Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings
Abstract
:1. Introduction
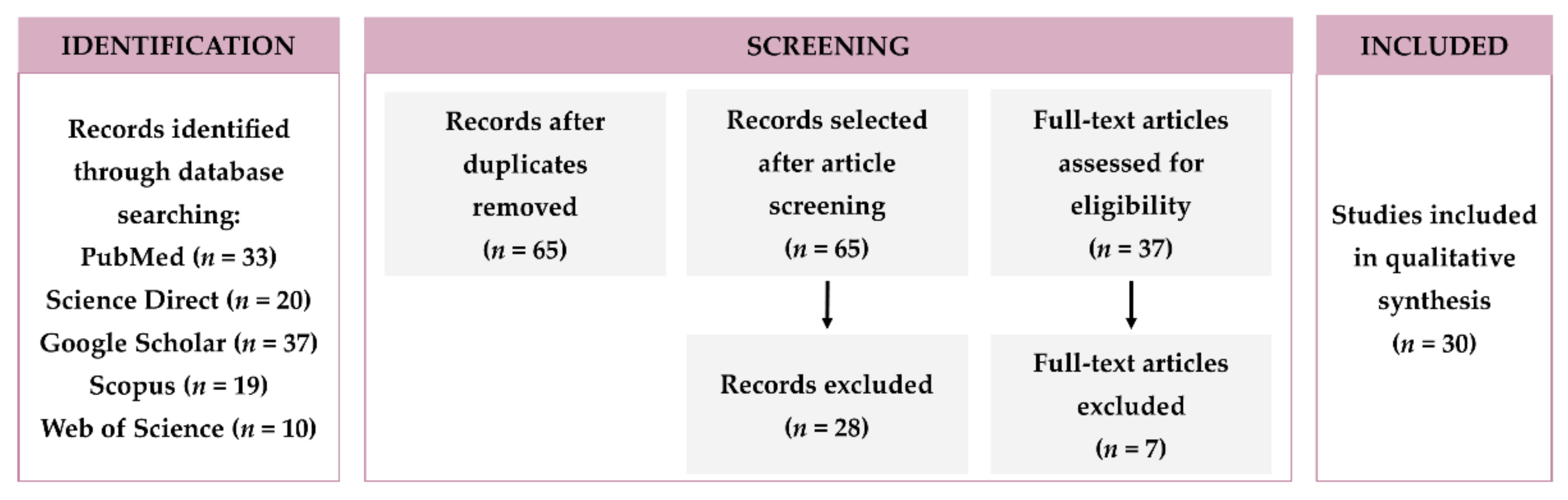
2. Research Methodology
3. NPs-Loaded Hydrogel System
4. Routes of Drugs Administration
4.1. Parenteral Administration of Polymeric NPs-Loaded Hydrogels
4.1.1. Subcutaneous Administration
4.1.2. Local Administration
4.2. Topical Administration of Polymeric NPs-Loaded Hydrogels
4.2.1. Ocular Administration by Instillation
4.2.2. Epidermic Administration
4.2.3. Vaginal Administration
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellotti, E.; Schilling, A.L.; Little, S.R.; Decuzzi, P. Injectable thermoresponsive hydrogels as drug delivery system for the treatment of central nervous system disorders: A review. J. Control. Release 2020, 329, 16–35. [Google Scholar] [CrossRef]
- El-Sayed, A.; Kamel, M. Advances in nanomedical applications: Diagnostic, therapeutic, immunization, and vaccine production. Environ. Sci. Pollut. Res. 2019, 27, 19200–19213. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Mozafari, M. Nanotechnology and Nanomedicine: Start small, think big. Mater. Today Proc. 2018, 5, 15492–15500. [Google Scholar] [CrossRef]
- Nobile, L.; Nobile, S. Recent advances of nanotechnology in medicine and engineering. AIP Conf. Proc. 2016, 1736, 20058. [Google Scholar] [CrossRef]
- Saxena, S.K.; Nyodu, R.; Kumar, S.; Maurya, V.K. Current Advances in Nanotechnology and Medicine. NanoBioMedicine 2020, 3–16. [Google Scholar] [CrossRef]
- Souto, E.B.; Silva, G.F.; Dias-Ferreira, J.; Zielinska, A.; Ventura, F.; Durazzo, A.; Lucarini, M.; Novellino, E.; Santini, A. Nanopharmaceutics: Part I—Clinical Trials Legislation and Good Manufacturing Practices (GMP) of Nanotherapeutics in the EU. Pharmaceutics 2020, 12, 146. [Google Scholar] [CrossRef] [Green Version]
- Zhao, F.; Yao, D.; Guo, R.; Deng, L.; Dong, A.; Zhang, J. Composites of Polymer Hydrogels and Nanoparticulate Systems for Biomedical and Pharmaceutical Applications. Nanomaterials 2015, 5, 2054–2130. [Google Scholar] [CrossRef] [Green Version]
- Operti, M.C.; Bernhardt, A.; Grimm, S.; Engel, A.; Figdor, C.G.; Tagit, O. PLGA-based nanomedicines manufacturing: Technologies overview and challenges in industrial scale-up. Int. J. Pharm. 2021, 605, 120807. [Google Scholar] [CrossRef]
- Wang, K.; Hao, Y.; Wang, Y.; Chen, J.; Mao, L.; Deng, Y.; Chen, J.; Yuan, S.; Zhang, T.; Ren, J.; et al. Functional Hydrogels and Their Application in Drug Delivery, Biosensors, and Tissue Engineering. Int. J. Polym. Sci. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.J.; Jia, S.R.; Bai, H.; Zhong, C. Nanocomposite hydrogels as multifunctional systems for biomedical applications: Current state and perspectives. Compos. Part B Eng. 2020, 200, 108208. [Google Scholar] [CrossRef]
- Mellati, A.; Hasanzadeh, E.; Gholipourmalekabadi, M.; Enderami, S.E. Injectable nanocomposite hydrogels as an emerging platform for biomedical applications: A review. Mater. Sci. Eng. C 2021, 131, 112489. [Google Scholar] [CrossRef] [PubMed]
- Biondi, M.; Borzacchiello, A.; Mayol, L.; Ambrosio, L. Nanoparticle-Integrated Hydrogels as Multifunctional Composite Materials for Biomedical Applications. Gels 2015, 1, 162–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Krishnan, N.; Heo, J.; Fang, R.H.; Zhang, L. Nanoparticle–hydrogel superstructures for biomedical applications. J. Control. Release 2020, 324, 505–521. [Google Scholar] [CrossRef] [PubMed]
