybee-Collected Pollen and Beebread Harvested from Transylvania (Romania). Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Anim. Sci. Biotechnol. 2009, 66, 1–2. [Google Scholar]
- Adaškevičiūtė, V.; Kaškonienė, V.; Kaškonas, P.; Barčauskaitė, K.; Maruška, A. Comparison of Physicochemical Properties of Bee Pollen with Other Bee Products. Biomolecules 2019, 9, 819. [Google Scholar] [CrossRef] [Green Version]
- Kalaycıoğlu, Z.; Kaygusuz, H.; Döker, S.; Kolayli, S.; Erim, F.B. Characterization of Turkish Honeybee Pollens by Principal Component Analysis Based on Their Individual Organic Acids, Sugars, Minerals, and Antioxidant Activities. LWT Food Sci. Technol. 2017, 84, 402–408. [Google Scholar] [CrossRef]
- Sandikci, S.; Tarhan, D.; Yılmaz Aksu, F.; Barutçu, Ü.; Or, M.E. Mineral Element and Heavy Metal (Cadmium, Lead and Arsenic) Levels of Bee Pollen in Turkey. Food Sci. Technol. Camp. 2017, 37, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Spulber, R.; Doğaroğlu, M.; Băbeanu, N.; Popa, O. Physicochemical Characteristics of Fresh Bee Pollen from Different Botanical Origins. Rom. Biotechnol. Lett. 2018, 23, 13357–13365. [Google Scholar]
- Aldgini, H.; Abbadi, A.; Abu-Nameh, E.; Alghazeer, R. Determination of Metals as Bio Indicators in Some Selected Bee Pollen Samples from Jordan. Saudi J. Biol. Sci. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Formicki, G.; Greń, A.; Stawarz, R.; Zyśk, B.; Gał, A. Metal Content in Honey, Propolis, Wax, and Bee Pollen and Implications for Metal Pollution Monitoring. Pol. J. Environ. Stud. 2013, 22, 99–106. [Google Scholar]
- Kostić, A.Ž.; Barać, M.B.; Stanojević, S.P.; Milojković-Opsenica, D.M.; Tešić, Ž.L.; Šikoparija, B.; Radišić, P.; Prentović, M.; Pešić, M.B. Physicochemical Composition and Techno-Functional Properties of Bee Pollen Collected in Serbia. LWT Food Sci. Technol. 2015, 62, 301–309. [Google Scholar] [CrossRef]
- Liolios, V.; Tananaki, C.; Papaioannou, A.; Kanelis, D.; Rodopoulou, M.-A.; Argena, N. Mineral Content in Monofloral Bee Pollen: Investigation of the Effect of the Botanical and Geographical Origin. J. Food Meas. Charact. 2019, 13, 1674–1682. [Google Scholar] [CrossRef]
- Máriássyová, M.I.; Kropková, Z.K.; Kačániová, M.; Fatrcová-Šramková, K.; Nóźková, J. Microbial Properties, Nutritional Composition and Antioxidant Activity of Brassica napus subsp. napus L. Bee Pollen Used in Human Nutrition. Ecol. Chem. Eng. A 2010, 17, 45–54. [Google Scholar]
- Serra Bonvehí, J.; Escolà Jordà, R. Nutrient Composition and Microbiological Quality of Honeybee-Collected Pollen in Spain. J. Agric. Food Chem. 1997, 45, 725–732. [Google Scholar] [CrossRef]
- Yang, K.; Wu, D.; Ye, X.; Liu, D.; Chen, J.; Sun, P. Characterization of Chemical Composition of Bee Pollen in China. J. Agric. Food Chem. 2013, 61, 708–718. [Google Scholar] [CrossRef]
- Serra Bonvehí, J.; Orantes-Bermej, F.J. Element Content of Propolis Collected from Different Areas of South Spain. Environ. Monit. Assess. 2013, 185, 6035–6047. [Google Scholar] [CrossRef]
- Cantarelli, M.Á.; Camiña, J.M.; Pettenati, E.M.; Marchevsky, E.J.; Pellerano, R.G. Trace Mineral Content of Argentinean Raw Propolis by Neutron Activation Analysis (NAA): Assessment of Geographical Provenance by Chemometrics. LWT Food Sci. Technol. 2011, 44, 256–260. [Google Scholar] [CrossRef]
- DOĞAN, M.; Silici, S.; Saraymen, R.; Ilhan, I.O. Element Content of Propolis from Different Regions of Turkey. Acta Aliment. 2006, 35, 127–130. [Google Scholar] [CrossRef]
