Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Grapes Encapsulation and Grape Pomace Drying
2.2.1. Encapsulation of Grapes
2.2.2. Grape Pomace Drying
2.3. Preparation of Infusions
2.3.1. Preparation of Encapsulated/Coated Grape Infusions
2.3.2. Grape Pomace Infusions
2.4. Sugar (°Brix) Content, pH, and Colorimetric Parameters
2.5. Total Phenolic Content Quantification
2.6. Antioxidant Activity (AA)
2.7. Infusions’ Sensory Profiles
2.7.1. Sensory Evaluation of Moscatel Galego Grape Pomace Infusions
2.7.2. CATA Test of Infusions Prepared with Coated Grapes
2.8. Data Analysis
3. Results
3.1. Sugar (°Brix) Content and pH
3.2. Infusions’ Colorimetric Parameters
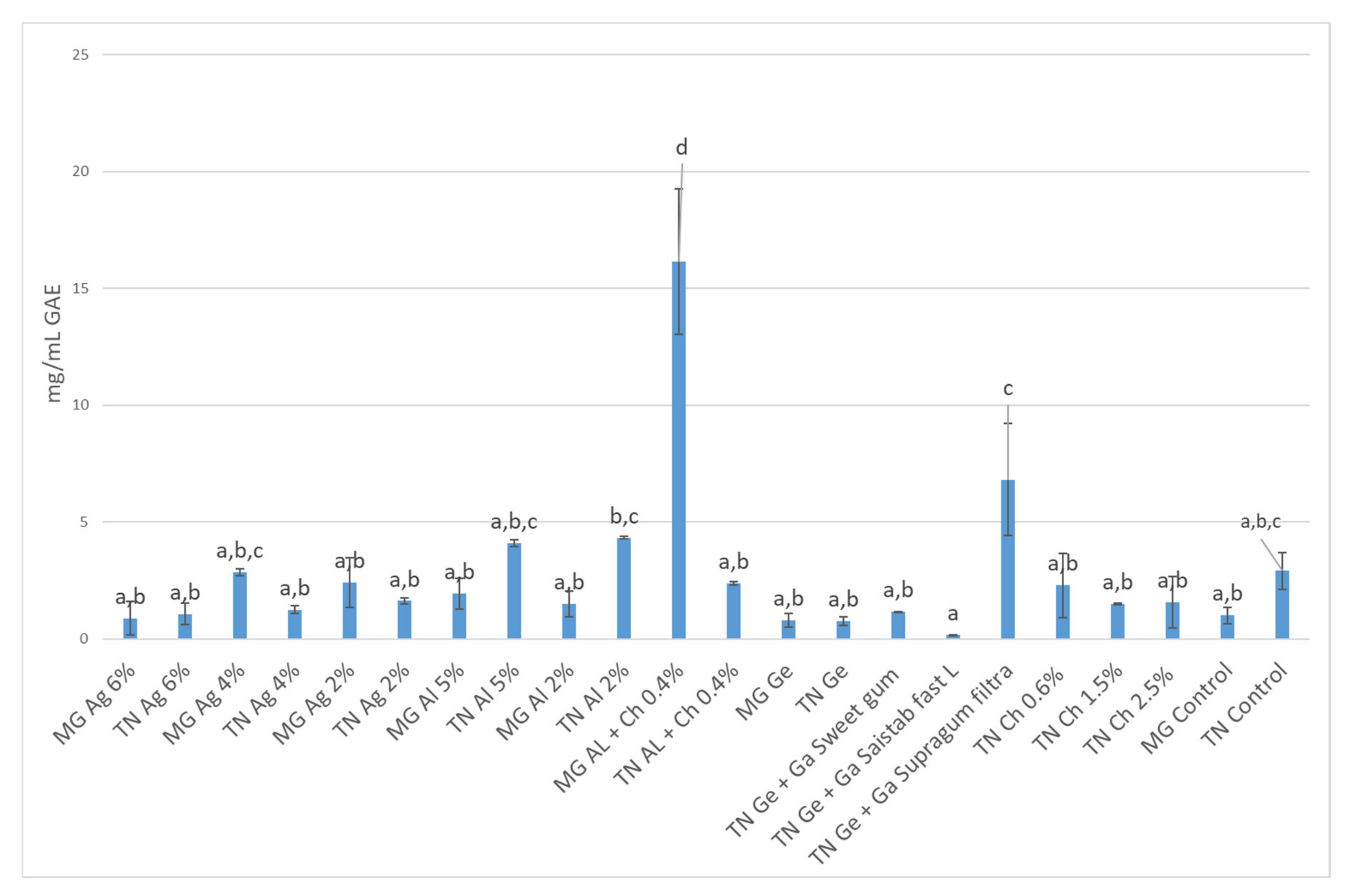
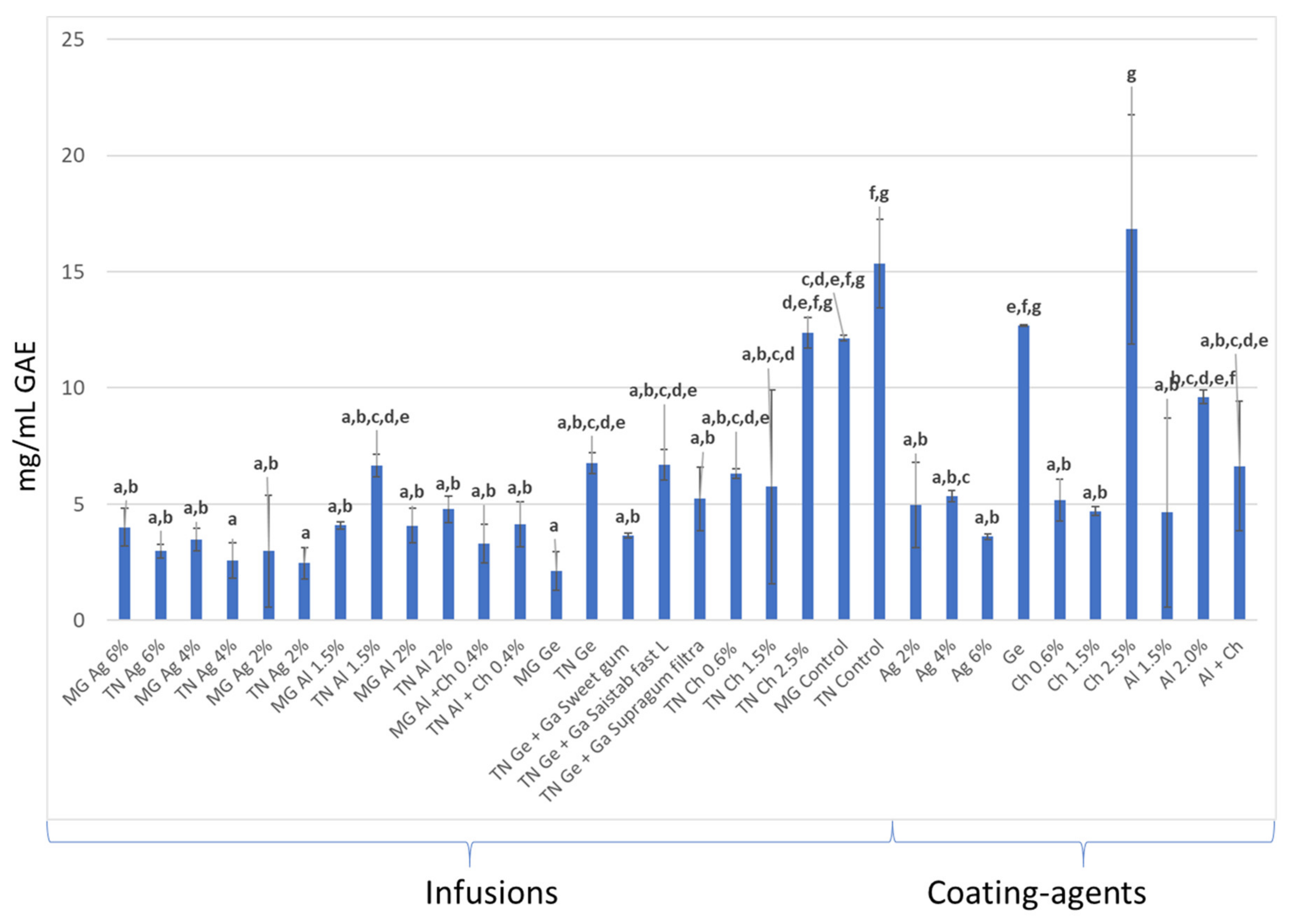
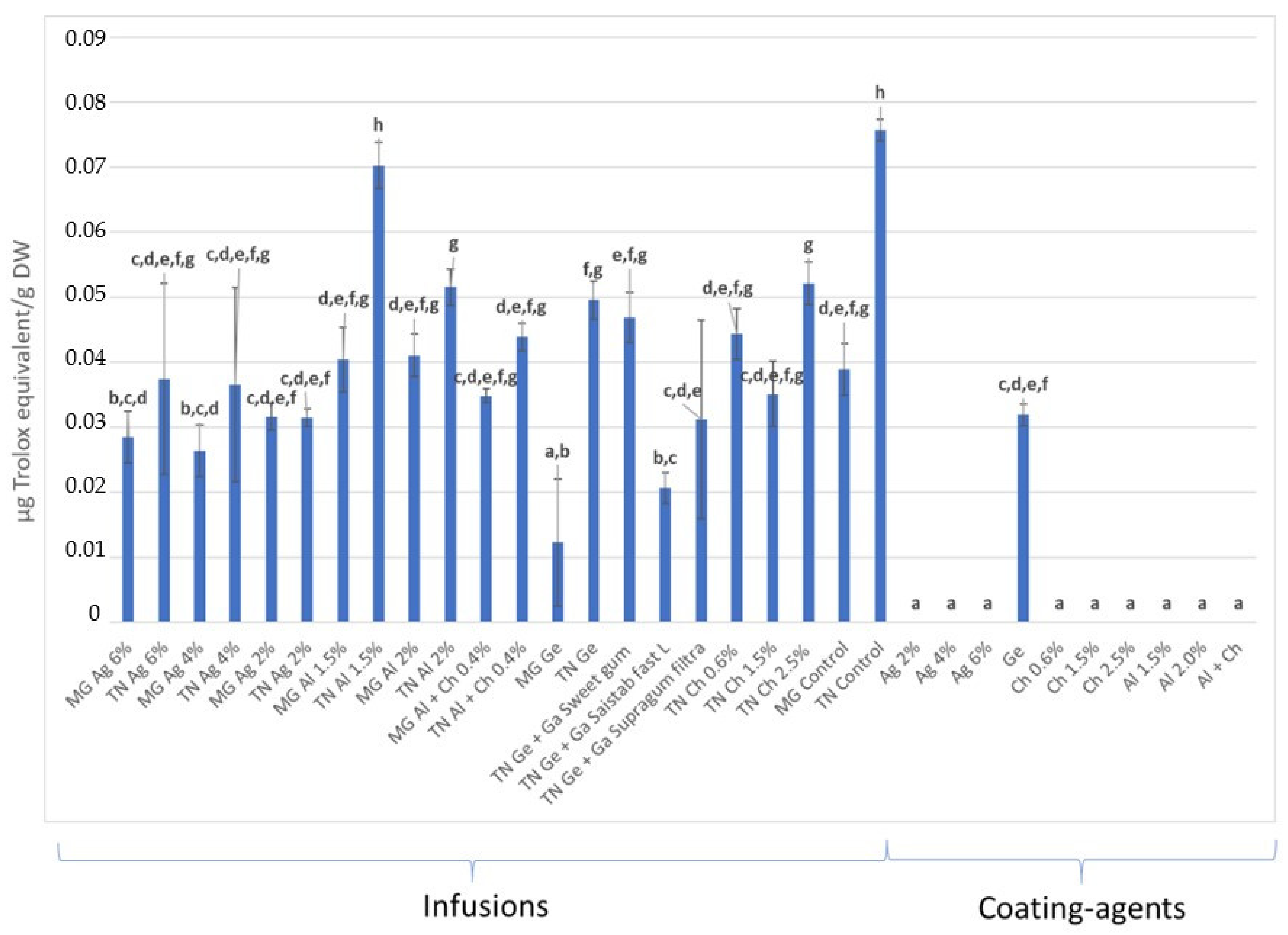
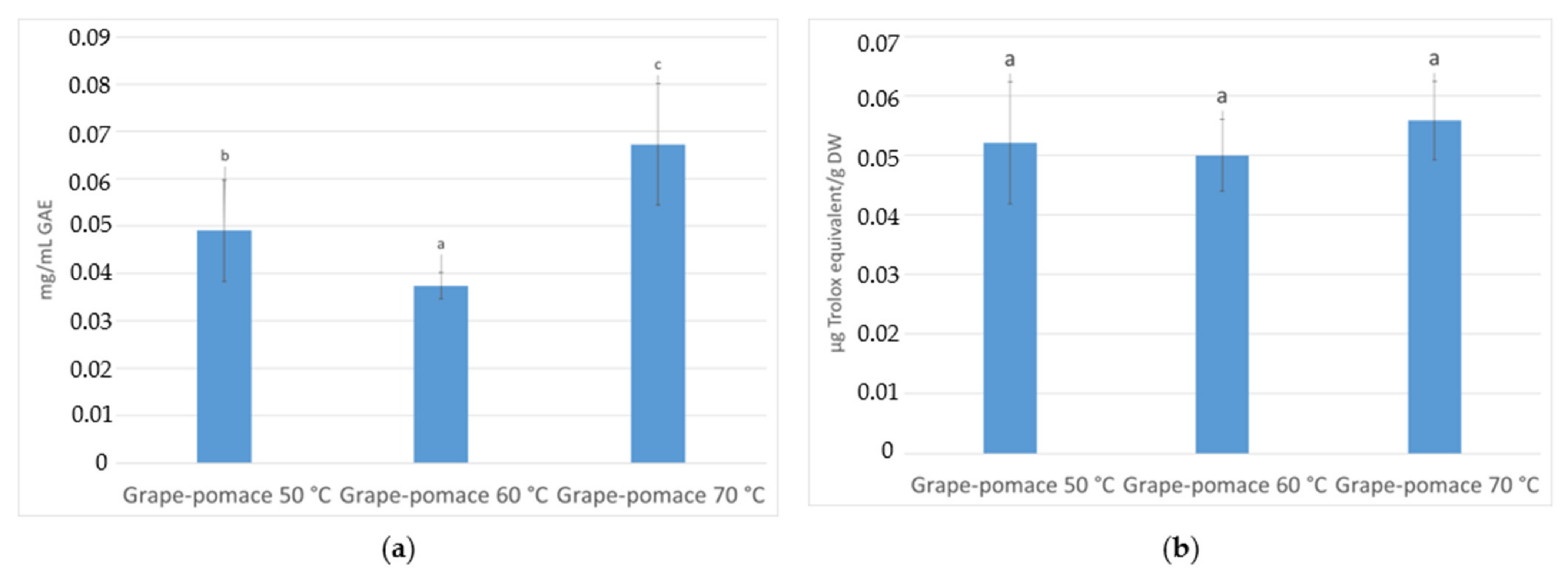
3.3. Total Phenolic Content and Antioxidant Activity (AA) Quantification