- Meis, C.M.; Grosskopf, A.K.; Correa, S.; Appel, E.A. Injectable Supramolecular Polymer-Nanoparticle Hydrogels for Cell and Drug Delivery Applications. JoVE 2021, 168, e62234. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049–2061. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, C.; Pereira, P.; Gama, M. Self-Assembled Hydrogel Nanoparticles for Drug Delivery Applications. Materials 2010, 3, 1420–1460. [Google Scholar] [CrossRef] [Green Version]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid Nanoparticles and Their Hydrogel Composites for Drug Delivery: A Review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef] [Green Version]
- Mauri, E.; Negri, A.; Rebellato, E.; Masi, M.; Perale, G.; Rossi, F. Hydrogel-Nanoparticles Composite System for Controlled Drug Delivery. Gels 2018, 4, 74. [Google Scholar] [CrossRef] [Green Version]
- Welch, V.; Petticrew, M.; Petkovic, J.; Moher, D.; Waters, E.; White, H.; Tugwell, P.; Atun, R.; Awasthi, S.; Barbour, V.; et al. Extending the PRISMA statement to equity-focused systematic reviews (PRISMA-E 2012): Explanation and elaboration. J. Clin. Epidemiol. 2015, 70, 68–89. [Google Scholar] [CrossRef] [Green Version]
- Begines, B.; Ortiz, T.; Pérez-Aranda, M.; Martínez, G.; Merinero, M.; Argüelles-Arias, F.; Alcudia, A. Polymeric Nanoparticles for Drug Delivery: Recent Developments and Future Prospects. Nanomaterials 2020, 10, 1403. [Google Scholar] [CrossRef]
- Adhikari, C. Polymer nanoparticles-preparations, applications and future insights: A concise review. Polym. Technol. Mater. 2021, 1–29. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2020, 20, 101–124. [Google Scholar] [CrossRef]
- Bernal-Chávez, S.A.; Alcalá-Alcalá, S.; Cerecedo, D.; Ganem-Rondero, A. Platelet lysate-loaded PLGA nanoparticles in a thermo-responsive hydrogel intended for the treatment of wounds. Eur. J. Pharm. Sci. 2020, 146, 105231. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2012, 64, 18–23. [Google Scholar] [CrossRef]
- Chang, D.; Park, K.; Famili, A. Hydrogels for sustained delivery of biologics to the back of the eye. Drug Discov. Today 2019, 24, 1470–1482. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Zhou, J.-E.; Tan, J.; Li, M.; Xu, N.; Qu, F.; Chen, J.; Li, J.; Wang, J.; et al. A Photopolymerized Semi-Interpenetrating Polymer Networks-Based Hydrogel Incorporated with Nanoparticle for Local Chemotherapy of Tumors. Pharm. Res. 2021, 38, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Madduma-Bandarage, U.S.K.; Madihally, S.V. Synthetic hydrogels: Synthesis, novel trends, and applications. J. App. Polym. Sci. 2021, 138, 50376. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Liu, J.; Wang, Z.; Wang, W.; Kong, D.; Leng, X. ICG/l-Arginine Encapsulated PLGA Nanoparticle-Thermosensitive Hydrogel Hybrid Delivery System for Cascade Cancer Photodynamic-NO Therapy with Promoted Collagen Depletion in Tumor Tissues. Mol. Pharm. 2021, 18, 928–939. [Google Scholar] [CrossRef]
- Boffito, M.; Gioffredi, E.; Chiono, V.; Calzone, S.; Ranzato, E.; Martinotti, S.; Ciardelli, G. Novel polyurethane-based thermosensitive hydrogels as drug release and tissue engineering platforms: Design and in vitro characterization. Polym. Int. 2016, 65, 756–769. [Google Scholar] [CrossRef]
- Ren, Y.; Li, X.; Han, B.; Zhao, N.; Mu, M.; Wang, C.; Du, Y.; Wang, Y.; Tong, A.; Liu, Y.; et al. Improved anti-colorectal carcinomatosis effect of tannic acid co-loaded with oxaliplatin in nanoparticles encapsulated in thermosensitive hydrogel. Eur. J. Pharm. Sci. 2018, 128, 279–289. [Google Scholar] [CrossRef]