- Finger, D.; Kelte Filho, I.; Torres, Y.R.; Quináia, S.P. Propolis as an Indicator of Environmental Contamination by Metals. Bull. Environ. Contam. Toxicol. 2014, 92, 259–264. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, M.I.; Escuredo, O.; Revilla, I.; Vivar-Quintana, A.M.; Coello, M.C.; Riocerezo, C.P.; Moncada, G.W. Determination of the Mineral Composition and Toxic Element Contents of Propolis by near Infrared Spectroscopy. Sensors 2015, 15, 27854–27868. [Google Scholar] [CrossRef] [PubMed]
- Lima, B.; Tapia, A.; Luna, L.; Fabani, M.; Schmeda-Hirschmann, G.; Podio, N.; Wunderlin, D.; Feresin, G. Main Flavonoids, DPPH Activity, and Metal Content Allow Determination of the Geographical Origin of Propolis from the Province of San Juan (Argentina). J. Agric. Food Chem. 2009, 57, 2691–2698. [Google Scholar] [CrossRef] [PubMed]
- Maragou, N.; Pavlidis, G.; Karasali, H.; Hatjina, F. Major and Minor Element Levels in Greek Apicultural Products. Glob. Nest J. 2017, 19, 423–429. [Google Scholar]
- Roman, A.; Madras-Majewska, B.; Popiela-Pleban, E. Comparative Study of Selected Toxic Elements in Propolis and Honey. J. Apic. Sci. 2011, 55, 97–106. [Google Scholar]
- Souza, E.; Zaluski, R.; Veiga, N.; Orsi, R. Effects of Seasonal Variations and Collection Methods on the Mineral Composition of Propolis from Apis Mellifera Linnaeus Beehives. Braz. J. Biol. 2016, 76, 396–401. [Google Scholar] [CrossRef] [Green Version]
- Tosic, S.; Stojanovic, G.; Mitic, S.; Pavlovic, A.; Alagic, S. Mineral Composition of Selected Serbian Propolis Samples. J. Apic. Sci. 2017, 61, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Suarez, J.M.; Giampieri, F.; Damiani, E.; Astolfi, P.; Fattorini, D.; Regoli, F.; Quiles, J.L.; Battino, M. Radical-Scavenging Activity, Protective Effect against Lipid Peroxidation and Mineral Contents of Monofloral Cuban Honeys. Plant Foods Hum. Nutr. 2012, 67, 31–38. [Google Scholar] [CrossRef]
- EL-Kazafy, A.; Ali, M. Determination of Heavy Metals Content in Cotton Honey in Kafr El-Shiekh Province, Egypt. J. Plant Prot. Pathol. 2012, 3, 1211–1219. [Google Scholar] [CrossRef]
- Grembecka, M.; Szefer, P. Evaluation of Honeys and Bee Products Quality Based on Their Mineral Composition Using Multivariate Techniques. Environ. Monit. Assess. 2013, 185, 4033–4047. [Google Scholar] [CrossRef] [Green Version]
- Kek, S.P.; Chin, N.L.; Tan, S.W.; Yusof, Y.A.; Chua, L.S. Classification of Honey from Its Bee Origin via Chemical Profiles and Mineral Content. Food Anal. Methods 2017, 10, 19–30. [Google Scholar] [CrossRef]
- Laaroussi, H.; Bouddine, T.; Bakour, M.; Ousaaid, D.; Lyoussi, B. Physicochemical Properties, Mineral Content, Antioxidant Activities, and Microbiological Quality of Bupleurum Spinosum Gouan Honey from the Middle Atlas in Morocco. J. Food Qual. 2020, 2020, 7609454. [Google Scholar] [CrossRef] [Green Version]
- Liberato, M.D.C.T.C.; Morais, S.M.D.; Magalhães, C.E.D.C.; Magalhães, I.L.; Cavalcanti, D.B.; Silva, M.M.D.O. Physicochemical Properties and Mineral and Protein Content of Honey Samples from Ceará State, Northeastern Brazil. Food Sci. Technol. 2013, 33, 38–46. [Google Scholar] [CrossRef]
- Maiyo, W.; Kituyi, J.; Mitei, Y.; Kagwanja, S. Heavy Metal Contamination in Raw Honey, Soil and Flower Samples Obtained from Baringo and Keiyo Counties, Kenya. Int. J. Emerg. Sci. Eng. 2014, 2, 5–9. [Google Scholar]
