Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Screening the Oils, Surfactants and Co-Surfactants on the Solubility of Genipin
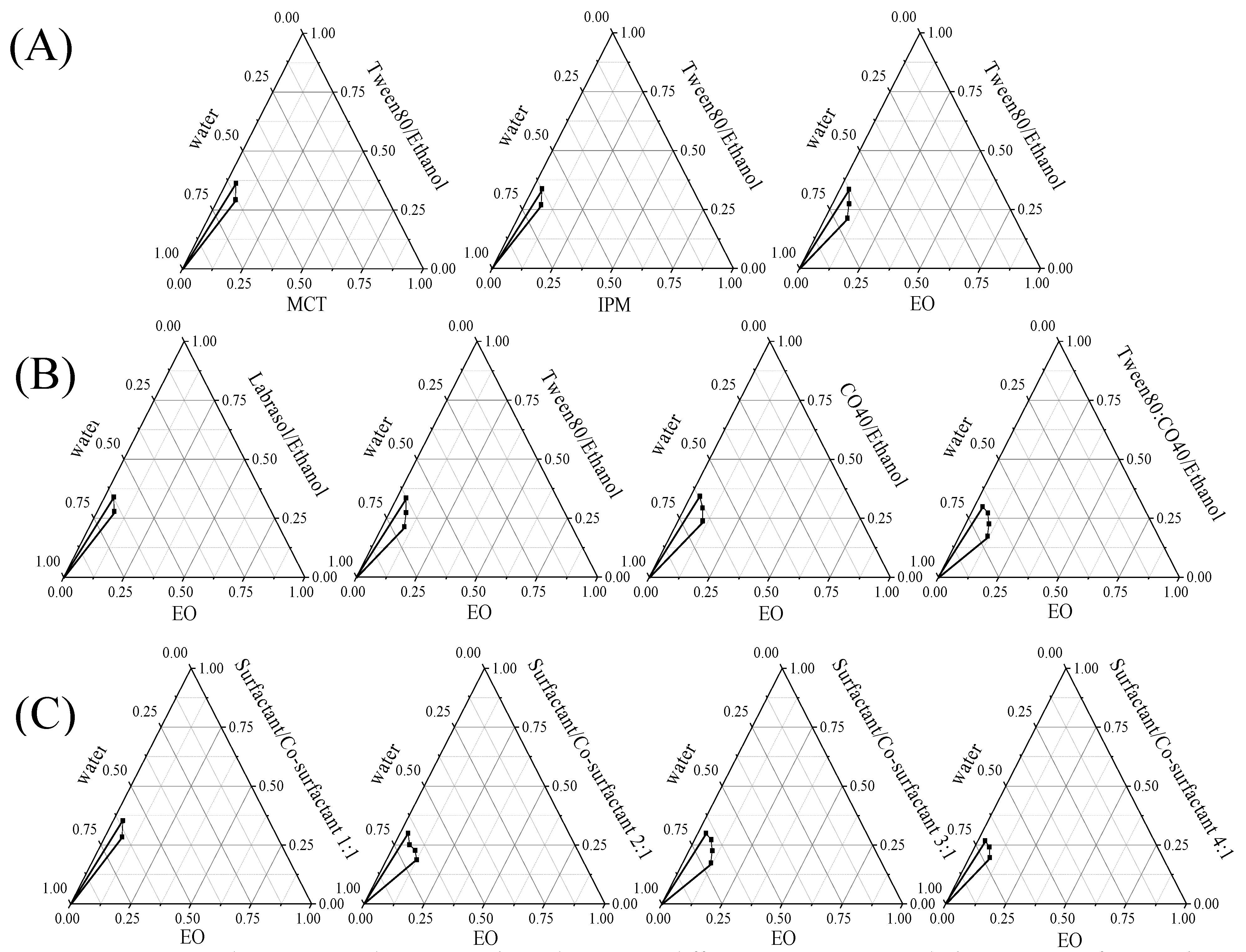
2.3. Construction of Pseudo-Ternary Phase Diagrams and Formulation of Microemulsions
2.4. Characterization of GME
2.4.1. Entrapment Efficiency (EE) and Drug Loading Efficiency (DL)
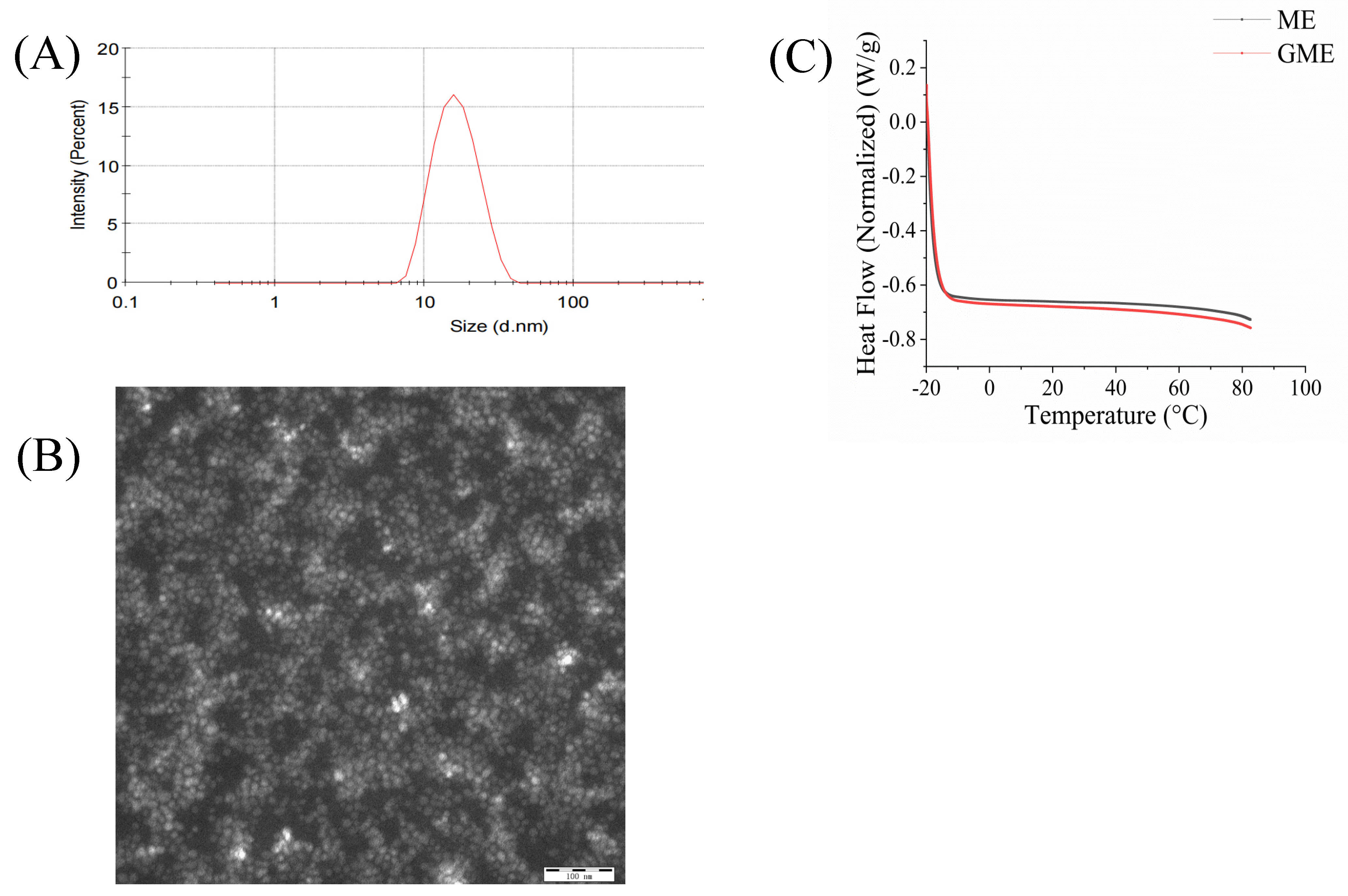
2.4.2. Particle Size, Polydispersity Index (PDI), Zeta Potential, and TEM Analysis
2.4.3. Differential Scanning Calorimeter (DSC) Analysis
2.5. Evaluation the Effect of Ionic Strength, Temperature and pH on GME Stability
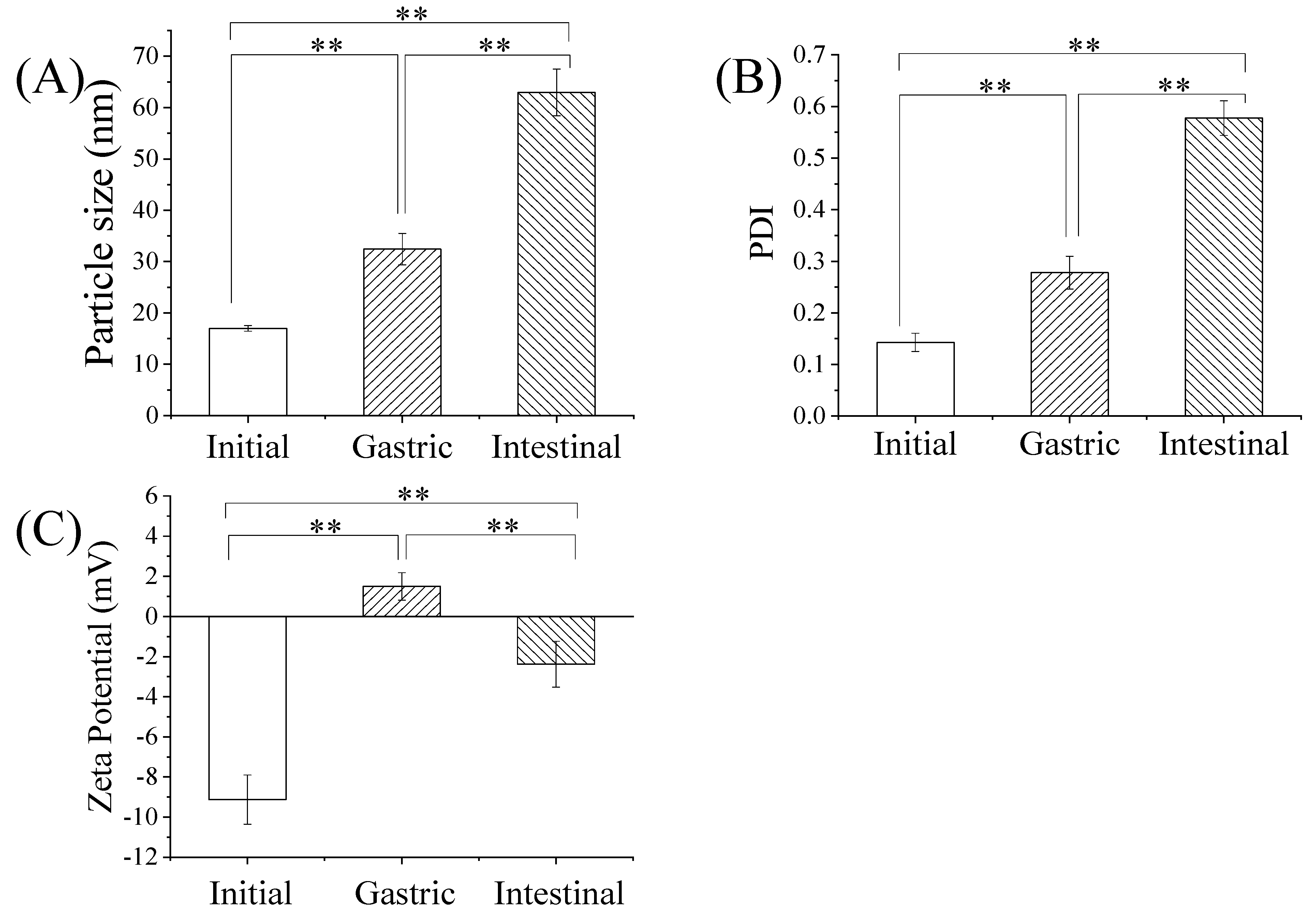
2.6. Characteristics of GME during Simulated Gastrointestinal Digestion
2.7. In Vitro Release Studies
- (1)
- Zero-order modelwhere K0 is the zero-order release rate constant;Q = a + K0 * t,
- (2)
- First-order modelwhere K1 is the first-order release rate constant;Q = a * (1 − exp (−K1 * t)),
- (3)
- Higuchi modelwhere KH represents the Higuchi release rate constant;Q = KH * t1/2 + a
- (4)
- Weibull modelwhere KW represents the Weibull release rate constant.Q = a * (1 − exp (−(KW * (t − tc)) d)),
2.8. Cell Culture and Cell Cytotoxic Studies
2.9. Cellular Uptake Studies
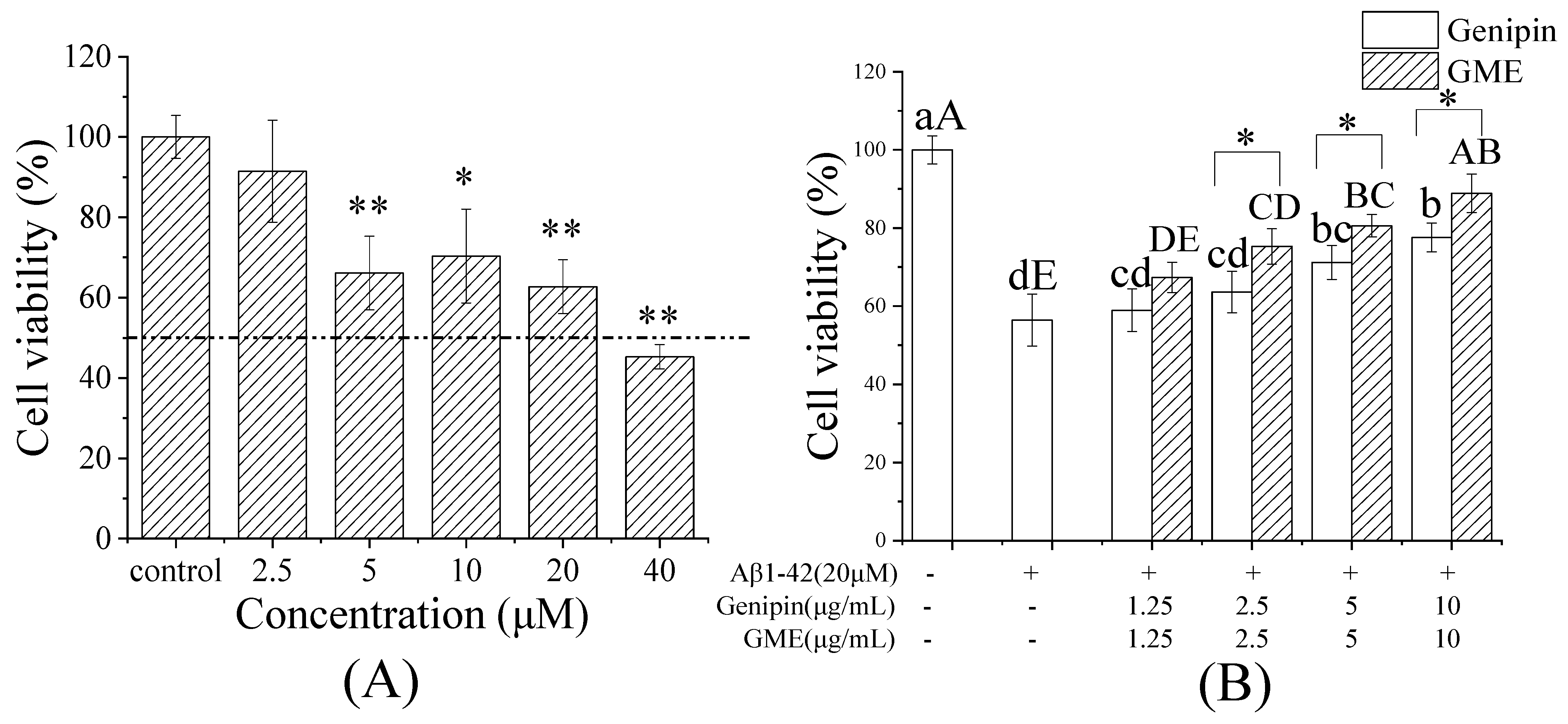
2.10. Protective Effect on Aβ1-42-Induced PC12 Cell Cytotoxicity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Solubility of Genipin in Oils, Surfactants, and Co-Surfactants
3.2. Construction of Pseudo-Ternary Phase Diagrams and Formulation of Microemulsions
3.3. Characterization of Genipin-Containing Microemulsions (GME)
3.4. Effect of Environmental Stresses on GME Stability
3.5. Stability of GME in Simulated Gastrointestinal Digestion
3.6. In Vitro Release Kinetics of GME
3.7. In Vitro Cellular Uptake of GME Study
3.8. Protective Effect of GME on Aβ-Induced Cytotoxicity of PC12 Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, W.; Li, S.; Wang, S.; Ho, C.T. Chemistry and bioactivity of Gardenia jasminoides. J. Food Drug Anal. 2017, 25, 43–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, M.; Yang, Z.; Tao, W.; Wang, P.; Tian, X.; Li, X.; Wang, W. Gardenia jasminoides Ellis: Ethnopharmacology, phytochemistry, and pharmacological and industrial applications of an important traditional Chinese medicine. J. Ethnopharmacol. 2020, 257, 112829. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Lin, L.; Cui, B.; Zhao, T.; Mao, L.; Song, Y.; Wang, X.; Feng, H.; Qingxiang, Y.; Zhang, J.; et al. Therapeutic potential of genipin in various acute liver injury, fulminant hepatitis, NAFLD and other non-cancer liver diseases: More friend than foe. Pharmacol. Res. 2020, 159, 104945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, Y.N.; Jia, T.; Huang, H.; Zhang, D.; Xu, X. Genipin and insulin combined treatment improves implant osseointegration in type 2 diabetic rats. J. Orthop. Surg. Res. 2021, 16, 59. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, L.; Liang, Z.; Li, Y.; Yuan, F.; Liu, J.; Tian, Y.; Hao, Z.; Zhou, F.; Liu, X.; et al. Genipin Inhibits LPS-Induced Inflammatory Response in BV2 Microglial Cells. Neurochem. Res. 2017, 42, 2769–2776. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Lee, S.M. Protective Effects of Geniposide and Genipin against Hepatic Ischemia/Reperfusion Injury in Mice. Biomol. Ther. 2013, 21, 132–137. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Li, J.; Zhang, Y.; Ma, X.; Zhu, P.; Zhang, Y. Design, synthesis, and evaluation of genipin derivatives for the treatment of Alzheimer’s Disease. Chem. Biol. Drug Des. 2019, 93, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Tian, J.S.; Cui, Y.L.; Gao, S. Genipin is active via modulating monoaminergic transmission and levels of brain-derived neurotrophic factor (BDNF) in rat model of depression. Neuroscience 2014, 275, 365–373. [Google Scholar] [CrossRef]
- Kang, M.J.; Khanal, T.; Kim, H.G.; Lee, D.H.; Yeo, H.K.; Lee, Y.S.; Ahn, Y.T.; Kim, D.H.; Jeong, H.G.; Jeong, T.C. Role of metabolism by human intestinal microflora in geniposide-induced toxicity in HepG2 cells. Arch. Pharmacal Res. 2012, 35, 733–738. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, C.J.; Ma, J.Y. Enhancement of active compound, genipin, from Gardeniae Fructus using immobilized glycosyl hydrolase family 3 β-glucosidase from Lactobacillus antri. AMB Express 2017, 7, 64. [Google Scholar] [CrossRef]
- Yamazaki, M.; Sakura, N.; Chiba, K.; Mohri, T. Prevention of the neurotoxicity of the amyloid beta protein by genipin. Biol. Pharm. Bull. 2001, 24, 1454–1455. [Google Scholar] [CrossRef]
- Akao, T.; Kobashi, K.; Aburada, M. Enzymic studies on the animal and intestinal bacterial metabolism of geniposide. Biol. Pharm. Bull. 1994, 17, 1573–1576. [Google Scholar] [CrossRef]
- Paik, Y.; Lee, C.; Cho, M.; Hahn, T. Physical stability of the blue pigments formed from geniposide of gardenia fruits: Effects of pH, temperature, and light. J. Agric. Food Chem. 2001, 49, 430–432. [Google Scholar] [CrossRef]
- Mishra, V.; Nayak, P.; Yadav, N.; Singh, M.; Tambuwala, M.M.; Aljabali, A.A.A. Orally administered self-emulsifying drug delivery system in disease management: Advancement and patents. Expert Opin. Drug Deliv. 2021, 18, 315–332. [Google Scholar] [CrossRef]
- Zu, Y.; Yu, X.; Zhao, X.; Wang, W.; Wang, K. Nanocrystallization of the Pharmaceutically Active Agent Genipin by an Emulsion Solvent Evaporation Method. J. Nanomater. 2014, 2014, 240950. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, F.C.; Cui, Y.L.; Song, Y.F. Enhancing effect of hydroxypropyl-beta-cyclodextrin on the intestinal absorption process of genipin. J. Agric. Food Chem. 2011, 59, 10919–10926. [Google Scholar] [CrossRef]
- Qi, X.-J.; Xu, D.; Tian, M.-L.; Zhou, J.-F.; Wang, Q.-S.; Cui, Y.-L. Thermosensitive hydrogel designed for improving the antidepressant activities of genipin via intranasal delivery. Mater. Des. 2021, 206, 109816. [Google Scholar] [CrossRef]