- Cao, D.; Zhang, X.; Akabar, M.D.; Luo, Y.; Wu, H.; Ke, X.; Ci, T. Liposomal doxorubicin loaded PLGA-PEG-PLGA based thermogel for sustained local drug delivery for the treatment of breast cancer. Artif. Cells Nanomed. Biotechnol. 2019, 47, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brachi, G.; Ruiz-Ramírez, J.; Dogra, P.; Wang, Z.; Cristini, V.; Ciardelli, G.; Rostomily, R.C.; Ferrari, M.; Mikheev, A.M.; Blanco, E.; et al. Intratumoral injection of hydrogel-embedded nanoparticles enhances retention in glioblastoma. Nanoscale 2020, 12, 23838–23850. [Google Scholar] [CrossRef] [PubMed]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S. Hydrogels as Drug Delivery Systems; Pros and Cons. Trends Pharm. Sci. 2019, 5, 7–24. [Google Scholar] [CrossRef]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- Malik, D.S.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Patents 2015, 26, 213–228. [Google Scholar] [CrossRef]
- Patil, J.S.; Sarasija, S. Pulmonary drug delivery strategies: A concise, systematic review. Lung India 2012, 29, 44–49. [Google Scholar]
- Huang, H.; Qi, X.; Chen, Y.; Wu, Z. Thermo-sensitive hydrogels for delivering biotherapeutic molecules: A review. Saudi Pharm. J. 2019, 27, 990–999. [Google Scholar] [CrossRef]
- Rafael, D.; Melendres, M.M.R.; Andrade, F.; Montero, S.; Martinez-Trucharte, F.; Vilar-Hernandez, M.; Durán-Lara, E.F.; Schwartz, S., Jr.; Abasolo, I. Thermo-responsive hydrogels for cancer local therapy: Challenges and state-of-art. Int. J. Pharm. 2021, 606, 120954. [Google Scholar] [CrossRef]
- Peng, Q.; Sun, X.; Gong, T.; Wu, C.-Y.; Zhang, T.; Tan, J.; Zhang, Z.-R. Injectable and biodegradable thermosensitive hydrogels loaded with PHBHHx nanoparticles for the sustained and controlled release of insulin. Acta Biomater. 2012, 9, 5063–5069. [Google Scholar] [CrossRef]
- Lee, A.L.Z.; Voo, Z.X.; Chin, W.; Ono, R.J.; Yang, C.; Gao, S.; Hedrick, J.L.; Yang, Y.Y. Injectable Coacervate Hydrogel for Delivery of Anticancer Drug-Loaded Nanoparticles in vivo. ACS Appl. Mater. Interfaces 2018, 10, 13274–13282. [Google Scholar] [CrossRef] [PubMed]
- Chao, Y.; Chen, Q.; Liu, Z. Smart Injectable Hydrogels for Cancer Immunotherapy. Adv. Funct. Mater. 2019, 30. [Google Scholar] [CrossRef]
- Thakur, S.; Singh, H.; Singh, A.; Kaur, S.; Sharma, A.; Singh, S.K.; Kaur, S.; Kaur, G.; Jain, S.K. Thermosensitive injectable hydrogel containing carboplatin loaded nanoparticles: A dual approach for sustained and localized delivery with improved safety and therapeutic efficacy. J. Drug Deliv. Sci. Technol. 2020, 58, 101817. [Google Scholar] [CrossRef]
- Gao, B.; Luo, J.; Liu, Y.; Su, S.; Fu, S.; Yang, X.; Li, B. Intratumoral Administration of Thermosensitive Hydrogel Co-Loaded with Norcantharidin Nanoparticles and Doxorubicin for the Treatment of Hepatocellular Carcinoma. Int. J. Nanomed. 2021, 16, 4073–4085. [Google Scholar] [CrossRef] [PubMed]
- Segovia, N.; Pont, M.; Oliva, N.; Ramos, V.; Borrós, S.; Artzi, N. Hydrogel Doped with Nanoparticles for Local Sustained Release of siRNA in Breast Cancer. Adv. Healthc. Mater. 2015, 4, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Men, K.; Liu, W.; Li, L.; Duan, X.; Wang, P.; Gou, M.; Wei, X.; Gao, X.; Wang, B.; Du, Y.; et al. Delivering instilled hydrophobic drug to the bladder by a cationic nanoparticle and thermo-sensitive hydrogel composite system. Nanoscale 2012, 4, 6425–6433. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Xu, Y.-Y.; Sun, Y.; Han, B.-S.; Duan, Y.-R. Preparation of a Thermosensitive Gel Composed of a mPEG-PLGA-PLL-cRGD Nanodrug Delivery System for Pancreatic Tumor Therapy. ACS Appl. Mater. Interfaces 2015, 7, 20530–20537. [Google Scholar] [CrossRef]
- Kulsirirat, T.; Sathirakul, K.; Kamei, N.; Takeda-Morishita, M. The in vitro and in vivo study of novel formulation of andrographolide PLGA nanoparticle embedded into gelatin-based hydrogel to prolong delivery and extend residence time in joint. Int. J. Pharm. 2021, 602, 120618. [Google Scholar] [CrossRef]