3.3.1. In Infusions Prepared with Dried–Minced Coated Grapes
3.3.2. In Infusions Prepared with Dried Grape Pomace at Different Temperatures
3.4. Infusions Sensory Evaluation
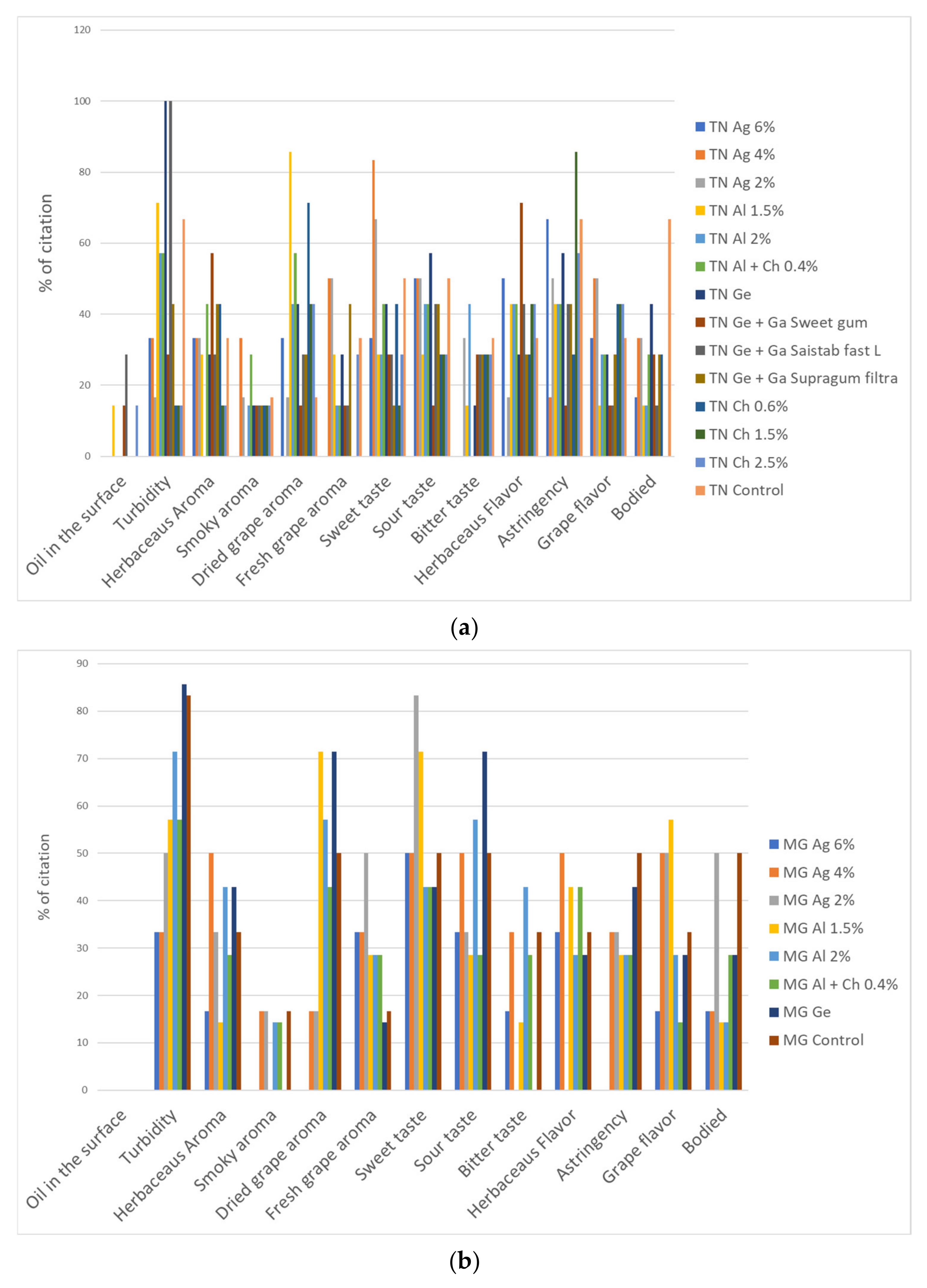
3.4.1. CATA Test of the Dried–Minced Coated Grape Infusions
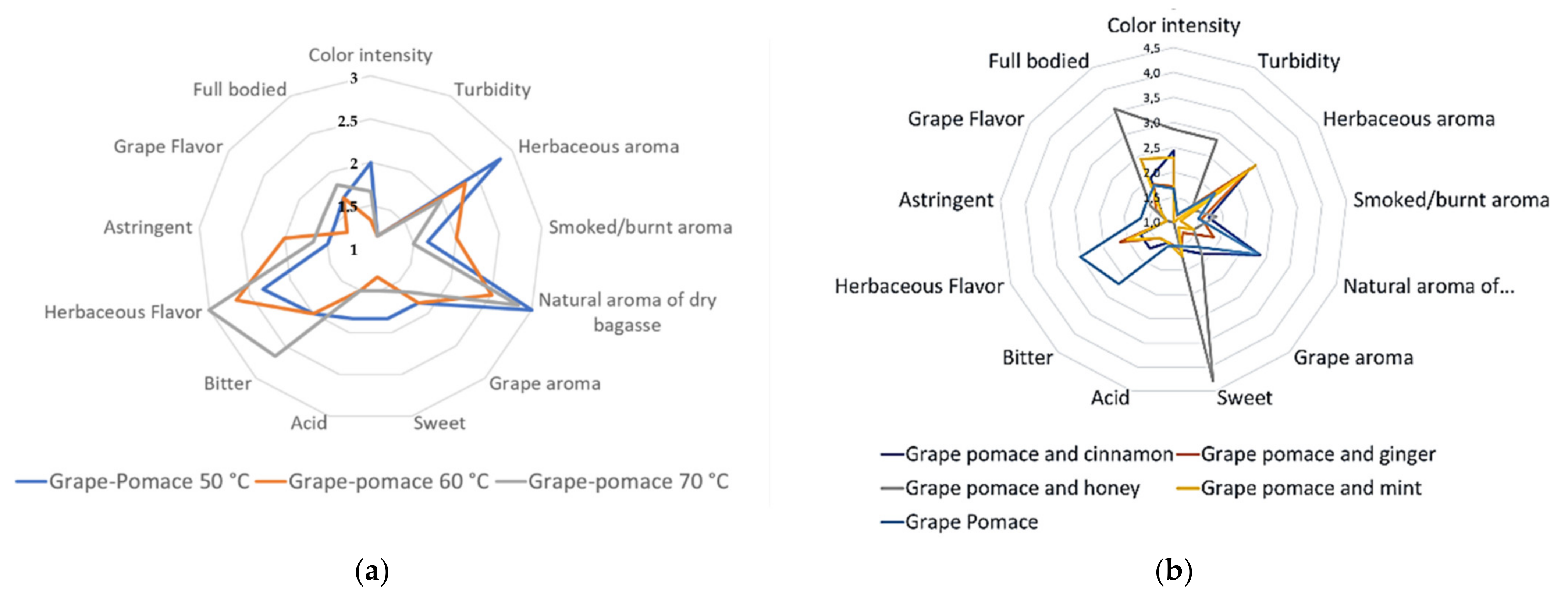
3.4.2. QDA Test of the Grape Pomace Infusions
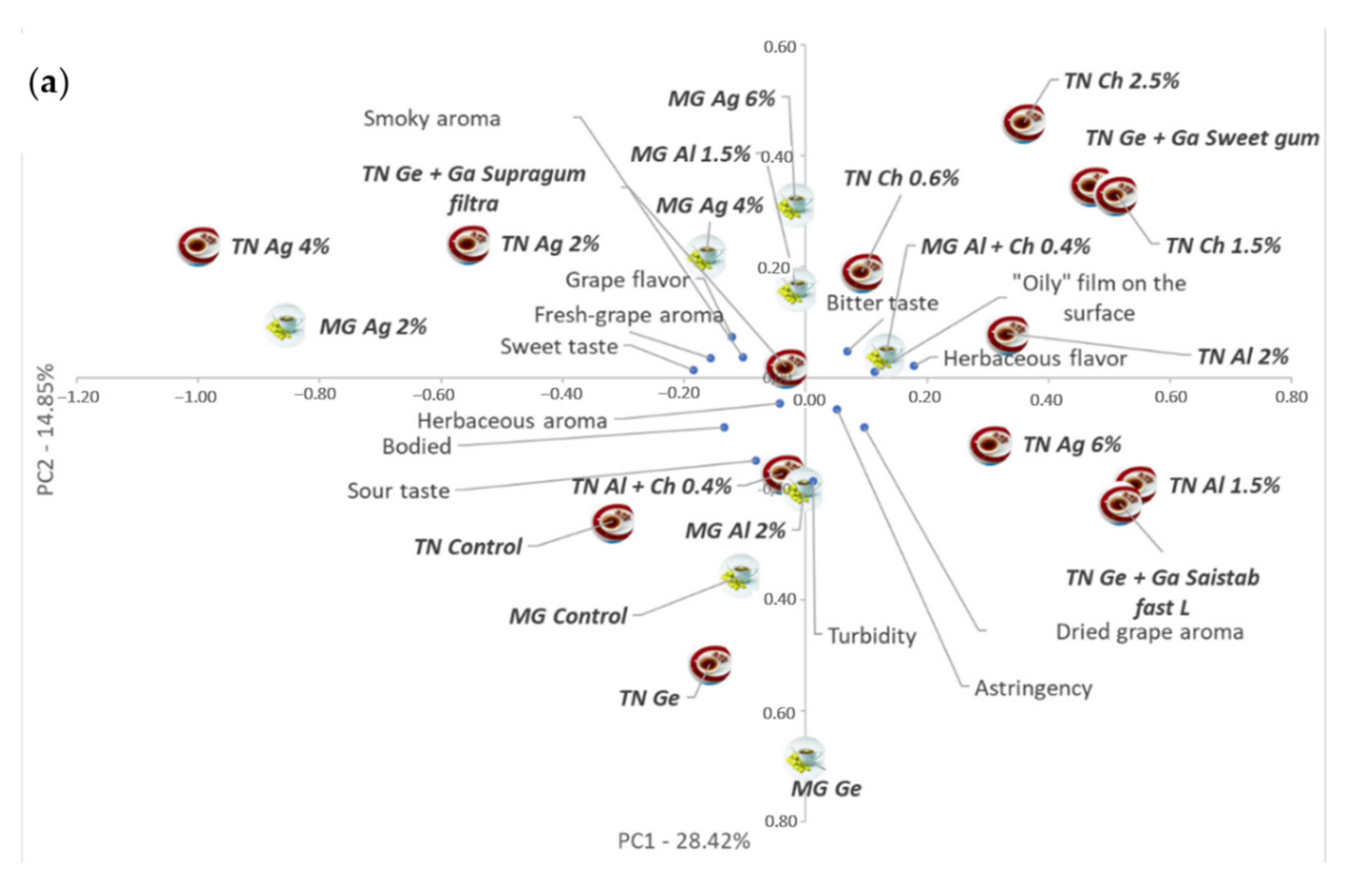
3.5. PCA Integrated Analysis of Coated Dried–Minced Grape Infusions Data
4. Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV, International Organisation of Vine and Wine Intergovernmental Organisation. Statistical Report on World Vitiviniculture. 2019. Available online: https://www.oiv.int/en/oiv-life/oiv-2019-report-on-the-world-vitivinicultural-situation (accessed on 23 February 2021).
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 31 July 2020).