- Mondragón-Cortez, P.; Ulloa, J.; Rosas-Ulloa, P.; Rodríguez-Rodríguez, R.; Resendiz Vázquez, J. Physicochemical Characterization of Honey from the West Region of México. CyTA-J. Food 2013, 11, 7–13. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Khalil, I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and Antioxidant Properties of Malaysian Honeys Produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Complement. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nigussie, K.; Subramanian, P.; Mebrahtu, G. Physicochemical Analysis of Tigray Honey: An Attempt to Determine Major Quality Markers of Honey. Bull. Chem. Soc. Ethiop. 2012, 26, 26. [Google Scholar] [CrossRef] [Green Version]
- Mbiri, A.; Onditi, A.; Oyaro, N.; Murago, E. Determination of Essential and Heavy Metals in Kenyan Honey by Atomic Absorption and Emission Spectroscopy. J. Agric. Sci. Technol. 2011, 13, 107–115. [Google Scholar]
- Ru, Q.-M.; Feng, Q.; He, J.-Z. Risk Assessment of Heavy Metals in Honey Consumed in Zhejiang Province, Southeastern China. Food Chem. Toxicol. 2013, 53, 256–262. [Google Scholar] [CrossRef]
- Sakač, M.B.; Jovanov, P.T.; Marić, A.Z.; Pezo, L.L.; Kevrešan, Ž.S.; Novaković, A.R.; Nedeljković, N.M. Physicochemical Properties and Mineral Content of Honey Samples from Vojvodina (Republic of Serbia). Food Chem. 2019, 276, 15–21. [Google Scholar] [CrossRef]
- Swaileh, K.M.; Abdulkhaliq, A. Analysis of Aflatoxins, Caffeine, Nicotine and Heavy Metals in Palestinian Multifloral Honey from Different Geographic Regions. J. Sci. Food Agric. 2013, 93, 2116–2120. [Google Scholar] [CrossRef] [PubMed]
- Vanhanen, L.P.; Emmertz, A.; Savage, G.P. Mineral Analysis of Mono-Floral New Zealand Honey. Food Chem. 2011, 128, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Stocker, A.; Schramel, P.; Kettrup, A.; Bengsch, E. Trace and Mineral Elements in Royal Jelly and Homeostatic Effects. J. Trace Elem. Med. Biol. 2005, 19, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, L.; Zhang, W.; Cui, X.; Wang, H.; Xu, B. Comparison of the Nutrient Composition of Royal Jelly and Worker Jelly of Honey Bees (Apis mellifera). Apidologie 2016, 47, 48–56. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Zhang, W.; Cui, X.; Xu, B. Zinc Nutrition Increases the Antioxidant Defenses of Honey Bees. Entomol. Exp. Appl. 2015, 156, 201–210. [Google Scholar] [CrossRef]
- Kostić, A.Ž.; Pešić, M.B.; Mosić, M.D.; Dojčinović, B.P.; Natić, M.M.; Trifković, J.Đ. Mineral Content of Bee Pollen from Serbia. Arh. Hig. Rada Toksikol. 2015, 66, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Carpes, S.T.; Cabral, I.S.R.; Luz, C.F.P.; Capeletti, J.P.; Alencar, S.M. Palynological and Physicochemical Characterization of Apis mellifera L. Bee Pollen in the Southern Region of Brazil. J. Food Agric. Environ. 2009, 7, 667–673. [Google Scholar]
- Costa, M.C.A.; Morgano, M.A.; Ferreira, M.M.C.; Milani, R.F. Quantification of Mineral Composition of Brazilian Bee Pollen by near Infrared Spectroscopy and PLS Regression. Food Chem. 2019, 273, 85–90. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Chowdhury, M.A.Z.; Rahman, M.A.; Sulaiman, S.A.; Gan, S.H. Determination of Mineral, Trace Element, and Pesticide Levels in Honey Samples Originating from Different Regions of Malaysia Compared to Manuka Honey. BioMed Res. Int. 2014, 2014, 359890. [Google Scholar] [CrossRef]
- European Commission. EU Register of Nutrition and Health Claims Made on Food; European Commission. 2016. Available online: https://ec.europa.eu/food/safety/labelling_nutrition/claims/register/public/?event=register.home (accessed on 8 February 2022).
