Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Counting
2.3. Set-Up of Experimental Cell Cultures
2.3.1. Cells for Biochemical Analyses
2.3.2. Cells for MRS Measurements
2.3.3. Cells for [U-13C6]Glucose Metabolomics
2.4. Cellular Uptake of 14C-Pyruvate
2.5. Metabolite Analyses
2.5.1. Glucose Quantification in Conditioned Media
2.5.2. Lactate Quantification in Cells and Conditioned Media
2.5.3. Quantification of NADH and NAD+
2.6. Measurements of LDH and Pyruvate Kinase Activities
2.7. Analyses of Hyperpolarized [1-13C]Pyruvate-to-[1-13C]Lactate Conversions
2.7.1. Hyperpolarization of [1-13C]Pyruvate
2.7.2. 13C-Magnetic Resonance Spectroscopy (MRS)
2.7.3. Data Analysis and Kinetic Modelling of Hyperpolarized 13C-Metabolites
2.8. Measurements of 13C-Glucose-Derived Metabolites
2.8.1. Cell Culture and 13C-Glucose Labelling
2.8.2. Extraction of Polar Metabolites and GC-MS Separation
2.8.3. 13C-Metabolomic Data Analysis
2.9. Statistics
3. Results
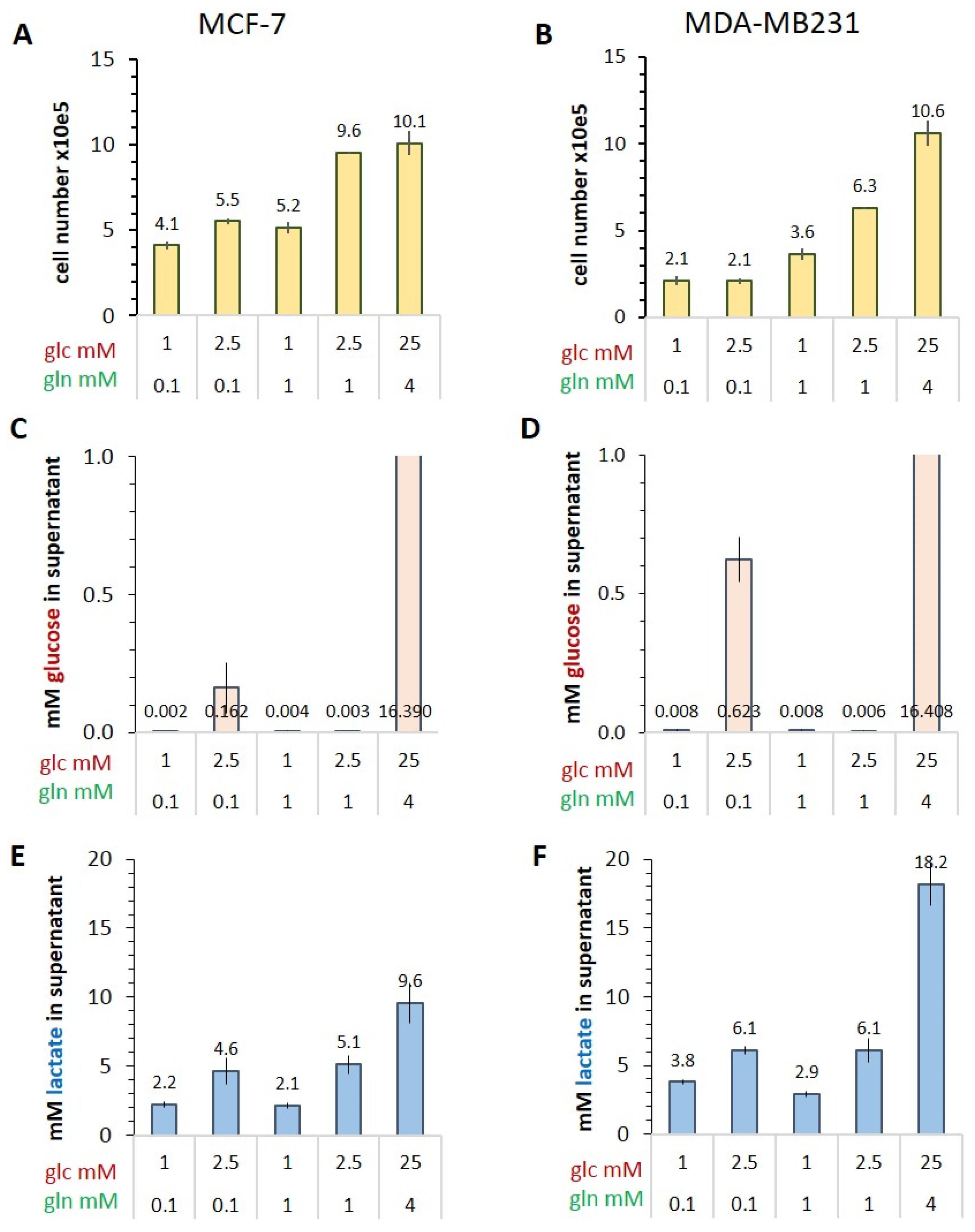
3.1. Characterizing the Warburg Effect in MCF-7 and MDA-MB-231 Cells in Limiting Nutrient Conditions
Glucose Consumption and Lactate Release
3.2. Conversion of Hyperpolarized [1-13C]Pyruvate in Variable Glucose/Glutamine Conditions
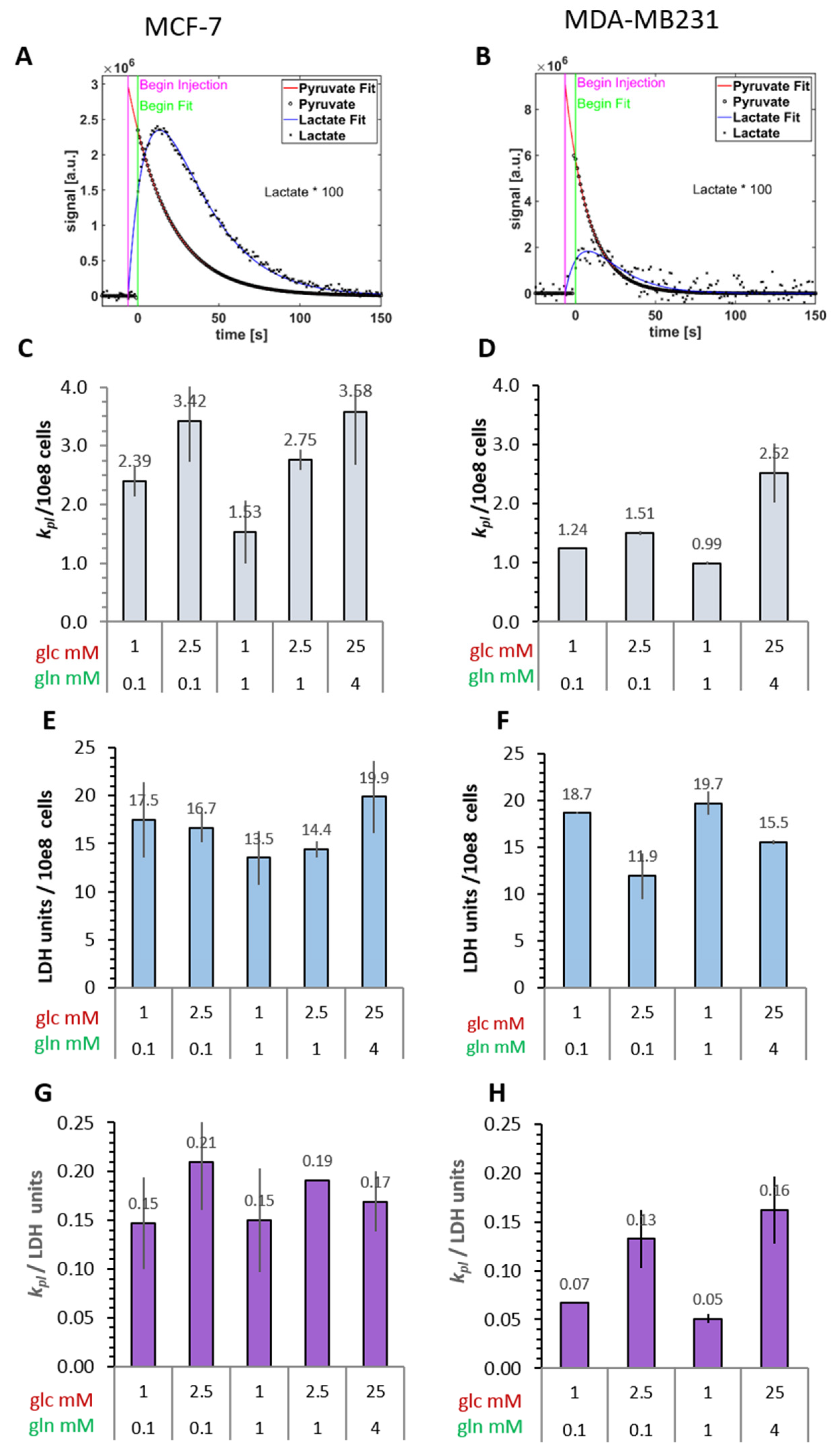
3.2.1. Conversion Rates of Exogenous [1-13C]Pyruvate to [1-13C]Lactate