- Dhaval, M.; Vaghela, P.; Patel, K.; Sojitra, K.; Patel, M.; Patel, S.; Dudhat, K.; Shah, S.; Manek, R.; Parmar, R. Lipid-based emulsion drug delivery systems—A comprehensive review. Drug Deliv. Transl. Res. 2021. ahead of print. [Google Scholar] [CrossRef]
- Buya, A.B.; Witika, B.A. Application of Lipid-Based Nanocarriers for Antitubercular Drug Delivery: A Review. Pharmaceutics 2021, 13, 2041. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Tavakkoli Yaraki, M.; Bourbour, M.; Noorbazargan, H.; Lajevardi, A.; Sadat Shilsar, S.M.; Heidari, F.; Mousavian, S.M. Optimized doxycycline-loaded niosomal formulation for treatment of infection-associated prostate cancer: An in-vitro investigation. J. Drug Deliv. Sci. Technol. 2020, 57, 101715. [Google Scholar] [CrossRef]
- Akbarzadeh, I.; Tavakkoli Yaraki, M.; Ahmadi, S.; Chiani, M.; Nourouzian, D. Folic acid-functionalized niosomal nanoparticles for selective dual-drug delivery into breast cancer cells: An in-vitro investigation. Adv. Powder Technol. 2020, 31, 4064–4071. [Google Scholar] [CrossRef]
- Callender, S.P.; Mathews, J.A.; Kobernyk, K.; Wettig, S.D. Microemulsion utility in pharmaceuticals: Implications for multi-drug delivery. Int. J. Pharm. 2017, 526, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Koli, A.R.; Ranch, K.M.; Patel, H.P.; Parikh, R.K.; Shah, D.O.; Maulvi, F.A. Oral bioavailability improvement of felodipine using tailored microemulsion: Surface science, ex vivo and in vivo studies. Int. J. Pharm. 2021, 596, 120202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Cao, C.; Li, N.; Yuan, S. SYL3C aptamer-anchored microemulsion co-loading beta-elemene and PTX enhances the treatment of colorectal cancer. Drug Deliv. 2019, 26, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Wang, F.; Zhou, J.; Luo, J.; Li, J.; Yi, X. Oral delivery of coix seed oil in o/w microemulsion: Preparation, characterization, and in vitro and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2019, 54, 101325. [Google Scholar] [CrossRef]
- Wang, W.T.; Wang, M.; Zhang, J.; Liu, H.; Pan, H.C. Cloud point thermodynamics of paclitaxel-loaded microemulsion in the presence of glucose and NaCl. Colloids Surf. A Physicochem. Eng. Asp. 2016, 507, 76–82. [Google Scholar] [CrossRef]
- Pangeni, R.; Kang, S.-W.; Oak, M.; Park, E.Y.; Park, J.W. Oral delivery of quercetin in oil-in-water nanoemulsion: In vitro characterization and in vivo anti-obesity efficacy in mice. J. Funct. Foods 2017, 38, 571–581. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, X.; Zhang, J.; Sun, P.; Wang, H.; Zhang, Y.; Ma, Y.; Xu, S.; Lv, R.; Liu, X. Oral delivery of lycopene-loaded microemulsion for brain-targeting: Preparation, characterization, pharmacokinetic evaluation and tissue distribution. Drug Deliv. 2019, 26, 1191–1205. [Google Scholar] [CrossRef]