- Saygili, E.; Kaya, E.; Ilhan-Ayisigi, E.; Saglam-Metiner, P.; Alarcin, E.; Kazan, A.; Girgic, E.; Kim, Y.-W.; Gunes, K.; Eren-Ozcan, G.G.; et al. An alginate-poly(acrylamide) hydrogel with TGF-β3 loaded nanoparticles for cartilage repair: Biodegradability, biocompatibility and protein adsorption. Int. J. Biol. Macromol. 2021, 172, 381–393. [Google Scholar] [CrossRef]
- Gong, Y.; Wang, Y.; Qu, Q.; Hou, Z.; Guo, T.; Xu, Y.; Qing, R.; Deng, J.; Wang, B.; Hao, S. Nanoparticle encapsulated core-shell hydrogel for on-site BMSCs delivery protects from iron overload and enhances functional recovery. J. Control. Release 2020, 320, 381–391. [Google Scholar] [CrossRef]
- Ai, A.; Behforouz, A.; Ehterami, A.; Sadeghvaziri, N.; Jalali, S.; Farzamfar, S.; Yousefbeigi, A.; Ai, A.; Goodarzi, A.; Salehi, M.; et al. Sciatic nerve regeneration with collagen type I hydrogel containing chitosan nanoparticle loaded by insulin. Int. J. Polym. Mater. Polym. Biomater. 2018, 68, 1133–1141. [Google Scholar] [CrossRef]
- Mahya, S.; Ai, J.; Shojae, S.; Khonakdar, H.A.; Darbemamieh, G.; Shirian, S. Berberine loaded chitosan nanoparticles encapsulated in polysaccharide-based hydrogel for the repair of spinal cord. Int. J. Biol. Macromol. 2021, 182, 82–90. [Google Scholar] [CrossRef]
- Gan, D.; Xing, W.; Jiang, L.; Fang, J.; Zhao, C.; Ren, F.; Fang, L.; Wang, K.; Lu, X. Plant-inspired adhesive and tough hydrogel based on Ag-Lignin nanoparticles-triggered dynamic redox catechol chemistry. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, M.J.; Loureiro, J.A.; Gomes, B.; Frasco, M.F.; Coelho, M.A.N.; Pereira, M.C. PLGA nanoparticles for calcitriol delivery. In Proceedings of the 2015 IEEE 4th Portuguese Meeting on Bioengineering, ENBENG, Porto, Portugal, 26–28 February 2015. [Google Scholar]
- Fonseca-Gomes, J.; Loureiro, J.A.; Tanqueiro, S.R.; Mouro, F.M.; Ruivo, P.; Carvalho, T.; Sebastião, A.M.; Diógenes, M.J.; Pereira, M.C. In vivo bio-distribution and toxicity evaluation of polymeric and lipid-based nanoparticles: A potential approach for chronic diseases treatment. Int. J. Nanomed. 2020, 15, 8609. [Google Scholar] [CrossRef]
- Spang, M.T.; Christman, K.L. Extracellular matrix hydrogel therapies: In vivo applications and development. Acta Biomater. 2018, 68, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gaudana, R.; Ananthula, H.K.; Parenky, A.; Mitra, A.K. Ocular drug delivery. AAPS J. 2010, 12, 348–360. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; Ko, Y.-C.; Chang, Y.-F.; Huang, S.-H.; Liu, C.J.-L. Thermosensitive chitosan-gelatin-based hydrogel containing curcumin-loaded nanoparticles and latanoprost as a dual-drug delivery system for glaucoma treatment. Exp. Eye Res. 2018, 179, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Tyagi, P.; Kadam, R.S.; Holden, C.A.; Kompella, U.B. Hybrid Dendrimer Hydrogel/PLGA Nanoparticle Platform Sustains Drug Delivery for One Week and Antiglaucoma Effects for Four Days Following One-Time Topical Administration. ACS Nano 2012, 6, 7595–7606. [Google Scholar] [CrossRef]
- Cheng, Y.-H.; Chang, Y.-F.; Ko, Y.-C.; Liu, C.J.-L. Development of a dual delivery of levofloxacin and prednisolone acetate via PLGA nanoparticles/thermosensitive chitosan-based hydrogel for postoperative management: An in-vitro and ex-vivo study. Int. J. Biol. Macromol. 2021, 180, 365–374. [Google Scholar] [CrossRef]
- Abrego, G.; Alvarado, H.; Souto, E.B.; Guevara, B.; Bellowa, L.H.; Parra, A.; Calpena, A.C.; Garcia, M.L. Biopharmaceutical profile of pranoprofen-loaded PLGA nanoparticles containing hydrogels for ocular administration. Eur. J. Pharm. Biopharm. 2015, 95, 261–270. [Google Scholar] [CrossRef] [Green Version]