3.2.2. Cellular LDH Activity of MRS Samples
3.3. Monocarboxylate Transporters and Uptake of [1-14C]Pyruvate
3.4. Concentrations of Intracellular Metabolites and LDH Activity
3.4.1. Lactate
3.4.2. Pyruvate
3.4.3. NADH and NAD+
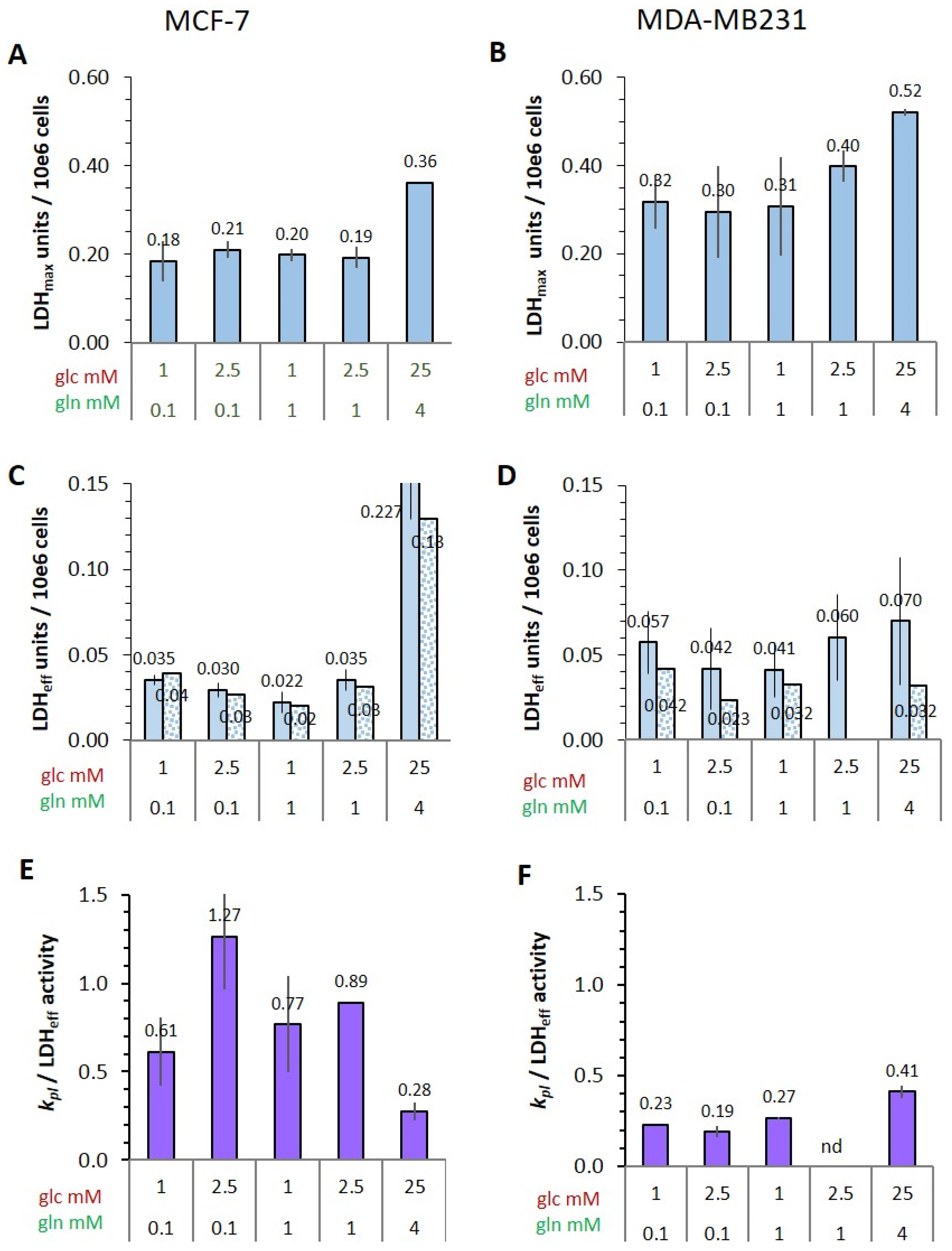
3.4.4. Potential and Effective Activity of LDH in Cells
3.5. Metabolic Flux of Pyruvate in MCF-7 and MDA-MB-231 Cells
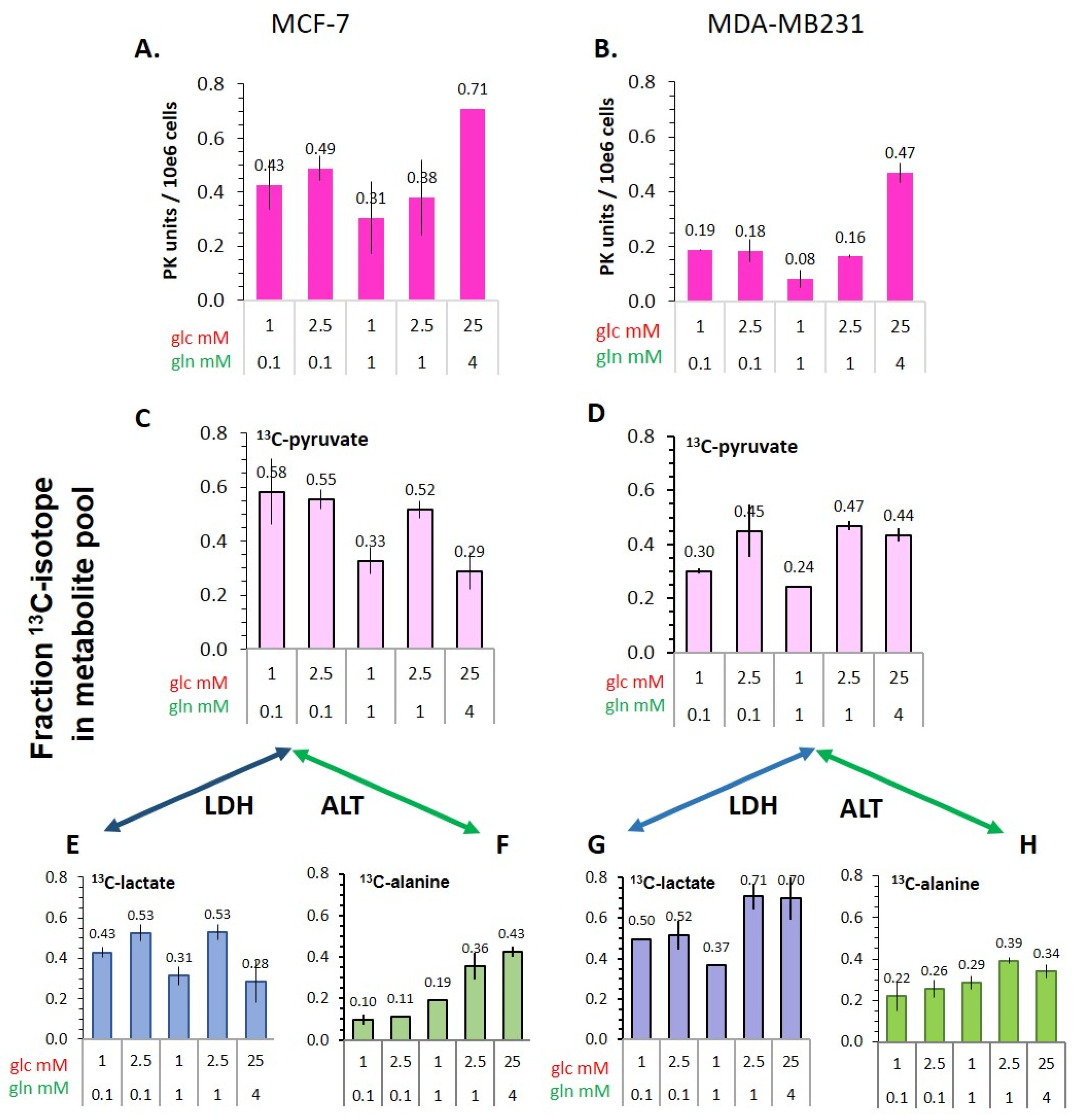
3.5.1. Pyruvate Kinase Activity and 13C-Glucose-Derived Pyruvate Levels as Indicators of Glycolytic Activity
3.5.2. Flux of 13C-Glucose-Derived Pyruvate to Lactate and Alanine
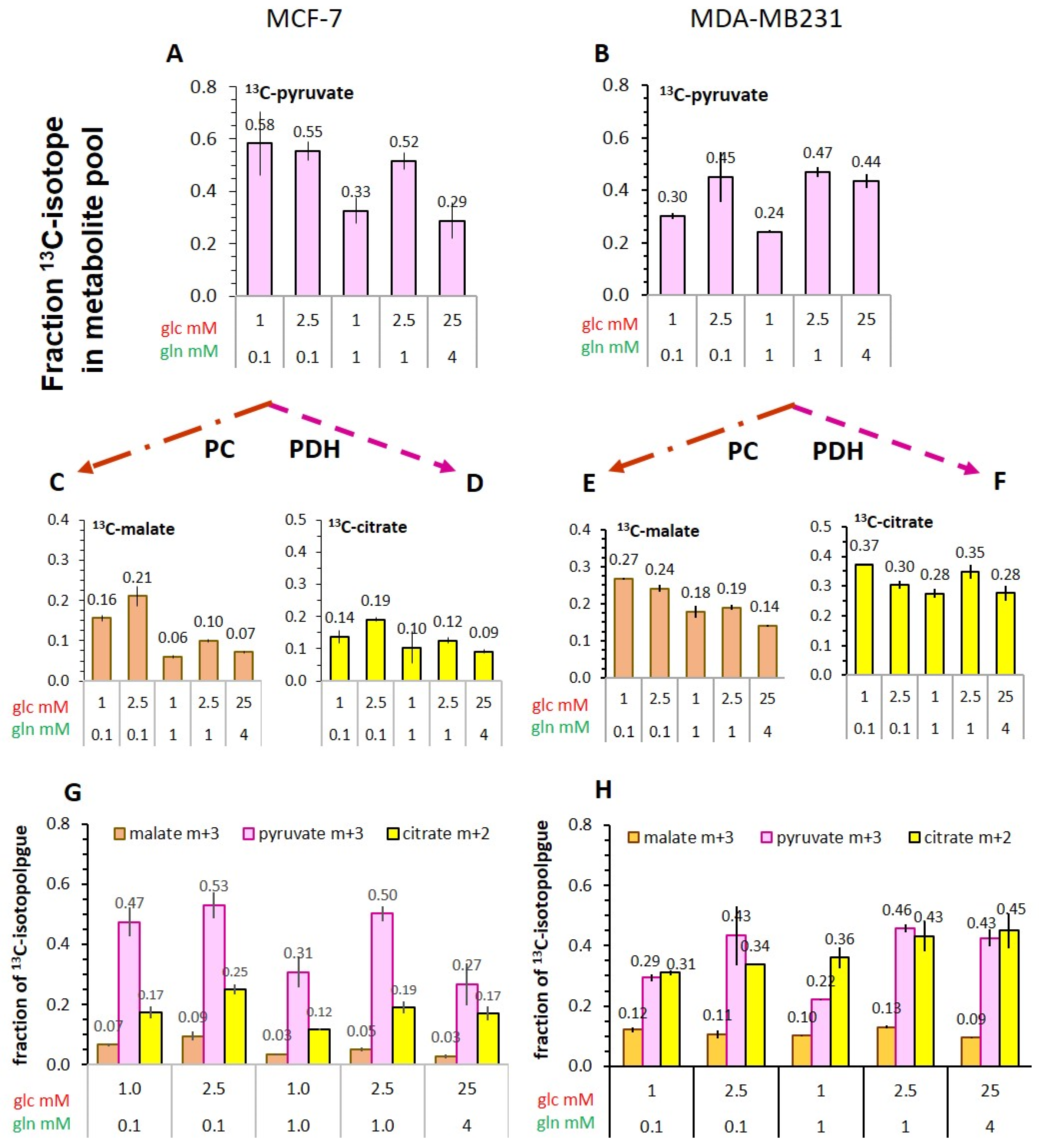