- Barnes, D.E.; Yaffe, K. The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 2011, 10, 819–828. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef]
- Fan, Y.; Li, X.; Ding, L.; Zhou, W.; Xu, G.; Wang, Y.; Zhang, Y.; Ni, Q. Accelerated Solvent Extraction of Antioxidant Compounds from Gardeniae Fructus and Its Acetylcholinesterase Inhibitory and PC12 Cell Protective Activities. Foods 2021, 10, 2805. [Google Scholar] [CrossRef]
- Tashtoush, B.M.; Bennamani, A.N.; Al-Taani, B.M. Preparation and characterization of microemulsion formulations of nicotinic acid and its prodrugs for transdermal delivery. Pharm. Dev. Technol. 2013, 18, 834–843. [Google Scholar] [CrossRef]
- Sun, R.; Xia, Q. In vitro digestion behavior of (W1/O/W2) double emulsions incorporated in alginate hydrogel beads: Microstructure, lipolysis, and release. Food Hydrocoll. 2020, 107, 105950. [Google Scholar] [CrossRef]
- Hart, S.M.; Lin, X.L.; Thilakarathna, S.H.; Wright, A.J. Emulsion droplet crystallinity attenuates early in vitro digestive lipolysis and beta-carotene bioaccessibility. Food Chem. 2018, 260, 145–151. [Google Scholar] [CrossRef]
- Chen, J.; Ma, X.H.; Yao, G.L.; Zhang, W.T.; Zhao, Y. Microemulsion-based anthocyanin systems: Effect of surfactants, cosurfactants, and its stability. Int. J. Food Prop. 2018, 21, 1152–1165. [Google Scholar] [CrossRef]
- Shi, J.; Xue, S.J.; Wang, B.; Wang, W.; Ye, X.; Quek, S.Y. Optimization of formulation and influence of environmental stresses on stability of lycopene-microemulsion. LWT-Food Sci. Technol. 2015, 60, 999–1008. [Google Scholar] [CrossRef]
- Mohammed, A.N.; Ishwarya, S.P.; Nisha, P. Nanoemulsion Versus Microemulsion Systems for the Encapsulation of Beetroot Extract: Comparison of Physicochemical Characteristics and Betalain Stability. Food Bioprocess Technol. 2021, 14, 133–150. [Google Scholar] [CrossRef]
- Ma, J.-J.; Huang, X.-N.; Yin, S.-W.; Yu, Y.-G.; Yang, X.-Q. Bioavailability of quercetin in zein-based colloidal particles-stabilized Pickering emulsions investigated by the in vitro digestion coupled with Caco-2 cell monolayer model. Food Chem. 2021, 360, 130152. [Google Scholar] [CrossRef]
- Subongkot, T.; Ngawhirunpat, T. Development of a novel microemulsion for oral absorption enhancement of all-trans retinoic acid. Int. J. Nanomed. 2017, 12, 5585–5599. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.; Xie, Y.; Liu, G.; Lu, Y.; Wu, W.; Chen, L. Oat protein-shellac nanoparticles as a delivery vehicle for resveratrol to improve bioavailability in vitro and in vivo. Nanomedicine 2019, 14, 2853–2871. [Google Scholar] [CrossRef]
- Zhao, X.; Wang, Z. A pH-sensitive microemulsion-filled gellan gum hydrogel encapsulated apigenin: Characterization and in vitro release kinetics. Colloids Surf. B Biointerfaces 2019, 178, 245–252. [Google Scholar] [CrossRef]
- Ersoz, M.; Erdemir, A.; Derman, S.; Arasoglu, T.; Mansuroglu, B. Quercetin-loaded nanoparticles enhance cytotoxicity and antioxidant activity on C6 glioma cells. Pharm. Dev. Technol. 2020, 25, 757–766. [Google Scholar] [CrossRef]