- Alruwaili, N.K.; Zafar, A.; Imam, S.S.; Alharbi, K.S.; Alotaibi, N.H.; Alshehri, S.; Alhakamy, N.A.; Alzarea, A.I.; Afzal, M.; Elmowafy, M. Stimulus Responsive Ocular Gentamycin-Ferrying Chitosan Nanoparticles Hydrogel: Formulation Optimization, Ocular Safety and Antibacterial Assessment. Int. J. Nanomed. 2020, 15, 4717–4737. [Google Scholar] [CrossRef] [PubMed]
- Fabiano, A.; Piras, A.M.; Guazzelli, L.; Storti, B.; Bizzarri, R.; Zambito, Y. Impact of Different Mucoadhesive Polymeric Nanoparticles Loaded in Thermosensitive Hydrogels on Transcorneal Administration of 5-Fluorouracil. Pharmaceutics 2019, 11, 623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karpecki, P.; Paterno, M.R.; Comstock, T.L. Limitations of Current Antibiotics for the Treatment of Bacterial Conjunctivitis. Optom. Vis. Sci. 2010, 87, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.; Qindeel, M.; Ahmed, N.; Asad, M.I.; Shah, K.U.; Rehman, A.U. Development of an intelligent, stimuli-responsive transdermal system for efficient delivery of Ibuprofen against rheumatoid arthritis. Int. J. Pharm. 2021, 610, 121242. [Google Scholar] [CrossRef]
- Shafique, M.; Sohail, M.; Minhas, M.U.; Khaliq, T.; Kousar, M.; Khan, S.; Hussain, Z.; Mahmood, A.; Abbasi, M.; Aziz, H.C.; et al. Bio-functional hydrogel membranes loaded with chitosan nanoparticles for accelerated wound healing. Int. J. Biol. Macromol. 2020, 170, 207–221. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, X.; Duan, Y.; Huang, Y. Nanoparticle-Hydrogel Systems Containing Platensimycin for Local Treatment of Methicillin-Resistant Staphylococcus aureus Infection. Mol. Pharm. 2021, 18, 4099–4110. [Google Scholar] [CrossRef]
- Saleh, B.; Dhaliwal, H.K.; Portillo-Lara, R.; Shirzaei Sani, E.; Abdi, R.; Amiji, M.M.; Annabi, N. Local Immunomodulation Using an Adhesive Hydrogel Loaded with miRNA-Laden Nanoparticles Promotes Wound Healing. Small 2019, 15, 1902232. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment Strategies for Infected Wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [Green Version]
- Onaciu, A.; Munteanu, R.A.; Moldovan, C.S.; Berindan-Neagoe, I. Hydrogels Based Drug Delivery Synthesis, Characterization and Administration. Pharmaceutics 2019, 11, 432. [Google Scholar] [CrossRef] [Green Version]
- Jacob, S.; Nair, A.; Shah, J.; Sreeharsha, N.; Gupta, S.; Shinu, P. Emerging Role of Hydrogels in Drug Delivery Systems, Tissue Engineering and Wound Management. Pharmaceutics 2021, 13, 357. [Google Scholar] [CrossRef]
- Aly, U.F.; Aboutaleb, H.A.; Abdellatif, A.A.; Tolba, N.S. Formulation and evaluation of simvastatin polymeric nanoparticles loaded in hydrogel for optimum wound healing purpose. Drug Des. Dev. Ther. 2019, 13, 1567–1580. [Google Scholar] [CrossRef] [Green Version]
- Zeng, Q.; Qian, Y.; Huang, Y.; Ding, F.; Qi, X.; Shen, J. Polydopamine nanoparticle-dotted food gum hydrogel with excellent antibacterial activity and rapid shape adaptability for accelerated bacteria-infected wound healing. Bioact. Mater. 2021, 6, 2647–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, J.; Chen, M.; Gong, H.; Thamphiwatana, S.; Eckmann, L.; Gao, W.; Zhang, L. A Bioadhesive Nanoparticle–Hydrogel Hybrid System for Localized Antimicrobial Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 18367–18374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, K.-L.; Fan, Z.-L.; Yuan, J.-D.; Chen, P.-P.; Yang, J.-J.; Xu, J.; ZhuGe, D.-L.; Jin, B.-H.; Zhu, Q.-Y.; Shen, B.-X.; et al. Skin-penetrating polymeric nanoparticles incorporated in silk fibroin hydrogel for topical delivery of curcumin to improve its therapeutic effect on psoriasis mouse model. Colloids Surf. B Biointerfaces 2017, 160, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.; Trintchina, E.; Forero-Shelton, M.; Vogel, V.; Sokurenko, E.V. Bacterial Adhesion to Target Cells Enhanced by Shear Force. Cell 2002, 109, 913–923. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent advances in hydrogels as strategy for drug delivery intended to vaginal infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, E.S.; Ferreira, L.M.; Denardi, L.B.; Sari, M.H.M.; Cervi, V.F.; Nogueira, C.W.; Alves, S.H.; Cruz, L. Mucoadhesive gellan gum hydrogel containing diphenyl diselenide-loaded nanocapsules presents improved anti-candida action in a mouse model of vulvovaginal candidiasis. Eur. J. Pharm. Sci. 2021, 167, 106011. [Google Scholar] [CrossRef]