- Polak, E.; Stępień, A.E.; Gol, O.; Tabarkiewicz, J. Potential Immunomodulatory Effects from Consumption of Nutrients in Whole Foods and Supplements on the Frequency and Course of Infection: Preliminary Results. Nutrients 2021, 13, 1157. [Google Scholar] [CrossRef]
- Ferreira-Santos, P.; Genisheva, Z.; Botelho, C.; Rocha, C.; Teixeira, J.A. Valorization of Natural Antioxidants for Nutritional and Health Applications. In Antioxidants; IntechOpen: London, UK, 2021. [Google Scholar]
- Zhao, B.; Ling, Y.; Li, J.; Peng, Y.; Huang, J.; Wang, Y.; Qu, H.; Gao, Y.; Li, Y.; Hu, B. Beneficial Aspects of High Dose Intravenous Vitamin C on Patients with COVID-19 Pneumonia in Severe Condition: A Retrospective Case Series Study. Ann. Palliat. Med. 2021, 10, 1599–1609. [Google Scholar] [CrossRef] [PubMed]
- Hakamifard, A.; Soltani, R.; Maghsoudi, A.; Rismanbaf, A.; Aalinezhad, M.; Tarrahi, M.; Mashayekhbakhsh, S.; Dolatshahi, K. The Effect of Vitamin E and Vitamin C in Patients with COVID-19 Pneumonia; a Randomized Controlled Clinical Trial. Immunopathol. Persa 2021, 7, e08. [Google Scholar]
- Azeem, M.; Hanif, M.; Mahmood, K.; Ameer, N.; Chughtai, F.R.S.; Abid, U. An Insight into Anticancer, Antioxidant, Antimicrobial, Antidiabetic and Anti-Inflammatory Effects of Quercetin: A Review. Polym. Bull. 2022, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Alijotas-Reig, J.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miró-Mur, F. Immunomodulatory Therapy for the Management of Severe COVID-19. Beyond the Anti-Viral Therapy: A Comprehensive Review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef] [PubMed]
- Derwand, R.; Scholz, M. Does Zinc Supplementation Enhance the Clinical Efficacy of Chloroquine/Hydroxychloroquine to Win Today’s Battle against COVID-19? Med. Hypotheses 2020, 142, 109815. [Google Scholar] [CrossRef]
- Pastick, K.A.; Okafor, E.C.; Wang, F.; Lofgren, S.M.; Skipper, C.P.; Nicol, M.R.; Pullen, M.F.; Rajasingham, R.; McDonald, E.G.; Lee, T.C. Hydroxychloroquine and Chloroquine for Treatment of SARS-CoV-2 (COVID-19); Oxford University Press: New York, NY, USA, 2020; Volume 7, p. ofaa130. [Google Scholar]
- Xue, J.; Moyer, A.; Peng, B.; Wu, J.; Hannafon, B.N.; Ding, W.-Q. Chloroquine Is a Zinc Ionophore. PLoS ONE 2014, 9, e109180. [Google Scholar] [CrossRef] [Green Version]
- Te Velthuis, A.J.W.; van den Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; van Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef]
- Carlucci, P.M.; Ahuja, T.; Petrilli, C.; Rajagopalan, H.; Jones, S.; Rahimian, J. Zinc Sulfate in Combination with a Zinc Ionophore May Improve Outcomes in Hospitalized COVID-19 Patients. J. Med. Microbiol. 2020, 69, 1228. [Google Scholar] [CrossRef]
- Al Sulaiman, K.; Aljuhani, O.; Al Shaya, A.I.; Kharbosh, A.; Kensara, R.; Al Guwairy, A.; Alharbi, A.; Algarni, R.; Al Harbi, S.; Vishwakarma, R. Evaluation of Zinc Sulfate as an Adjunctive Therapy in COVID-19 Critically Ill Patients: A Two Center Propensity-Score Matched Study. Crit. Care 2021, 25, 363. [Google Scholar] [CrossRef]
- Szarpak, L.; Pruc, M.; Gasecka, A.; Jaguszewski, M.J.; Michalski, T.; Peacock, F.W.; Smereka, J.; Pytkowska, K.; Filipiak, K.J. Should We Supplement Zinc in COVID-19 Patients? Evidence from Meta-Analysis. Pol. Arch. Intern. Med. 2021, 131, 802–807. [Google Scholar] [CrossRef]
- Gupta, S.; Read, S.A.; Shackel, N.A.; Hebbard, L.; George, J.; Ahlenstiel, G. The Role of Micronutrients in the Infection and Subsequent Response to Hepatitis C Virus. Cells 2019, 8, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giménez, V.M.M.; Bergam, I.; Reiter, R.J.; Manucha, W. Metal Ion Homeostasis with Emphasis on Zinc and Copper: Potential Crucial Link to Explain the Non-Classical Antioxidative Properties of Vitamin D and Melatonin. Life Sci. 2021, 281, 119770. [Google Scholar] [CrossRef] [PubMed]