- Mundada, V.P.; Patel, M.H.; Mundada, P.K.; Sawant, K.K. Enhanced bioavailability and antihypertensive activity of nisoldipine loaded nanoemulsion: Optimization, cytotoxicity and uptake across Caco-2 cell line, pharmacokinetic and pharmacodynamic studies. Drug Dev. Ind. Pharm. 2020, 46, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chi, J.; Ye, X.; Wang, S.; Liang, J.; Yue, P.; Xiao, H.; Gao, X. Nanoliposomes as delivery system for anthocyanins: Physicochemical characterization, cellular uptake, and antioxidant properties. LWT 2021, 139, 110554. [Google Scholar] [CrossRef]
- Kostiv, U.; Kotelnikov, I.; Proks, V.; Slouf, M.; Kucka, J.; Engstova, H.; Jezek, P.; Horak, D. RGDS- and TAT-Conjugated Upconversion of NaYF4:Yb(3+)/Er(3+)&SiO2 Nanoparticles: In Vitro Human Epithelioid Cervix Carcinoma Cellular Uptake, Imaging, and Targeting. ACS Appl. Mater. Interfaces 2016, 8, 20422–20431. [Google Scholar] [CrossRef] [PubMed]
- Kogan, A.; Garti, N. Microemulsions as transdermal drug delivery vehicles. Adv. Colloid Interface Sci. 2006, 123–126, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.T.; Huang, Z.B.; Zhang, S.J.; Zhao, J.H.; Wang, Z.; Liu, Y.; Feng, N.P. In vitro cellular uptake of evodiamine and rutaecarpine using a microemulsion. Int. J. Nanomed. 2012, 7, 2465–2472. [Google Scholar] [CrossRef][Green Version]
- Bookstaff, R.C.; Stuard, S.B.; Ward, S.R.; Pesik, P.K.; Henwood, S.M. The safety of ethyl oleate is supported by a 91-day feeding study in rats. Regul. Toxicol. Pharmacol. 2004, 39, 202–213. [Google Scholar] [CrossRef]
- Jin, J.; Wang, H.; Jing, Y.; Liu, M.; Wang, D.; Li, Y.; Bao, M. An efficient and environmental-friendly dispersant based on the synergy of amphiphilic surfactants for oil spill remediation. Chemosphere 2019, 215, 241–247. [Google Scholar] [CrossRef]
- Shaaban, H.A.; Sadek, Z.; Edris, A.E.; Saad-Hussein, A. Analysis and antibacterial activity of Nigella sativa essential oil formulated in microemulsion system. J. Oleo Sci. 2015, 64, 223–232. [Google Scholar] [CrossRef]
- Wan, J.; Long, Y.; Liu, S.; Zhang, Y.; Xiang, Y.; Li, D.; Shi, A.; Shuang, Y.; Li, Y.; He, Y.; et al. Geniposide-Loaded Liposomes for Brain Targeting: Development, Evaluation, and In Vivo Studies. AAPS PharmSciTech 2021, 22, 222. [Google Scholar] [CrossRef]
- Ghaferi, M.; Asadollahzadeh, M.J.; Akbarzadeh, A.; Ebrahimi Shahmabadi, H.; Alavi, S.E. Enhanced Efficacy of PEGylated Liposomal Cisplatin: In Vitro and In Vivo Evaluation. Int. J. Mol. Sci. 2020, 21, 559. [Google Scholar] [CrossRef]
- Alavi, S.E.; Muflih Al Harthi, S.; Ebrahimi Shahmabadi, H.; Akbarzadeh, A. Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved Properties as an Anticancer Agent. Int. J. Mol. Sci. 2019, 20, 1531. [Google Scholar] [CrossRef]
- Alany, R.G.; Rades, T.; Agatonovic-Kustrin, S.; Davies, N.M.; Tucker, I.G. Effects of alcohols and diols on the phase behaviour of quaternary systems. Int. J. Pharm. 2000, 196, 141–145. [Google Scholar] [CrossRef]