- García-Lomillo, J.; González-SanJosé, M.L. Applications of Wine Pomace in the Food Industry: Approaches and Functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bender, A.B.B.; Speroni, C.S.; Salvador, P.R.; Loureiro, B.B.; Lovatto, N.M.; Goulart, F.R.; Lovatto, M.T.; de Miranda, M.Z.; da Silva, L.P.; Penna, N.G. Grape Pomace Skins and the Effects of Its Inclusion in the Technological Properties of Muffins. J. Culin. Sci. Technol. 2017, 15, 143–157. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel application and industrial exploitation of winery by-products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.S.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.C.; Cruz, A.P.G.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef]
- Balbinoti, T.C.V.; Stafussa, A.P.; Haminiuk, C.W.I.; Maciel, G.M.; Sassaki, G.L.; Jorge, L.M.D.M.; Jorge, R.M.M. Addition of grape pomace in the hydration step of parboiling increases the antioxidant properties of rice. Int. J. Food Sci. Technol. 2020, 55, 2370–2380. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A. Healthy Drinks with Lovely Colors: Phenolic Compounds as Constituents of Functional Beverages. Beverages 2021, 7, 12. [Google Scholar] [CrossRef]
- Abbas, M.; Saeed, F.; Anjum, F.M.; Afzaal, M.; Tufail, T.; Bashir, M.S.; Ishtiaq, A.; Hussain, S.; Suleria, H.A.R. Natural polyphenols: An overview. Int. J. Food Prop. 2017, 20, 1689–1699. [Google Scholar] [CrossRef]
- Negro, C.; Tommasi, L.; Miceli, A. Phenolic compounds and antioxidant activity from red grape marc extracts. Bioresour. Technol. 2003, 87, 41–44. [Google Scholar] [CrossRef]
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic content and in vitro antioxidant characteristics of wine industry and other agri-food solid waste extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Boussetta, N.; Lanoisellé, J.-L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; de Souza Schmidt Gonçalves, A.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Braibante, M.; Silva, D.; Braibante, H.; Pazinato, M. A Química Dos Chás. Quím. Nova Esc. 2014. Available online: http://qnesc.sbq.org.br/online/prelo/QS-47-13.pdf (accessed on 31 July 2020).
- Cosme, F.; Pinto, T.; Vilela, A. Oenology in the Kitchen: The Sensory Experience Offered by Culinary Dishes Cooked with Alcoholic Drinks, Grapes and Grape Leaves. Beverages 2017, 3, 42. [Google Scholar] [CrossRef]
- Vilela, A.; Pinto, T. Grape Infusions: Between Nutraceutical and Green Chemistry. Sustain. Chem. 2021, 2, 25. [Google Scholar] [CrossRef]
- Vashisth, T.; Singh, R.K.; Pegg, R.B. Effects of drying on the phenolics content and antioxidant activity of muscadine pomace. LWT—Food Sci. Technol. 2011, 44, 1649–1657. [Google Scholar] [CrossRef]
- Vilela, A.; Cruz, I.; Moreira, J.; Pinto, T. Grape-infusions made with dried grape berries immobilized in organic matrices: Preservation of flavour and nutritional characteristics. In Proceedings of the XV Encontro de Química dos Alimentos, Funchal, Portugal, 5–8 September 2021; Universidade da Madeira: Funchal, Portugal; pp. 223–224, PC-A12. ISBN 978-989-8805-68-3. [Google Scholar]
- Souza, V. Extração e Encapsulação por Coacervação Complexa das Proantocianidinas da Canela (Cinnamomum zeylanicum Blume). Ph.D. Thesis, Faculty of Animal Science and Food Engineering, University of São Paulo, Pirassununga, Brazil, 2016. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as Promising Natural Polymer for Pharmaceutical, Food, and Biomedical Applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef]
- Rinaudo, M. Biomaterials based on a natural polysaccharide: Alginate. TIP Revista Especializada en Ciencias Químico-Biológicas 2014, 17, 92–96. [Google Scholar] [CrossRef]
- Lou, X.; Guo, X.; Wang, K.; Wu, C.; Jin, Y.; Lin, Y.; Xu, H.; Hanna, M.; Yuan, L. Phenolic profiles and antioxidant activity of Crataegus pinnatifida fruit infusion and decoction and influence of in vitro gastrointestinal digestion on their digestive recovery. LWT 2020, 135, 110171. [Google Scholar] [CrossRef]
- Park, S.H.; Lee, C.-R.; Hong, S.-K. Implications of agar and agarase in industrial applications of sustainable marine biomass. Appl. Microbiol. Biotechnol. 2020, 104, 2815–2832. [Google Scholar] [CrossRef]
- Adsare, S.R.; Annapure, U.S. Microencapsulation of curcumin using coconut milk whey and Gum Arabic. J. Food Eng. 2021, 298, 110502. [Google Scholar] [CrossRef]
- No, H.; Meyers, S.; Prinyawiwatkul, W.; Xu, Z. Applications of Chitosan for Improvement of Quality and Shelf Life of Foods: A Review. J. Food Sci. 2007, 72, R87–R100. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Junior, E.N.O.; Franco, T.T. Chitosan tailor-made films: The effects of additives on barrier and mechanical properties. Packag. Technol. Sci. 2009, 22, 161–170. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Matté, G.; Rosa, S. A Tecnologia da microencapsulação através de microesferas de quitosana. Rev. Iberoam. Polim. 2013, 14, 206–218. [Google Scholar]
- Liu, J.; Sun, H.; Dong, F.; Xue, Q.; Wang, G.; Qin, S.; Guo, Z. The influence of the cation of quaternized chitosans on antioxidant activity. Carbohydr. Polym. 2009, 78, 439–443. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, X.; Lu, Y.; Dong, S.; Zhao, Y.; Zeng, M. Effects of chitosan molecular weight and degree of deacetylation on the properties of gelatine-based films. Food Hydrocoll. 2012, 26, 311–317. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of active gelatin films by means of valorisation of food processing waste: A review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Qin, W.; Dai, J.; Zhang, Q.; Lee, K.; Liu, Y. Physicochemical properties of gelatin films containing tea polyphenol-loaded chitosan nanoparticles generated by electrospray. Mater. Des. 2020, 185, 108277. [Google Scholar] [CrossRef]
- Etchepare, M.A.; Menezes, M.F.D.S.C.D.; Rodrigues, L.Z.; Codevilla, C.F. Microencapsulação de compostos bioativos pelo método de extrusão. Ciência e Natura 2015, 37, 97. [Google Scholar] [CrossRef][Green Version]
- Brasileiro, J. Microencapsulação de Compostos Bioativos: Inovação em Diferentes Areas. Master’s Thesis, Faculty of Health Sciences, University Fernando Pessoa, Porto, Portugal, 2011. [Google Scholar]
- Vilela, A.; Schuller, D.; Mendes-Faia, A.; Côrte-Real, M. Reduction of volatile acidity of acidic wines by immobilized Saccharomyces cerevisiae cells. Appl. Microbiol. Biotechnol. 2013, 97, 4991–5000. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.; Rossi, J. Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- ISO 8589; Sensory Analysis—General Guidance for the Design of Test Rooms. ISO: Geneva, Switzerland, 2007. Available online: https://www.iso.org/standard/36385.html (accessed on 1 June 2021).
- ISO 3591; Sensory Analysis—Apparatus—Wine-Tasting Glass. ISO: Geneva, Switzerland, 1977. Available online: https://www.iso.org/standard/9002.html (accessed on 1 June 2021).