- Von Bülow, V.; Dubben, S.; Engelhardt, G.; Hebel, S.; Plümäkers, B.; Heine, H.; Rink, L.; Haase, H. Zinc-Dependent Suppression of TNF-α Production Is Mediated by Protein Kinase A-Induced Inhibition of Raf-1, IκB Kinase β, and NF-ΚB. J. Immunol. 2007, 179, 4180–4186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalal, Z.; Bakour, M.; Lyoussi, B. Medicinal Plants and Zinc: Impact on COVID-19 Pandemic. Sci. World J. 2021, 2021, 9632034. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural Polyphenols: An Overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef] [Green Version]
- Bakour, M.; Campos, M.d.G.; Imtara, H.; Lyoussi, B. Antioxidant Content and Identification of Phenolic/Flavonoid Compounds in the Pollen of Fourteen Plants Using HPLC-DAD. J. Apic. Res. 2019, 59, 35–41. [Google Scholar] [CrossRef]
- Galeotti, F.; Capitani, F.; Fachini, A.; Volpi, N. Recent Advances in Analytical Approaches for the Standardization and Quality of Polyphenols of Propolis. J. Med. Plants Res. 2019, 13, 487–500. [Google Scholar]
- Rzepecka-Stojko, A.; Stojko, J.; Kurek-Górecka, A.; Górecki, M.; Kabała-Dzik, A.; Kubina, R.; Moździerz, A.; Buszman, E. Polyphenols from Bee Pollen: Structure, Absorption, Metabolism and Biological Activity. Molecules 2015, 20, 21732–21749. [Google Scholar] [CrossRef] [Green Version]
- Memariani, H.; Memariani, M.; Moravvej, H.; Shahidi-Dadras, M. Melittin: A Venom-Derived Peptide with Promising Anti-Viral Properties. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 5–17. [Google Scholar] [CrossRef]
- Sobral, F.; Sampaio, A.; Falcão, S.; Queiroz, M.J.R.; Calhelha, R.C.; Vilas-Boas, M.; Ferreira, I.C. Chemical Characterization, Antioxidant, Anti-Inflammatory and Cytotoxic Properties of Bee Venom Collected in Northeast Portugal. Food Chem. Toxicol. 2016, 94, 172–177. [Google Scholar] [CrossRef] [Green Version]
- Medana, C.; Carbone, F.; Aigotti, R.; Appendino, G.; Baiocchi, C. Selective Analysis of Phenolic Compounds in Propolis by HPLC-MS/MS. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2008, 19, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Dranca, F.; Ursachi, F.; Oroian, M. Bee Bread: Physicochemical Characterization and Phenolic Content Extraction Optimization. Foods 2020, 9, 1358. [Google Scholar] [CrossRef]
- Osés, S.M.; Marcos, P.; Azofra, P.; de Pablo, A.; Fernández-Muíño, M.Á.; Sancho, M.T. Phenolic Profile, Antioxidant Capacities and Enzymatic Inhibitory Activities of Propolis from Different Geographical Areas: Needs for Analytical Harmonization. Antioxidants 2020, 9, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulusoy, E.; Kolayli, S. Phenolic Composition and Antioxidant Properties of Anzer Bee Pollen. J. Food Biochem. 2014, 38, 73–82. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Giordano, A.; Retamal, M.; Valenzuela, L. Bioactivities of Phenolic Blend Extracts from Chilean Honey and Bee Pollen. CyTA-J. Food 2019, 17, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic Acids and Flavonoids Profiles of Commercial Honey from Different Floral Sources and Geographic Sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Elamine, Y.; Lyoussi, B.; Miguel, M.G.; Anjos, O.; Estevinho, L.; Alaiz, M.; Girón-Calle, J.; Martín, J.; Vioque, J. Physicochemical Characteristics and Antiproliferative and Antioxidant Activities of Moroccan Zantaz Honey Rich in Methyl Syringate. Food Chem. 2021, 339, 128098. [Google Scholar] [CrossRef] [PubMed]