- Golwala, P.; Rathod, S.; Patil, R.; Joshi, A.; Ray, D.; Aswal, V.K.; Bahadur, P.; Tiwari, S. Effect of cosurfactant addition on phase behavior and microstructure of a water dilutable microemulsion. Colloids Surf. B Biointerfaces 2020, 186, 110736. [Google Scholar] [CrossRef]
- Ife, A.F.; Harding, I.H.; Shah, R.M.; Palombo, E.A.; Eldridge, D.S. Effect of pH and electrolytes on the colloidal stability of stearic acid–based lipid nanoparticles. J. Nanoparticle Res. 2018, 20, 318. [Google Scholar] [CrossRef]
- Fanun, M. Phase behavior, transport, diffusion and structural parameters of nonionic surfactants microemulsions. J. Mol. Liq. 2008, 139, 14–22. [Google Scholar] [CrossRef]
- Tesch, S.; Schubert, H. Influence of increasing viscosity of the aqueous phase on the short-term stability of protein stabilized emulsions. J. Food Eng. 2002, 52, 305–312. [Google Scholar] [CrossRef]
- Abbasi, S.; Radi, M. Food grade microemulsion systems: Canola oil/lecithin:n-propanol/water. Food Chem. 2016, 194, 972–979. [Google Scholar] [CrossRef]
- Ting, P.; Srinuanchai, W.; Suttisansanee, U.; Tuntipopipat, S.; Charoenkiatkul, S.; Praengam, K.; Chantong, B.; Temviriyanukul, P.; Nuchuchua, O. Development of Chrysin Loaded Oil-in-Water Nanoemulsion for Improving Bioaccessibility. Foods 2021, 10, 1912. [Google Scholar] [CrossRef]
- Verkempinck, S.H.E.; Salvia-Trujillo, L.; Moens, L.G.; Charleer, L.; Van Loey, A.M.; Hendrickx, M.E.; Grauwet, T. Emulsion stability during gastrointestinal conditions effects lipid digestion kinetics. Food Chem. 2018, 246, 179–191. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, S.; Ma, H.; Dong, P.; Ma, X.; Xu, M.; Tian, Y.; Tang, Z.; Peng, J.; Chen, H.; et al. In situ monitoring of the structural change of microemulsions in simulated gastrointestinal conditions by SAXS and FRET. Acta Pharm. Sin. B 2018, 8, 655–665. [Google Scholar] [CrossRef]
- Kuang, J.; Gao, J.; Xie, S.; Lei, Q.; Fang, W.; Xie, H.; Lu, X. Phase behaviors and curcumin encapsulation performance of Gemini surfactant microemulsion. J. Mol. Liq. 2020, 315, 113786. [Google Scholar] [CrossRef]
- Yao, Y.; Xia, M.; Wang, H.; Li, G.; Shen, H.; Ji, G.; Meng, Q.; Xie, Y. Preparation and evaluation of chitosan-based nanogels/gels for oral delivery of myricetin. Eur. J. Pharm. Sci. 2016, 91, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. In vitro release kinetics model fitting of liposomes: An insight. Chem. Phys. Lipids 2016, 201, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Modi, S.; Anderson, B.D. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Mol. Pharm. 2013, 10, 3076–3089. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.; Dupin, A.; Sassi, A.H.; Monteil, J.; Sotiroudis, G.T.; Leal-Calderon, F.; Xenakis, A. Hydroxytyrosol encapsulated in biocompatible water-in-oil microemulsions: How the structure affects in vitro absorption. Colloids Surf. B Biointerfaces 2019, 184, 110482. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Guan, Y.; Baek, S.J.; Zhong, Q. Caffeic Acid Phenethyl Ester Loaded in Microemulsions: Enhanced In Vitro Activity against Colon and Breast Cancer Cells and Possible Cellular Mechanisms. Food Biophys. 2018, 14, 80–89. [Google Scholar] [CrossRef]