- Jaeger, S.R.; Chheang, S.L.; Jin, D.; Roigard, C.M.; Ares, G. Check-all-that-apply (CATA) questions: Sensory term citation frequency reflects rated term intensity and applicability. Food Qual. Preference 2020, 86, 103986. [Google Scholar] [CrossRef]
- Corazza, M.; Rodrigues, D.; Nozaki, J. Preparação e caracterização do vinho de laranja. Química Nova 2001, 24, 449–452. [Google Scholar]
- Martinazzo, A.; Corrêa, P.; Melo, E.; Carneiro, A. Avaliação colorimétrica de folhas secas de Cymbopogon citratus (D.C.) Stapf durante o armazenamento em diferentes embalagens. Rev. Bras. De Prod. Agroind. 2008, 10, 131–140. [Google Scholar]
- Carrilha, F.; Guiné, R. Avaliação da cor de peras secadas por diferentes métodos. In Livro de Resumos e CD-Rom das Actas do 1° Encontro Português de Secagem de Alimentos; Instituto Politécnico de Viseu: Viseu, Portugal, 2010; p. 9. Available online: http://hdl.handle.net/10400.19/1356 (accessed on 15 May 2021).
- OIV. Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2021. [Google Scholar]
- Cabrita, M.; Silva, R.; Laureano, O. Os compostos fenólicos das uvas e dos vinhos. In Proceedings of the I Seminário Internacional de Vitivinicultura, INIFAP, Bento Gonçalves, Brazil, 3–5 December 2003; Universidade Federal do Amapá: Santana, Brazil, 2003. [Google Scholar]
- Ikawa, M.; Schaper, T.D.; Dollard, C.A.; Sasner, J.J. Utilization of Folin–Ciocalteu Phenol Reagent for the Detection of Certain Nitrogen Compounds. J. Agric. Food Chem. 2003, 51, 1811–1815. [Google Scholar] [CrossRef]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin–Ciocalteu reagent. J. Agric. Food Chem. 2010, 58, 8139–8144. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Dall’Antonia, L.H.; Archela, E. Determinação de Compostos Fenólicos em Vinho: Uma revisão. Semin. Ciências Exatas Tecnol. 2013, 34, 193. [Google Scholar] [CrossRef]
- Chen, L.-Y.; Cheng, C.-W.; Liang, J.-Y. Effect of esterification condensation on the Folin–Ciocalteu method for the quantitative measurement of total phenols. Food Chem. 2015, 170, 10–15. [Google Scholar] [CrossRef]
- Watson, R.; Preedly, V.; Zibadi, S. Polyphenols in Human Health and Disease; Watson, R., Preedy, V., Zibadi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; ISBN 978-0-12-398471-5. [Google Scholar]
- Roshanak, S.; Rahimmalek, M.; Goli, S.A.H. Evaluation of seven different drying treatments in respect to total flavonoid, phenolic, vitamin C content, chlorophyll, antioxidant activity and color of green tea (Camellia sinensis or C. assamica) leaves. J. Food Sci. Technol. 2016, 53, 721–729. [Google Scholar] [CrossRef]
- Prathapan, A.; Lukhman, M.; Arumughan, C.; Sundaresan, A.; Raghu, K.G. Effect of heat treatment on curcuminoid, colour value and total polyphenols of fresh turmeric rhizome. Int. J. Food Sci. Technol. 2009, 44, 1438–1444. [Google Scholar] [CrossRef]
- Paixão, N.; Perestrelo, R.; Marques, J.C.; Câmara, J. Relationship between antioxidant capacity and total phenolic content of red, rosé and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Vilela, A.; Matos, S.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Sucrose Replacement by Sweeteners in Strawberry, Raspberry, and Cherry Jams: Effect on the Textural Characteristics and Sensorial Profile—A Chemometric Approach. J. Food Process. 2015, 2015, 749740. [Google Scholar] [CrossRef]
- Vilela, A.; Sobreira, C.; Abraão, A.S.; Lemos, A.M.; Nunes, F.M. Texture quality of candied fruits as influenced by osmotic dehydration agents. J. Texture Stud. 2016, 47, 239–252. [Google Scholar] [CrossRef]
- Pinto, T.; Vilela, A.; Pinto, A.; Nunes, F.M.; Cosme, F.; Anjos, R. Influence of cultivar and of conventional and organic agricultural practices on phenolic and sensory profile of blackberries (Rubus fruticosus). J. Sci. Food Agric. 2018, 98, 4616–4624. [Google Scholar] [CrossRef]
- Oliveira, I.; Meyer, A.S.; Afonso, S.; Sequeira, A.; Vilela, A.; Goufo, P.; Trindade, H.; Gonçalves, B. Effects of Different Processing Treatments on Almond (Prunus dulcis) Bioactive Compounds, Antioxidant Activities, Fatty Acids, and Sensorial Characteristics. Plants 2020, 9, 1627. [Google Scholar] [CrossRef]
- Cabo, S.; Aires, A.; Carvalho, R.; Vilela, A.; Pascual-Seva, N.; Silva, A.P.; Gonçalves, B. Kaolin, Ascophyllum nodosum and salicylic acid mitigate effects of summer stress improving hazelnut quality. J. Sci. Food Agric. 2021, 101, 459–475. [Google Scholar] [CrossRef]