- Berretta, A.A.; Silveira, M.A.D.; Capcha, J.M.C.; De Jong, D. Propolis and Its Potential against SARS-CoV-2 Infection Mechanisms and COVID-19 Disease: Running Title: Propolis against SARS-CoV-2 Infection and COVID-19. Biomed. Pharmacother. 2020, 131, 110622. [Google Scholar] [CrossRef] [PubMed]
- Laforge, M.; Elbim, C.; Frère, C.; Hémadi, M.; Massaad, C.; Nuss, P.; Benoliel, J.-J.; Becker, C. Tissue Damage from Neutrophil-Induced Oxidative Stress in COVID-19. Nat. Rev. Immunol. 2020, 20, 515–516. [Google Scholar] [CrossRef]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [Green Version]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, J.-H.; Baek, S.J. Molecular Targets of Dietary Polyphenols with Anti-Inflammatory Properties. Yonsei Med. J. 2005, 46, 585–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahlan, M.; Irdiani, R.; Flamandita, D.; Aditama, R.; Alfarraj, S.; Ansari, M.J.; Khayrani, A.C.; Pratami, D.K.; Lischer, K. Molecular Interaction Analysis of Sulawesi Propolis Compounds with SARS-CoV-2 Main Protease as Preliminary Study for COVID-19 Drug Discovery. J. King Saud Univ.-Sci. 2021, 33, 101234. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Dhanjal, J.K.; Kaul, S.C.; Wadhwa, R.; Sundar, D. Withanone and Caffeic Acid Phenethyl Ester Are Predicted to Interact with Main Protease (Mpro) of SARS-CoV-2 and Inhibit Its Activity. J. Biomol. Struct. Dyn. 2021, 39, 3842–3854. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.S.; Sushma, P.; Dharmashekar, C.; Beelagi, M.S.; Prasad, S.K.; Shivamallu, C.; Prasad, A.; Syed, A.; Marraiki, N.; Prasad, K.S. In Silico Evaluation of Flavonoids as Effective Antiviral Agents on the Spike Glycoprotein of SARS-CoV-2. Saudi J. Biol. Sci. 2021, 28, 1040–1051. [Google Scholar] [CrossRef]
- Hashem, H. In Silico Approach of Some Selected Honey Constituents as SARS-CoV-2 Main Protease (COVID-19) Inhibitors. EJMO. 2020, 3, 196–200. [Google Scholar] [CrossRef]
- Vijayakumar, B.G.; Ramesh, D.; Joji, A.; Kannan, T. In Silico Pharmacokinetic and Molecular Docking Studies of Natural Flavonoids and Synthetic Indole Chalcones against Essential Proteins of SARS-CoV-2. Eur. J. Pharmacol. 2020, 886, 173448. [Google Scholar] [CrossRef]
- Pitsillou, E.; Liang, J.; Ververis, K.; Hung, A.; Karagiannis, T.C. Interaction of Small Molecules with the SARS-CoV-2 Papain-like Protease: In Silico Studies and in Vitro Validation of Protease Activity Inhibition Using an Enzymatic Inhibition Assay. J. Mol. Graph. Model. 2021, 104, 107851. [Google Scholar] [CrossRef]
- Bumrungpert, A.; Lilitchan, S.; Tuntipopipat, S.; Tirawanchai, N.; Komindr, S. Ferulic Acid Supplementation Improves Lipid Profiles, Oxidative Stress, and Inflammatory Status in Hyperlipidemic Subjects: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2018, 10, 713. [Google Scholar] [CrossRef] [Green Version]
- Abdollahi, S.; Salehi-Abargouei, A.; Toupchian, O.; Sheikhha, M.H.; Fallahzadeh, H.; Rahmanian, M.; Tabatabaie, M.; Mozaffari-Khosravi, H. The Effect of Resveratrol Supplementation on Cardio-metabolic Risk Factors in Patients with Type 2 Diabetes: A Randomized, Double-blind Controlled Trial. Phytother. Res. 2019, 33, 3153–3162. [Google Scholar] [CrossRef]
- Marinella, M.A. Indomethacin and Resveratrol as Potential Treatment Adjuncts for SARS-CoV-2/COVID-19. Int. J. Clin. Pract. 2020, 74, e13535. [Google Scholar] [CrossRef] [PubMed]
- McKee, D.L.; Sternberg, A.; Stange, U.; Laufer, S.; Naujokat, C. Candidate Drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020, 157, 104859. [Google Scholar] [CrossRef] [PubMed]