- Sorasitthiyanukarn, F.N.; Muangnoi, C.; Rojsitthisak, P.; Rojsitthisak, P. Chitosan-alginate nanoparticles as effective oral carriers to improve the stability, bioavailability, and cytotoxicity of curcumin diethyl disuccinate. Carbohydr. Polym. 2021, 256, 117426. [Google Scholar] [CrossRef]
- Hu, X.; Yang, F.; Liao, Y.; Li, L.; Zhang, L. Cholesterol-PEG comodified poly (N-butyl) cyanoacrylate nanoparticles for brain delivery: In vitro and in vivo evaluations. Drug Deliv. 2017, 24, 121–132. [Google Scholar] [CrossRef]
- Kettler, K.; Veltman, K.; van de Meent, D.; van Wezel, A.; Hendriks, A.J. Cellular uptake of nanoparticles as determined by particle properties, experimental conditions, and cell type. Environ. Toxicol. Chem. 2014, 33, 481–492. [Google Scholar] [CrossRef]
- Li, Y.; Li, L.; Hölscher, C. Therapeutic Potential of Genipin in Central Neurodegenerative Diseases. CNS Drugs 2016, 30, 889–897. [Google Scholar] [CrossRef]
- Md, S.; Gan, S.Y.; Haw, Y.H.; Ho, C.L.; Wong, S.; Choudhury, H. In vitro neuroprotective effects of naringenin nanoemulsion against beta-amyloid toxicity through the regulation of amyloidogenesis and tau phosphorylation. Int. J. Biol. Macromol. 2018, 118, 1211–1219. [Google Scholar] [CrossRef]





| Phases | Solubility (mg/mL) | |
|---|---|---|
| Oils | MCT | 0.603 ± 0.007 |
| EO | 0.570 ± 0.010 | |
| IPM | 0.446 ± 0.008 | |
| Soybean | 0.249 ± 0.017 | |
| Olive | 0.291 ± 0.006 | |
| Surfactants | Tween 20 | 2.494 ± 0.116 |
| Tween 80 | 3.327 ± 0.208 | |
| Span 80 | 0.808 ± 0.078 | |
| Labrasol | 3.206 ± 0.064 | |
| CO-40 | 2.863 ± 0.062 | |
| EL-35 | 2.755 ± 0.087 | |
| Tween80: CO-40 (1:1) | 3.353 ± 0.158 | |
| Co-surfactants | Glycerol | 22.29 ± 0.579 |
| Ethanol | 36.59 ± 0.922 | |
| PEG400 | 29.35 ± 0.840 | |
| Ethylene | 27.28 ± 1.175 |
| Average Particle Size/nm | PDI | Zeta Potential/mV | ||
|---|---|---|---|---|
| Temperature/(°C) | 20 | 16.69 ± 0.27 a | 0.183 ± 0.013 a | −8.11 ± 0.77 a |
| 30 | 16.31 ± 0.35 a | 0.172 ± 0.011 a | −7.86 ± 1.59 a | |
| 40 | 16.52 ± 0.54 a | 0.161 ± 0.022 a | −9.55 ± 0.83 a | |
| 50 | 16.11 ± 0.65 a | 0.175 ± 0.005 a | −6.72 ± 1.39 a | |
| 60 | 16.15 ± 0.38 a | 0.157 ± 0.017 a | −7.94 ± 0.90 a | |
| 70 | 16.43 ± 0.38 a | 0.162 ± 0.016 a | −9.41 ± 0.62 a | |
| pH | 2 | 17.62 ± 0.07 cd | 0.135 ± 0.029 b | −1.50 ± 1.33 a |
| 4 | 17.64 ± 0.40 cd | 0.153 ± 0.034 ab | −1.66 ± 0.22 a | |
| 5.6 | 16.53 ± 0.60 d | 0.057 ± 0.005 c | −7.25 ± 0.67 b | |
| 6 | 16.77 ± 0.27 d | 0.139 ± 0.015 b | −7.14 ± 0.38 b | |
| 8 | 18.54 ± 0.62 c | 0.192 ± 0.003 a | −10.63 ± 0.35 c | |