- Oliveira, J.; Carvalho, A.; Martins, L.; Moreira, D. Elaboração e caracterização físico-química e sensorial de estruturados de polpa concentrada de abacaxi. Alimentos e Nutrição 2012, 23, 23–31. [Google Scholar]
- Han, C.; Lederer, C.; McDaniel, M.; Zhao, Y. Sensory Evaluation of Fresh Strawberries (Fragaria ananassa) Coated with Chitosan-based Edible Coatings. J. Food Sci. 2006, 70, S172–S178. [Google Scholar] [CrossRef]



| Encapsulating Agent | Concentration (w/v) |
|---|---|
| Alginate (Sigma-Aldrich) | 1.5% |
| 2% | |
| Alginate/chitosan (Sigma-Aldrich) | 2%/0.4% |
| Gelatin (cooking gelatin in leaves) | 4 leaves/100 mL |
| Agar (Sigma-Aldrich) | 2% |
| 4% | |
| 6% | |
| Chitosan (low molecular weight, Sigma-Aldrich) | 0.6% |
| 1.5% | |
| 2.5% | |
| Arabic gum * + gelatin (cooking gelatin in leaves) | 4 leaves of gelatin/100 mL of Arabic gum |
| Sample Number | Composition of the Coating Matrix | Mass of a Coated Ball (g) | Sample Code |
|---|---|---|---|
| 1 | Agar 6% Moscatel Galego | 1.73061 | MG Ag 6% |
| 2 | Agar 6% Touriga Nacional | 1.91657 | TN Ag 6% |
| 3 | Agar 4% Moscatel Galego | 2.02412 | MG Ag 4% |
| 4 | Agar 4% Touriga Nacional | 2.04031 | TN Ag 4% |
| 5 | Agar 2% Moscatel Galego | 1.86336 | MG Ag 2% |
| 6 | Agar 2% Touriga Nacional | 1.75125 | TN Ag 2% |
| 7 | Alginate 1.5% Moscatel Galego | 1.72077 | MG Al 5% |
| 8 | Alginate 1.5% Touriga Nacional | 1.77616 | TN Al 5% |
| 9 | Alginate 2% Moscatel Galego | 1.55352 | MG Al 2% |
| 10 | Alginate 2% Touriga Nacional | 1.69500 | TN Al 2% |
| 11 | Alginate 2% + chitosan 0.4% Moscatel Galego | 1.64180 | MG AL + Ch 0.4% |
| 12 | Alginate 2% + chitosan 0.4% Touriga Nacional | 1.56874 | TN AL + Ch 0.4% |
| 13 | Gelatin (4 leaves/100 mL) Moscatel Galego | 1.57904 | MG Ge |
| 14 | Gelatin (4 leaves/100 mL Touriga Nacional | 1.55987 | TN Ge |
| 15 | Gelatin (4 leaves/100 mL) + Arabic gum Sweet Gum Touriga Nacional | 1.60356 | TN Ge + Ga Sweet Gum |
| 16 | Gelatin (4 leaves/100 mL) + Arabic gum Saistab Fast L Touriga Nacional | 1.70025 | TN Ge + Ga Saistab Fast L |
| 17 | Gelatin (4 leaves/100 mL) + Arabic gum Supragum Touriga Nacional | 1.63114 | TN Ge + Ga Supragum Filtra |
| 18 | Chitosan 0.6% (+ 6 drops of HCl at 37%) Touriga Nacional | 2.01055 | TN Ch 6% |
| 19 | Chitosan 1.5% (+14 drops of HCl at 37%) Touriga Nacional | 1.90835 | TN Ch 1.5% |
| 20 | Chitosan 2.5% (+20 drops of HCl at 37%) Touriga Nacional | 1.99261 | TN Ch 2.5% |
| 21 | Control Moscatel Galego | 1.67969 | MG control |
| 22 | Control Touriga Nacional | 1.73862 | TN control |
| 23 | Encapsulating agent agar 2% | - | Ag 2% |
| 24 | Encapsulating agent agar 4% | - | Ag 4% |
| 25 | Encapsulating agent agar 6% | - | Ag 6% |
| 26 | Encapsulating agent gelatin 4 leaves/ 100 mL | - | Ge |
| 27 | Encapsulating agent chitosan 0.6% | - | Ch 0.6% |
| 28 | Encapsulating agent chitosan 1.5% | - | Ch 1.5% |
| 29 | Encapsulating agent chitosan 2.5% | - | Ch 2.5% |
| 30 | Encapsulating agent alginate 1.5% | - | Al 1.5% |
| 31 | Encapsulating agent alginate 2.0% | - | Al 2.0% |
| 32 | Encapsulating agent alginate 2.0% + chitosan 0.4% | - | Al + Ch |
| Samples | pH (M ± SD) | Brix (M ± SD) |
|---|---|---|
| MG Ag 6% | 3.89 ± 0.03 a | 0.87 ± 0.05 a |
| TN Ag 6% | 4.59 ± 0.03 I | 0.93 ± 0.05 a |
| MG Ag 4% | 3.87 ± 0.03 a | 1.40 ± 0.00 h |
| TN Ag 4% | 4.62 ± 0.00 i | 1.00 ± 0.00 abc |
| MG Ag 2% | 4.23 ± 0.03 cde | 1.23 ± 0.05 defg |
| TN Ag 2% | 3.94 ± 0.04 ab | 1.27 ± 0.05 efgh |
| MG Al 1.5% | 3.93 ± 0.03 ab | 1.20 ± 0.00 def |
| TN Al 1.5% | 4.17 ± 0.05 c | 1.37 ± 0.05 gh |
| MG Al 2% | 4.02 ± 0.02 b | 1.27 ± 0.05 efgh |
| TN Al 2% | 4.28 ± 0.01 def | 1.33 ± 0.05 fgh |
| MG Al + Ch 0.4% | 4.04 ± 0.02 b | 1.37 ± 0.05 gh |
| TN Al + Ch 0.4% | 4.39 ± 0.06 fg | 1.27 ± 0.05 efgh |
| MG Ge | 3.95 ± 0.02 ab | 1.33 ± 0.05 fgh |
| TN Ge | 4.47 ± 0.03 gh | 0.97 ± 0.05 ab |
| TN Ge + Ga Sweet Gum | 4.17 ± 0.01 cd | 1.17 ± 0.05 de |
| TN Ge + Ga Saistab Fast L | 4.75 ± 0.02 j | 0.93 ± 0.05 a |
| TN Ge + Ga Supragum Filtra | 4.32 ± 0.03 ef | 1.00 ± 0.00 abc |
| TN Ch 0.6% | 4.35 ± 0.05 f | 1.10 ± 0.08 bcd |
| TN Ch 1.5% | 4.55 ± 0.00 hi | 1.00 ± 0.00 abc |
| TN Ch 2.5% | 4.61 ± 0.01 i | 1.00 ± 0.00 abc |
| MG control | 3.91 ± 0.01 a | 1.13 ± 0.05 cde |
| TN control | 4.30 ± 0.02 ef | 1.13 ± 0.05 cde |
| Grape pomace 50 °C | 3.55 ± 0.04 a | 0.07 ± 0.06 a |
| Grape pomace 60 °C | 3.65 ± 0.04 a | 0.10 ± 0.00 a |
| Grape pomace 70 °C | 3.69 ± 0.04 a | 0.13 ± 0.06 a |
| Samples | L*/10 | a* | b* | C | H |
|---|---|---|---|---|---|
| MG Ag 6% | 3.28 ± 0.006 n | 0.11 ± 0.010 a | 3.93 ± 0.006 g | 3.93 ± 0.006 g | 1.54 ± 0.003 i |