- Islam, S.; Bhuiyan, M.A.R.; Islam, M.N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J. Polym. Environ. 2016, 25, 854–866. [Google Scholar] [CrossRef]
- Nascimento, M.H.M.; Franco, M.K.K.D.; Yokaichyia, F.; de Paula, E.; Lombello, C.B.; de Araujo, D.R. Hyaluronic acid in Pluronic F-127/F-108 hydrogels for postoperative pain in arthroplasties: Influence on physico-chemical properties and structural requirements for sustained drug-release. Int. J. Biol. Macromol. 2018, 111, 1245–1254. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar]
- Cascone, S.; Lamberti, G. Hydrogel-based commercial products for biomedical applications: A review. Int. J. Pharm. 2019, 573, 118803. [Google Scholar] [CrossRef] [PubMed]



| Polymeric NPs | Hydrogel | Polymeric NLH | Refs. | |
|---|---|---|---|---|
| Multiple drug loading | + | + | Improved | [17,20,28] |
| Hydrophobic drugs loading | + | − | Maintained | [13,17,20,31] |
| Controlled and sustained release | + | +/− | Improved | [13,28] |
| Drug bioavailability improvement | + | + | Improved | [13] |
| Targeted drug delivery | + | − | Maintained | [20] |
| Local retention of drug | − | + | Maintained | [1,15,30,32] |
| Stimuli-responsive behavior | + | + | Improved | [12,25,29] |
| NPs Material | Hydrogel Material | Loaded Cargo | NPs Production Method | Hydrogel Crosslinking Nature | Biomedical Application | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
| PHBHHx | Chitosan | Insulin | Single-emulsion solvent-evaporation | Physical (β-GP) | Diabetes | NLH increased insulin bioavailability and prolonged hypoglycemic effect | [40] |
| PLGA | PCL–PEG–PCL | ICG and l-Arginine | Double-emulsion solvent-evaporation | Chemical | Various types of cancer | NLH increased ICG and l-Arg concentration and retention at the tumor site, inhibiting tumor growth and regression of the established tumors | [28] |
| Micelles of PEG-phenylboronic acid-polycarbonate | P(Bor)5-PEG-P(Bor)5 and P(Gu)5-PEG-P(Gu)5 | BTZ | Film hydration | Chemical | Cancer (myeloma) | BTZ-loaded NLH enhanced anti-cancer activity by decreasing the tumor size and inhibiting its progression | [41] |
| NPs Material | Hydrogel Material | Loaded Cargo | NPs Production Method | Hydrogel Crosslinking Nature | Biomedical Application | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
| Ethyl cellulose | Chitosan | Carboplatin | Double-emulsion solvent-evaporation | Physical (dibasic sodium phosphate) | Various types of cancer | NLH reduced systemic toxicity, increased drug concentration at the tumor site, and improved anti-tumor activity | [43] |
| PCL-PEG-PCL | Pluronic F-127 | Norcantharidin | Thin-film dispersion | Chemical | Cancer (hepatocellular carcinoma) | NLH provided high anti-tumor activity, with inhibition of the implanted tumors growth and prolonged the survival time of the tumor-bearing mice | [44] |
| PLA | PCL and Pluronic 10R5 | Oxaliplatin and Tannic acid | Double-emulsion solvent-evaporation | Chemical (Sn(Oct)2) | Cancer (colorectal peritoneal carcinoma) | NPs incorporation in the hydrogel allowed for a sustained release in vivo, improving tumor growth inhibition while reducing systemic toxicity | [30] |