| Clinical Trial Study | Dose/Route and Treatment Duration | Drugs/Dietary Supplement | Number of Participants/Age/Sex | ClinicalTrials.gov Identifier/Phase |
|---|---|---|---|---|
| Efficacy of subcutaneous ivermectin with or without zinc in COVID-19 patients | 20 mg/day of zinc sulfate, orally 3 times a day | Ivermectin and zinc | 180/18 years and older/males and females | NCT04472585/Phase II |
| A randomized, placebo-controlled study evaluating the efficacy of zinc for the treatment of COVID-19 in the outpatient setting | 220 mg/day of zinc Sulfate, once daily, orally for 5 days | Zinc sulfate | 300/18 years and older/males and females | NCT04621461/Phase IV |
| A study of quintuple therapy to treat COVID-19 infection Hydroxychloroquine | No available data | Hydroxychloroquine, Azithromycin/Vitamin C, vitamin D and zinc | 600/18 years and older/both sexes | NCT04334512/Phase II |
| Zinc versus multivitamin micronutrient supplementation to support immune health in the setting of COVID-19 pandemic: a randomized study | 11 mg/day of zinc, orally during 3 months | Vitamin E, vitamin C, zinc, copper and beta-carotene | 2700/between 18 to 90 years/both sexes | NCT04551339/Complited |
| Anti-inflammatory/antioxidant oral nutrition supplementation in COVID-19 | Oraldietary supplement containing 5.7 mg zinc for 14 days | Supplement enriched with zinc, selenium vitamin A | 30/between 18 to 65 years/both sexes | NCT04323228/Phase III |
| The study of quadruple therapy zinc, quercetin, bromelain, and vitamin C on the clinical outcomes of patients infected with COVID-19 | 50 mg/day, orally during 10 days | Quercetin, bromelain, vitamin C and Zinc | 60/18 years and older adult)/males and females | NCT04468139/Phase IV |
| A preventive treatment for migrant workers at high-risk of COVID-19 | 80 mg/day of zinc tablet, once a day for 42 days | Hydroxychloroquine, Ivermectin, povidone-iodine/vitamin C, and zinc | 5000/between 21 to 60 years/both sexes | NCT04446104/Phase III |
| Comparative study of hydroxychloroquine associated with zinc and ivermectin in COVID-19 prophylaxis | 20 mg/day of zinc twice a day for 45 days | Hydroxychloroquine and Ivermectin/zinc | 400/between 18 to 70 years/both sexes | NCT04384458/not available |
| A study of hydroxychloroquine and zinc in the prevention of COVID-19 infection in military healthcare workers | 15 mg/day of zinc sulfate, orally during 2 months | Hydroxychloroquine/zinc | 660/between 18 to 65 years/both sexes | NCT04377646/Phase III |
| International ALLIANCE study of therapies to prevent progression of COVID-19 | 30 mg/day of zinc citrate, orally for 14 days | Hydroxychloroquine and Azithromycin/Zinc Citrate, Vitamin C, vitamin D3, and vitamin B12 | 200/18 years and older/both sexes | NCT04395768/Phase II |
| Community-based intervention trial to compare theimpact of preventive and therapeutic zinc supplementation programs amoung young children | 20 mg/day of zinc, orally for 10 days during episodes of diarrhea | Zinc | 7680/18 years and older/both sexes | NCT00944359/not available |
| Hydroxychloroquine and zinc with either azithromycin or doxycycline for treatment of COVID-19 in outpatient setting | 220 mg of zinc sulfate once a day, orally for 5 days | Hydroxychloroquine, Azithromycin, Doxycycline/zinc | 750/30 years and older/males and females | NCT04370782/Phase IV |
| Trial of combination therapy to treat COVID-19 infection | No available data | Ivermectin, doxycycline Hcl/zinc, vitamin C, and vitamin D | 31/between 18 to 75 years/males and females | NCT04482686/Pahse I |