| TN Ag 6% | 3.34 ± 0.006 q | 0.16 ± 0.015 b | 6.55 ± 0.000 p | 6.55 ± 0.000 o | 1.55 ± 0.002 i |
| MG Ag 4% | 3.15 ± 0.026 m | 0.08 ± 0.012 a | 3.13 ± 0.012 d | 3.13 ± 0.011 d | 1.55 ± 0.004 i |
| TN Ag 4% | 3.40 ± 0.020 r | 0.25 ± 0.006 c | 8.10 ± 0.006 r | 8.11 ± 0.006 q | 1.54 ± 0.001 i |
| MG Ag 2% | 3.09 ± 0.012 k | 0.84 ± 0.012 i | 6.83 ± 0.021 q | 6.89 ± 0.020 p | 1.45 ± 0.002 h |
| TN Ag 2% | 3.31 ± 0.000 p | 0.27 ± 0.006 c | 5.20 ± 0.015 m | 5.20 ± 0.015 i | 1.52 ± 0.001 k |
| MG Al 1.5% | 3.15 ± 0.040 i | 0.37 ± 0.030 d | 4.07 ± 0.047 h | 4.08 ± 0.045 h | 1.48 ± 0.008 j |
| TN Al 1.5% | 2.90 ± 0.006 e | 1.01 ± 0.026 k | 5.06 ± 0.006 i | 5.16 ± 0.006 i | 1.37 ± 0.005 d |
| MG Al 2% | 3.05 ± 0.017 i | 0.25 ± 0.023 c | 3.07 ± 0.023 c | 3.08 ± 0.022 c | 1.49 ± 0.008 j |
| TN Al 2% | 2.85 ± 0.006 c | 1.13 ± 0.023 m | 4.76 ± 0.015 k | 4.89 ± 0.010 k | 1.34 ± 0.005 ab |
| MG Al + Ch 0.4% | 2.99 ± 0.006 h | 0.18 ± 0.012 b | 3.18 ± 0.006 e | 3.19 ± 0.005 e | 1.52 ± 0.004 k |
| TN Al + Ch 0.4% | 3.05 ± 0.010 i | 0.95 ± 0.000 j | 6.13 ± 0.012 o | 6.21 ± 0.011 n | 1.42 ± 0.000 ef |
| MG Ge | 2.88 ± 0.000 d | 0.46 ± 0.006 e | 2.45 ± 0.006 a | 2.50 ± 0.005 a | 1.38 ± 0.003 d |
| TN Ge | 2.76 ± 0.006 a | 0.67 ± 0.006 h | 2.76 ± 0.017 b | 2.84 ± 0.016 b | 1.33 ± 0.003 a |
| TN Ge + Ga Sweet Gum | 2.96 ± 0.006 g | 0.58 ± 0.000 g | 4.22 ± 0.010 i | 4.26 ± 0.010 i | 1.43 ± 0.000 g |
| TN Ge + Ga Saistab Fast L | 2.78 ± 0.006 b | 0.55 ± 0.006 fg | 3.66 ± 0.010 f | 3.70 ± 0.011 f | 1.42 ± 0.001 f |
| TN Ge + Ga Supragum Filtra | 3.05 ± 0.000 j | 1.09 ± 0.000 i | 4.78 ± 0.000 k | 4.90 ± 0.000 k | 1.35 ± 0.000 b |
| TN Ch 0.6% | 3.29 ± 0.000 o | 1.45 ± 0.006 p | 8.90 ± 0.006 s | 9.01 ± 0.005 r | 1.41 ± 0.001 e |
| TN Ch 1.5% | 3.32 ± 0.000 p | 1.33 ± 0.006 o | 8.95 ± 0.012 t | 9.05 ± 0.012 r | 1.42 ± 0.000 f |
| TN Ch 2.5% | 3.49 ± 0.010 s | 1.04 ± 0.006 k | 10.19 ± 0.006 u | 10.24 ± 0.006 s | 1.47 ± 0.001 i |
| MG control | 3.15 ± 0.017 m | 0.52 ± 0.010 f | 4.33 ± 0.010 j | 4.36 ± 0.009 j | 1.45 ± 0.003 h |
| TN control | 2.94 ± 0.006 f | 1.25 ± 0.010 n | 5.81 ± 0.017 n | 5.94 ± 0.015 m | 1.36 ± 0.002 c |
| Grape pomace 50 °C | 4.00 ± 0.001 c | −0.19 ± 0.000 b | 4.65 ± 0.010 c | 4.65 ± 0.010 c | 178.47 ± 0.000 a |
| Grape pomace 60 °C | 3.52 ± 0.001 a | −0.15 ± 0.010 a | 3.01 ± 0.006 a | 3.01 ± 0.006 a | 178.48 ± 0.000 b |
| Grape pomace 70 °C | 3.78 ± 0.005 b | −0.24 ± 0.015 c | 3.89 ± 0.012 b | 3.89 ± 0.011 b | 178.49 ± 0.000 c |
| Descriptors | DGP and Cinnamon | DGP and Ginger | DGP and Honey | DGP and Mint | DGP at 70 °C |
|---|---|---|---|---|---|
| Light intensity | 2.4 ± 1.4 | 1.7 ± 0.8 | 2.9 ± 1.8 | 2.3 ± 1.5 | 1.7 ± 0.5 |
| Turbidity | 1.0 ± 0.0 a | 1.1 ± 0.4 a | 2.9 ± 1.1 b | 1.0 ± 0.0 a | 1.2 ± 0.4 a |
| Herbaceous aroma | 2.9 ± 1.7 ab | 2.9 ± 1.5 ab | 1.4 ± 0.5 a | 3.0 ± 1.7 b | 2.0 ± 0.9 ab |
| Smoky/burnt aroma | 1.7 ± 1.1 | 1.6 ± 0.8 | 1.9 ± 1.6 | 1.1 ± 0.4 | 1.5 ± 0.5 |
| Natural aroma of dry pomace | 2.9 ± 1.1 c | 1.9 ± 0.9 ab | 1.4 ± 0.8 a | 1.4 ± 0. 8 a | 2.8 ± 1.5 c |
| Grape aroma | 1.9 ± 1.2 | 1.3 ± 0.8 | 1.9 ± 1.2 | 1.1 ± 0.4 | 1.7 ± 1.2 |
| Sweet taste | 1.6 ± 0.8 a | 1.6 ± 0.8 a | 4.3 ± 0.8 b | 1.7 ± 1.3 a | 1.5 ± 0.5 a |
| Acid taste | 1.4 ± 0.8 | 1.4 ± 0.8 | 1.0 ± 0.0 | 1.4 ± 0.8 | 1.5 ± 0.8 |
| Bitter taste | 1.7 ± 0.8 ab | 1.4 ± 0.5 a | 1.0 ± 0.0 a | 1.4 ± 0.5 a | 2.7 ± 1.6 b |
| Herbaceous flavor | 1.7 ± 0.8 ab | 2.1 ± 1.1 ab | 1.0 ± 0.0 a | 2.0 ± 1.2 ab | 3.0 ± 1.7 b |
| Astringency | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.7 ± 1.6 |
| Grape flavor | 1.3 ± 0.5 | 1.4 ± 0.8 | 1.6 ± 0.8 | 1.3 ± 0.5 | 1.7 ± 0.8 |
| Full bodied | 2.0 ± 1.2 a | 1.9 ± 1.1 a | 3.6 ± 1.6 b | 2.4 ± 1.5 ab | 1.8 ± 1.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilela, A.; Cruz, I.; Oliveira, I.; Pinto, A.; Pinto, T. Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Coatings 2022, 12, 443. https://doi.org/10.3390/coatings12040443
Vilela A, Cruz I, Oliveira I, Pinto A, Pinto T. Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Coatings. 2022; 12(4):443. https://doi.org/10.3390/coatings12040443
Chicago/Turabian StyleVilela, Alice, Irene Cruz, Ivo Oliveira, Ana Pinto, and Teresa Pinto. 2022. "Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes" Coatings 12, no. 4: 443. https://doi.org/10.3390/coatings12040443
APA StyleVilela, A., Cruz, I., Oliveira, I., Pinto, A., & Pinto, T. (2022). Sensory and Nutraceutical Properties of Infusions Prepared with Grape Pomace and Edible-Coated Dried–Minced Grapes. Coatings, 12(4), 443. https://doi.org/10.3390/coatings12040443