3.5.3. Flux of 13C-Glucose-Derived Pyruvate into TCA-Cycle Metabolites
4. Discussion
- (1)
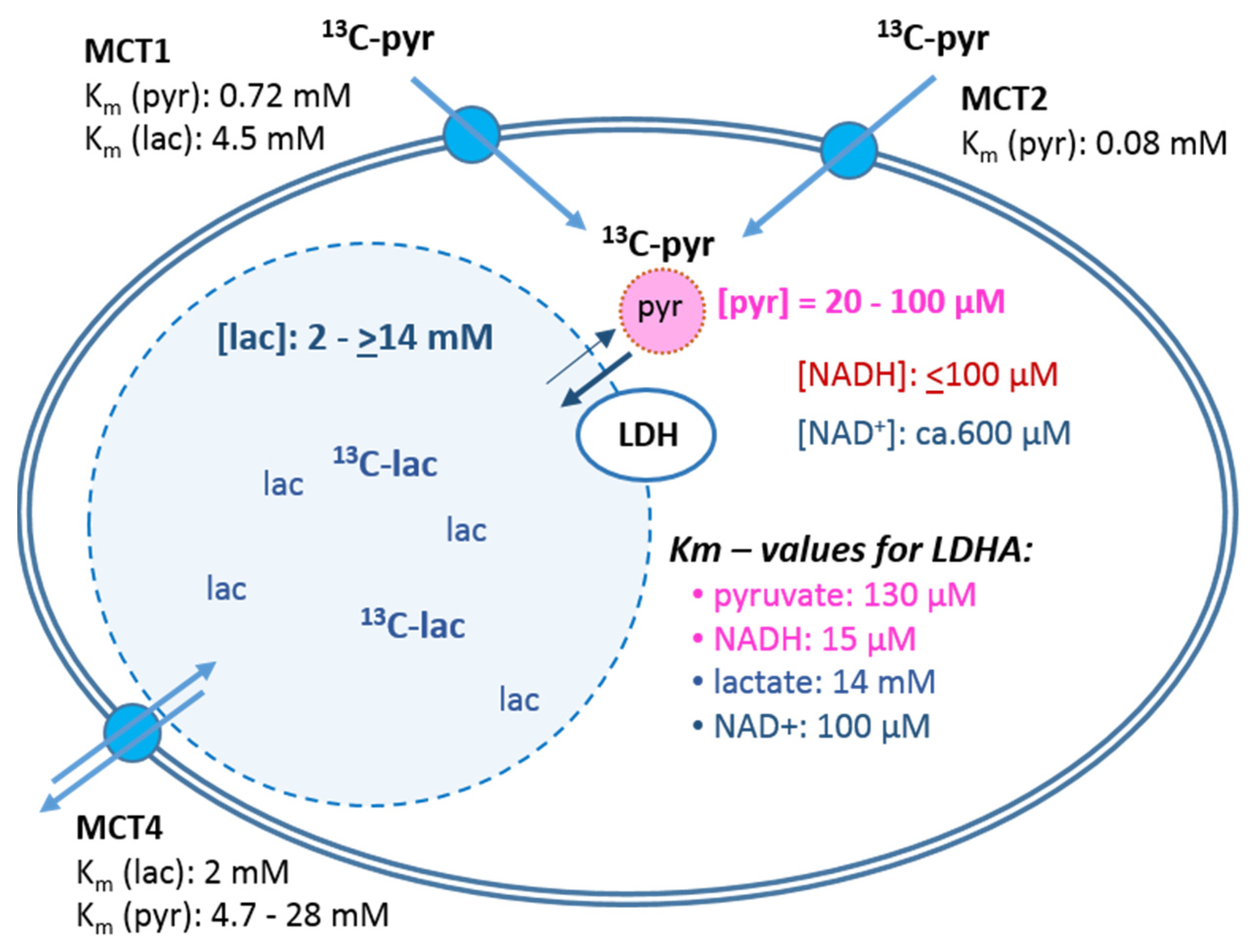
- The cellular uptake experiments performed with a physiological concentration of 1 mM 14C-pyruvate could not establish differences in initial uptake rates between MCF-7 and MDA-MB-231 cells. While pyruvate uptake in MCF-7 cells is mediated by MCT1 and MCT2 (Figure 3), MDA-MB-231 cells do not express MCT1 protein, but do express MCT2 and especially MCT4 [29], the latter mainly regulating lactate exports [31]. The fact that both cell lines express MCT2, which has the highest known affinity for pyruvate of the different transporters, may explain that MDA-MB-231 cells could take up pyruvate avidly in spite of the lack of MCT1. On the other hand, several studies do conclude that MCT1-expression is rate-limiting for the uptake and conversion of hyperpolarized 13C-pyruvate, albeit in conditions of MCT1 knockdown or genetic overexpression [27,34]. In those studies, a rescuing role of MCT 2 was not considered. Moreover, with the results presented here, no reliable differences in pyruvate uptake were observed in the different glucose/glutamine conditions, leaving open the question whether the expression and activity of these transporters are regulated by extracellular levels of glucose, glutamine, or extracellular lactate levels.