| Does zinc supplementation enhance the clinical efficacy of chloroquine/hydroxychloroquine in treatment of COVID-19? | No available data | Chloroquine/zinc | 200/adults/males and females | NCT04447534/Phase III |
| Type of the Study | Compounds Used | Key Results | Reference |
|---|---|---|---|
| In silico | glyasperin A and broussoflavonol F | Both compounds have favorable interaction profiles with SARS-CoV-2 main protease (PDB ID 6Y2F) catalytic sites (His41 and Cys145) with binding similarities of 75% and 63%, respectively, compared to potent inhibitors. | [199] |
| In silico | caffeic acid phenethyl ester | Caffeic acid phenethyl ester can bind to the substrate-binding pocket of SARS-CoV-2 Mprowith efficacy and binding energies equivalent to an already claimed N3 protease inhibitor | [200] |
| In silico | apigenin, chrysin, fisetin, galangin, hesperitin, luteolin, morin, naringin, quercetin, rutin, qercetin, kaempferol, p-coumaric acid, chrysin, luteolin, ribavirin | All the flavonoids studied have a high binding affinity with the active site of the spike protein of SARS-CoV-2 | [201] |
| In silico | 3-phenyllactic acid, caffeic acid phenethyl ester, caffeic acid, chrysin, galangin, lumichrome | caffeic acid phenethyl ester, caffeic acid, chrysin, galanginhave a strong binding affinity with a good glide score and may inhibit the COVID-19 Mpro and replication of the virus. | [202] |
| In silico | luteolin, apigenin, tangeritin, kaempferol, quercetin, myricetin, fisetin, hesperitin, naringenin, eriodicytol, luquiritin, genistein, daidzein, callophyllolide, cyanidin, delphenidin, malvidin, pelargonidin, peonidzin, arbutin, pheloretin, chalconaringenin | cyanidin may suppress rdrp by binding at asp761 catalytic residue and halting the viral replication process. daidzein, eriodictyol, fisetin, genistein, kaempferol, myricetin, quercetin, arbutin, chalconaringenin, phloretin, and liquiritin interact on the spike proteins’ key rbd and may inhibit spread to receptors. | [203] |
| In silico and in vitro | a total of 220 phenolic compounds were tested in the study | In silico and in vitro results indicate that hypericin, rutin, and cyanidin-3-O-glucoside can inhibit SARS-CoV-2 papain-like protease (PLpro) | [204] |
| In silico | rutin, caffeic acid phenethyl ester, quercetin, kaempferol, pinocembrin, pinobanksin, galangin, chrysin, p-cumaric acid, and benzoic acid. | Docking studies revealed that Rutin and Caffeic acid phenethyl ester showed the highest affinity to both targets (COVID-19 3CL-protease and S1 Spike) | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakour, M.; Laaroussi, H.; Ousaaid, D.; El Ghouizi, A.; Es-safi, I.; Mechchate, H.; Lyoussi, B. New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols. Nutrients 2022, 14, 942. https://doi.org/10.3390/nu14050942
Bakour M, Laaroussi H, Ousaaid D, El Ghouizi A, Es-safi I, Mechchate H, Lyoussi B. New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols. Nutrients. 2022; 14(5):942. https://doi.org/10.3390/nu14050942
Chicago/Turabian StyleBakour, Meryem, Hassan Laaroussi, Driss Ousaaid, Asmae El Ghouizi, Imane Es-safi, Hamza Mechchate, and Badiaa Lyoussi. 2022. "New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols" Nutrients 14, no. 5: 942. https://doi.org/10.3390/nu14050942
APA StyleBakour, M., Laaroussi, H., Ousaaid, D., El Ghouizi, A., Es-safi, I., Mechchate, H., & Lyoussi, B. (2022). New Insights into Potential Beneficial Effects of Bioactive Compounds of Bee Products in Boosting Immunity to Fight COVID-19 Pandemic: Focus on Zinc and Polyphenols. Nutrients, 14(5), 942. https://doi.org/10.3390/nu14050942