| pBAE | PAMAM crosslinked with dextran aldehyde | siRNA | Self-assembly | Chemical | Breast cancer | The formulation exhibited a sustained and controlled release in vivo, but the therapeutic effect was not improved | [45] |
| PCL-PEG and DOTAP | Pluronic F127 | Deguelin | Film hydration | Chemical | Bladder cancer | NLH acts as a drug depot, allowing for sustained local drug delivery | [46] |
| PUR | Poloxamer 407 | BODIPY (mimic) | Nanoprecipitation | Physical (saline solution) | Glioblastoma | NPs incorporation in the hydrogel increased drug retention time in the tumor tissue, without systemic toxicity | [32] |
| PLGA | PEGDA and HA | Paclitaxel | Single-emulsion solvent-evaporation | Physical (UV light) | Lung cancer | Incorporating the drug-loaded NPs into the hydrogel improved in vivo tumor growth inhibition | [26] |
| PLGA-PEG | Pluronic F-127, Pluronic F-68, HPMC, MC and SA | Paclitaxel | Single-emulsion solvent-evaporation | Chemical | Pancreatic cancer | NPs incorporation in the hydrogel increased drug retention time in the tumor tissue, improving tumor growth inhibition. | [47] |
| PLGA | Gelatin | Andrographolide | Single-emulsion solvent-evaporation | n.d. | Osteoarthritis | NPs incorporation in the hydrogel increases the retention time in the joint, maintaining a sustained release for over 8 weeks | [48] |
| PLGA | Alginate and PAM | TGF-β3 | Nanoprecipitation | Chemical (PAAm crosslinker) | Tissue Regeneration (cartilage) | TGF-β3-loaded NLH induces the formation of new cartilage tissue | [49] |
| PLGA | Keratin | EGF and bFGF | Double-emulsion solvent-evaporation | Physical (hydrogen peroxide) | Intracerebral hemorrhage (iron overload) | The NLH improved stem cell differentiation and accelerated neurological recovery in vivo | [50] |
| Chitosan | Collagen | Insulin | Ionic gelation | Chemical (EDC) | Tissue regeneration (peripheral nerve) | Collagen hydrogel has tissue regeneration ability, but the incorporation of insulin-loaded NPs enhances the effect | [51] |
| Chitosan | Alginate and Chitosan | Berberine | Ionic gelation | Physical (β-GP) | Spinal cord injury | The hydrogel containing berberine-loaded NPs and stem cells exhibited a higher tissue regeneration ability | [52] |
| Ag-Lignin | Pectin and PAA | None | Self-assembly | Chemical | Wound healing | NPs incorporation in the hydrogel improves wound healing ability-enhancing the formation of mature tissue | [53] |
| NPs Material | Hydrogel Material | Loaded Cargo | NPs Production Method | Hydrogel Crosslinking Nature | Biomedical Application | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
| PLGA | PAMAM | Brimonidine and Timolol maleate | Single-emulsion solvent-evaporation | Chemical | Glaucoma | NLH provided a controlled release of drugs, reduction of IOP, and higher concentrations of drugs at the target site. | [59] |
| PLGA | Chitosan and Gelatin | Curcumin and Latanoprost | Single-emulsion solvent-evaporation | Physical (β-GP) | Glaucoma | The loaded-NLH reduced the oxidative stress effect that causes glaucoma, provided an anti-inflammatory effect. | [58] |
| PLGA | Chitosan and Gelatin | Levofloxacin and Prednisolone acetate | Single-emulsion solvent-evaporation | Physical (β-GP) | Anti-inflammatory treatment following surgery | Incorporating NPs into a hydrogel, a longstanding anti-inflammatory and anti-bacterial treatment were obtained, reducing side effects. | [60] |
| PLGA | Carbomer 934 | Pranoprofen | Solvent displacement | Chemical | Anti-inflammatory treatment following surgery | NLH provided therapy with improved anti-inflammatory effects and edema reduction. | [61] |
| Chitosan | Carbopol 974P | Gentamycin | Ionotropic gelation | Chemical | Ophthalmic bacterial infections | NLH increased drug contact time in the cornea, extended release, and excellent antimicrobial properties. | [62] |