- (2)
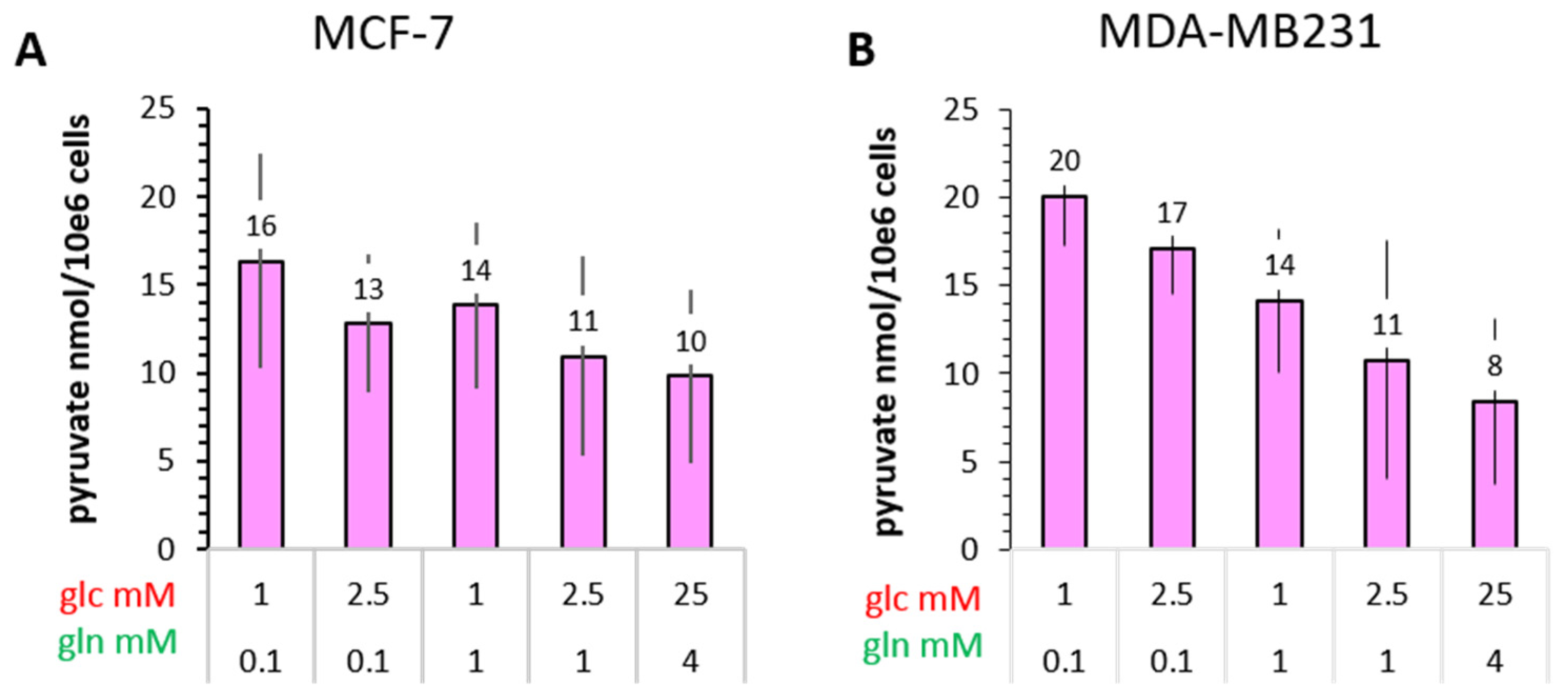
- Intracellular levels of metabolites are likely to vary with the metabolic state of the cells and thereby modulate enzyme activities. However, in the low glucose/glutamine conditions, both MCF-7 and MDA-MB-231 cells have similarly low intra-cellular pyruvate concentrations, in a range of 20–44 µM. Furthermore, NAD+ concentrations were found to be similar in both cell lines, in the range of 500–650 µM. In contrast, intracellular lactate concentrations of MDA-MB-231 cells are approximately three-fold higher than those of MCF-7 cells, while NADH concentrations, being around 100 µM for MCF-7 cells, were instead more than 30% lower in MDA-MB-231 cells growing in 1 mM glucose conditions. The combination of high intracellular lactate with a low NADH concentration could reduce pyruvate-to-lactate conversion rates. Indeed, a decrease in conversion rate was observed with MDA-MB-231 xenografts having reduced NADH levels upon treatment with doxorubicin [61]. Thus, a reduction in NADH levels provides a further explanation for lower kpl-values in MDA-MB-231 cells.