| 10 | 22.21 ± 0.53 b | 0.203 ± 0.007 a | −11.43 ± 0.68 c | |
| 12 | 28.43 ± 0.41 a | 0.169 ± 0.093 ab | −12.40 ± 0.72 c | |
| NaCl concentration /(mM) | 0 | 17.76 ± 0.27 d | 0.204 ± 0.013 b | −7.25 ± 0.67 b |
| 100 | 21.01 ± 0.99 d | 0.226 ± 0.023 b | −2.59 ± 0.62 a | |
| 200 | 25.02 ± 0.26 c | 0.220 ± 0.019 b | −3.06 ± 0.13 a | |
| 300 | 27.27 ± 1.05 c | 0.230 ± 0.026 b | −2.36 ± 0.33 a | |
| 400 | 33.19 ± 2.02 b | 0.244 ± 0.013 b | −2.01 ± 1.76 a | |
| 500 | 41.36 ± 1.61 a | 0.325 ± 0.019 a | −1.66 ± 1.78 a |
| Sample | Release Kinetic Models | |||
|---|---|---|---|---|
| Zero-Order | First-Order | Higuchi | Weibull | |
| Genipin (pH = 1.2 hydrochloric acid) | Q = 73.95 + 1.80 × t | Q = 87.86 × (1 − exp (−2.33 × t)) | Q = 10.58 × (t^(1/2)) + 61.62 | Q = 88.76 × (1 − exp( − (3.55 × (t − 0.13))^0.64)) |
| R2 = 0.1672 | R2 = 0.98524 | R2 = 0.39335 | R2 = 0.9889 | |
| GME (pH = 1.2 hydrochloric acid) | Q = 16.70 + 4.19 × t | Q = 80.67 × (1 – exp (−0.41 × t)) | Q = 22.67 × (t^(1/2)) − 1.37 | Q = 78.75 × (1 − exp( − (0.71 × (t − 0.17))^0.98)) |
| R2 = 0.59977 | R2 = 0.99077 | R2 = 0.84307 | R2 = 0.99972 | |
| Genipin (water) | Q = 80.78 + 0.70 × t | Q = 93.13 × (1 – exp (−2.93 × t)) | Q = 5.61 × (t^(1/2)) + 74.35 | Q = 93.43 × (1 − exp( − (0.40 × (t + 2.13))^4.48)) |
| R2 = 0.00827 | R2 = 0.96665 | R2 = 0.17475 | R2 = 0.97942 | |
| GME (water) | Q = 69.91 + 8.85 × t | Q = 92.91 × (1 − exp (−0.70 × t)) | Q = 13.38 × (t^(1/2)) + 50.35 | Q = 92.06 × (1 − exp( − (0.57 × (t + 0.25))^1.26)) |
| R2 = 0.09699 | R2 = 0.9938 | R2 = 0.36512 | R2 = 0.99458 | |
| Genipin (pH = 7.4 PBS) | Q = 94.74 + 0.16 × t | Q = 98.51 × (1 − exp (−3.04 × t)) | Q = 1.34 × (t^(1/2)) + 91.95 | Q = 89.74 × (1 − exp( − (0.05 × (t + 40.19))^3.05)) |
| R2 = −0.02465 | R2 = 0.92898 | R2 = 0.05309 | R2 = −25.8365 | |
| GME (pH = 7.4 PBS) | Q = 55.46 + 2.93 × t | Q = 90.30 × (1 − exp (−0.75 × t)) | Q = 19.49 × (t^(1/2)) + 29.19 | Q = 90.09 × (1 − exp( − (0.72 × (t−0.05))^1.08)) |
| R2 = 0.28428 | R2 = 0.99861 | R2 = 0.61602 | R2 = 0.99834 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Xu, G.; Ni, Q.; Wang, Y.; Gao, Q.; Zhang, Y. Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity. Pharmaceutics 2022, 14, 617. https://doi.org/10.3390/pharmaceutics14030617
Zheng Y, Xu G, Ni Q, Wang Y, Gao Q, Zhang Y. Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity. Pharmaceutics. 2022; 14(3):617. https://doi.org/10.3390/pharmaceutics14030617
Chicago/Turabian StyleZheng, Yujie, Guangzhi Xu, Qinxue Ni, Yan Wang, Qianxin Gao, and Youzuo Zhang. 2022. "Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity" Pharmaceutics 14, no. 3: 617. https://doi.org/10.3390/pharmaceutics14030617
APA StyleZheng, Y., Xu, G., Ni, Q., Wang, Y., Gao, Q., & Zhang, Y. (2022). Microemulsion Delivery System Improves Cellular Uptake of Genipin and Its Protective Effect against Aβ1-42-Induced PC12 Cell Cytotoxicity. Pharmaceutics, 14(3), 617. https://doi.org/10.3390/pharmaceutics14030617