| Chitosan | Chitosan or its derivatives (TSOH) | 5-fluorouracil | Self-assembly | Physical (β-GP) | Several ophthalmic diseases | NLH increased drug bioavailability and prolonged drug retention at the cornea. | [63] |
| NPs Material | Hydrogel Material | Loaded Cargo | NPs Production Method | Hydrogel Crosslinking Nature | Biomedical Applications | Main Conclusions | Ref. |
|---|---|---|---|---|---|---|---|
| PLGA | Pluronic F-127 | Platelet lysate | Double-emulsion solvent-evaporation | Chemical | Wound healing | NLH accelerates wound closure by promoting the cell migration and proliferation of fibroblasts | [23] |
| HA | Gelatin and methacryloyl (GelMA) | miR-223 5p mimic | n.d. | Chemical | Wound healing | Promotion of wound healing by initiating the resolution of the inflammatory phase and stimulating the formation of new vascularized skin tissue | [68] |
| PEG 4000 | Carbopol | Simvastatin | Nanoprecipitation | Chemical | Wound healing | Acceleration of the wound healing by forming a normal epithelial layer and mature collagen fibers, with minimal inflammatory cell infiltration | [72] |
| Polydopamine | Xanthan gum and Konjac glucomannan | - | Nanoprecipitation | Chemical | Wound healing | NLH significantly accelerates the healing of wounds by reducing the inflammatory response and promoting vascular reconstruction | [73] |
| Chitosan | HA, pullulan and PVA | Cefepime | Ionic gelation | Physical (sodium tripolyphosphate) | Wound healing | Accelerates the wound healing process by inhibiting Gram-positive and Gram-negative bacteria growth, with no cytotoxicity against a human cell line | [66] |
| PAMAM | PAM | Platensimycin | Double-emulsion solvent-evaporation | Physical (PEG di-methacrylate) | Wound healing and subcutaneous bacterial infections | Accelerates wound closure and treats subcutaneous infections by exhibiting antibacterial activity | [67] |
| PLGA | Acrylamide, PEG dimethacrylate and PVA | Ciprofloxacin | Double-emulsion solvent-evaporation | Physical (PEG di-methacrylate) | Bacterial infections | The bioadhesive NLH showed superior adhesion and antibiotic retention under high shear stress, with no skin toxicity | [74] |
| RRR-α-tocopheryl succinate-grafted-ε-polylysine | Silk fibroin | Curcumin | Self-assembly | Chemical | Psoriasis | NPs incorporation in the hydrogel improved the therapeutic effect of curcumin by inhibiting skin inflammation | [75] |
| Eudragit L 100 | Carbopol 934 and argan oil | Ibuprofen | Nanoprecipitation | Physical (glutaraldehyde) | Rheumatoid arthritis | Incorporating the drug-loaded NPs into the hydrogels improved the anti-inflammatory effect of ibuprofen compared to the commercially available ibuprofen cream | [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, D.; Andrade, S.; Ramalho, M.J.; Loureiro, J.A.; Pereira, M.C. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers 2022, 14, 1010. https://doi.org/10.3390/polym14051010
Nunes D, Andrade S, Ramalho MJ, Loureiro JA, Pereira MC. Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers. 2022; 14(5):1010. https://doi.org/10.3390/polym14051010
Chicago/Turabian StyleNunes, Débora, Stéphanie Andrade, Maria João Ramalho, Joana A. Loureiro, and Maria Carmo Pereira. 2022. "Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings" Polymers 14, no. 5: 1010. https://doi.org/10.3390/polym14051010
APA StyleNunes, D., Andrade, S., Ramalho, M. J., Loureiro, J. A., & Pereira, M. C. (2022). Polymeric Nanoparticles-Loaded Hydrogels for Biomedical Applications: A Systematic Review on In Vivo Findings. Polymers, 14(5), 1010. https://doi.org/10.3390/polym14051010