- (3)
- The conversion of [1-13C]pyruvate to [1-13C]lactate is facilitated by LDH activity, and its rate will depend not only on the number of LDH-complexes in the cell, but also on the substrate and coenzyme availability at the site of the enzyme. LDHA expression reported as either mRNA level or as protein levels determined by Western blots or by immunohistochemistry can provide information on LDH availability, which has been associated with the malignant state of the tumor [23,62]. Moreover, LDHA activity is generally quantified with cell homogenates or lysates using saturating pyruvate and NADH concentrations, conditions which gives an indication of potentially maximal activity (LDHmax). Comparing the LDH activities of MCF-7 and MDA-MB-231 cells confirms these observations in that LDH activities were about two-fold higher in MDA-MB-231 than in MCF-7 cells. However, based on the actual cellular levels of pyruvate and NADH, only about 15 to 30%, maximally 65%, of the potentially available LDH activity would be operative, without affecting the differences in LDH activities between MCF-7 and MDA-MB-231 cells (Figure 6). Thus, contrary to the expectations, neither maximal nor effective LDH activity correlated with the lower pyruvate-to-lactate conversion rate in the more malignant MDA-MB-231 cells. This is corroborated by a recent study on different breast cancers in patients, where LDH expression did not correlate with differences in lactate signals following uptake and conversion of hyperpolarized [1-13C]pyruvate [63]. Considering that pyruvate is a metabolite at a metabolic junction [40,64], the fate of hyperpolarized 13C-pyruvate is unlikely to be confined to its conversion to 13C-lactate. Furthermore, 13C-glucose-derived metabolomics have revealed that 13C-pyruvate is also channeled to 13C-alanine and to 13C-labelled TCA-metabolites, i.e., into the mitochondrial compartment (Figure 9). While the 13C-enrichments of lactate and alanine are similar for the two cell lines (Figure 7E–H), they differ markedly between 13C-citrate and 13C-malate (Figure 8G,H). The increase in TCA-cycle channeling and flux could divert available 13C-pyruvate and thus reduce the fraction of 13C-pyruvate available for conversion to 13C-lactate. Therefore, the expression and activity of MCTs and of LDH are not the only rate-determining factors for the conversion of hyperpolarized 13C-pyruvate to 13C-lactate in physiological conditions, as already discussed above [60,63].
- (4)
- To explain why the kpl for the LDH-catalyzed reaction correlated better with glycolytic activity, namely pyruvate kinase activity, than with LDH activity, a hypothesis of metabolic micro-compartmentation of glycolysis and LDH is proposed. It is known that metabolic enzymes are not uniformly distributed in the cell. LDH proteins are considered to be cytosolic, but are also localized in mitochondria [29], where they are involved in oxidative metabolism [67]. Moreover, LDH has been found in peroxisomes, i.e., organelles of fatty acid oxidation, in which LDH activity is proposed to maintain redox-balance [68]. Likewise, glycolytic enzymes are found in the cytosol fraction, but in the intact cytoplasm are clustered into micro-compartments, which in contact with actin filaments and microtubules form a metabolon (Figure 9) [69,70]. This includes pyruvate kinase as the terminal enzyme of the glycolytic metabolon. The pyruvate produced here is rapidly converted depending on the enzymes “waiting” for it, one of them being “cytosolic” LDH. In support of this hypothesis, MCF-7 and MDA-MB231 cells differ in their actin skeleton in that MCF-7 cells have a more pronounced actin network than MDA-MB-231 cells [57]. Upon entry into the cells, the exogenous hyperpolarized [1-13C]pyruvate could easily diffuse in the quasi-aqueous milieu between such macromolecular structures [71] and find access to the LDH localized in the immediate vicinity of the glycolytic site. This hypothesis could thus explain the metabolic dependency of the exogenous pyruvate-to-lactate conversion on glucose and the lack of correlation with total LDH activity, which has been also observed and discussed in other studies with breast cancer models [60,63].
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kurhanewicz, J.; Vigneron, D.B.; Ardenkjaer-Larsen, J.H.; Bankson, J.A.; Brindle, K.; Cunningham, C.H.; Gallagher, F.A.; Keshari, K.R.; Kjaer, A.; Laustsen, C.; et al. Hyperpolarized 13C MRI: Path to Clinical Translation in Oncology. Neoplasia 2019, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Racker, E. Bioenergetics and the Problem of Tumor Growth: An understanding of the mechanism of the generation and control of biological energy may shed light on the problem of tumor growth. Am. Sci. 1972, 60, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Pantel, A.R.; Ackerman, D.; Lee, S.C.; Mankoff, D.A.; Gade, T.P. Imaging Cancer Metabolism: Underlying Biology and Emerging Strategies. J. Nucl. Med. 2018, 59, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A. Mammalian hexokinases and their abnormal expression in cancer. Br. J. Biomed. Sci. 2000, 57, 170–178. [Google Scholar] [PubMed]
- Gutte, H.; Hansen, A.E.; Johannesen, H.H.; Clemmensen, A.E.; Ardenkjaer-Larsen, J.H.; Nielsen, C.H.; Kjaer, A. The use of dynamic nuclear polarization (13)C-pyruvate MRS in cancer. Am. J. Nucl. Med. Mol. Imaging 2015, 5, 548–560. [Google Scholar] [PubMed]
- Ardenkjær-Larsen, J.H.; Fridlund, B.; Gram, A.; Hansson, G.; Hansson, L.; Lerche, M.H.; Servin, R.; Thaning, M.; Golman, K. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. USA 2003, 100, 10158–10163. [Google Scholar] [CrossRef] [Green Version]
- Gallagher, F.A.; Kettunen, M.I.; Hu, D.E.; Jensen, P.R.; Zandt, R.I.; Karlsson, M.; Gisselsson, A.; Nelson, S.K.; Witney, T.H.; Bohndiek, S.E.; et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc. Natl. Acad. Sci. USA 2009, 106, 19801–19806. [Google Scholar] [CrossRef] [Green Version]
- Duwel, S.; Durst, M.; Gringeri, C.V.; Kosanke, Y.; Gross, C.; Janich, M.A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; Schulte, R.F.; et al. Multiparametric human hepatocellular carcinoma characterization and therapy response evaluation by hyperpolarized C-13 MRSI. NMR Biomed. 2016, 29, 952–960. [Google Scholar] [CrossRef]
- Cavallari, E.; Carrera, C.; Aime, S.; Reineri, F. 13C MR Hyperpolarization of Lactate by Using ParaHydrogen and Metabolic Transformation in vitro. Chemistry 2017, 23, 1200–1204. [Google Scholar] [CrossRef] [Green Version]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.Z.; Harzstark, A.L.; Ferrone, M.; van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I.; et al. Metabolic Imaging of Patients with Prostate Cancer Using Hyperpolarized [1-13C] Pyruvate. Sci. Transl. Med. 2013, 5, 198ra108. [Google Scholar] [CrossRef] [Green Version]
- Payen, V.L.; Porporato, P.E.; Baselet, B.; Sonveaux, P. Metabolic changes associated with tumor metastasis, part 1: Tumor pH, glycolysis and the pentose phosphate pathway. Cell. Mol. Life Sci. 2016, 73, 1333–1348. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Baselet, B.; Sonveaux, P. Metabolic changes associated with tumor metastasis, part 2: Mitochondria, lipid and amino acid metabolism. Cell. Mol. Life Sci. 2016, 73, 1349–1363. [Google Scholar] [CrossRef] [PubMed]
- Unterlass, J.E.; Curtin, N.J. Warburg and Krebs and related effects in cancer. Expert Rev. Mol. Med. 2019, 21, e4. [Google Scholar] [CrossRef]
- Warburg, O. The Metabolism of Carcinoma Cells. J. Cancer Res. 1925, 9, 148–163. [Google Scholar] [CrossRef] [Green Version]
- Walenta, S.; Wetterling, M.; Lehrke, M.; Schwickert, G.; Sundfor, K.; Rofstad, E.K.; Mueller-Klieser, W. High lactate levels predict likelihood of metastases, tumor recurrence, and restricted patient survival in human cervical cancers. Cancer Res. 2000, 60, 916–921. [Google Scholar] [PubMed]
- Harrison, C.; Yang, C.; Jindal, A.; DeBerardinis, R.J.; Hooshyar, M.A.; Merritt, M.; Sherry, A.D.; Malloy, C.R. Comparison of kinetic models for analysis of pyruvate-to-lactate exchange by hyperpolarized 13C NMR. NMR Biomed. 2012, 25, 1286–1294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witney, T.H.; Kettunen, M.I.; Brindle, K.M. Kinetic Modeling of Hyperpolarized C-13 Label Exchange between Pyruvate and Lactate in Tumor Cells. J. Biol. Chem. 2011, 286, 24572–24580. [Google Scholar] [CrossRef] [Green Version]
- Keshari, K.R.; Sriram, R.; Van Criekinge, M.; Wilson, D.M.; Wang, Z.J.; Vigneron, D.B.; Peehl, D.M.; Kurhanewicz, J. Metabolic reprogramming and validation of hyperpolarized 13C lactate as a prostate cancer biomarker using a human prostate tissue slice culture bioreactor. Prostate 2013, 73, 1171–1181. [Google Scholar] [CrossRef] [Green Version]
- Harris, T.; Degani, H.; Frydman, L. Hyperpolarized 13C NMR studies of glucose metabolism in living breast cancer cell cultures. NMR Biomed. 2013, 26, 1831–1843. [Google Scholar] [CrossRef]
- Scroggins, B.T.; Matsuo, M.; White, A.O.; Saito, K.; Munasinghe, J.P.; Sourbier, C.; Yamamoto, K.; Diaz, V.; Takakusagi, Y.; Ichikawa, K.; et al. Hyperpolarized [1-13C]-Pyruvate Magnetic Resonance Spectroscopic Imaging of Prostate Cancer In Vivo Predicts Efficacy of Targeting the Warburg Effect. Clin. Cancer Res. 2018, 24, 3137–3148. [Google Scholar] [CrossRef] [Green Version]
- Golman, K.; Zandt, R.i.t.; Lerche, M.; Pehrson, R.; Ardenkjaer-Larsen, J.H. Metabolic Imaging by Hyperpolarized 13C Magnetic Resonance Imaging for In vivo Tumor Diagnosis. Cancer Res. 2006, 66, 10855–10860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboagye, E.O.; Mori, N.; Bhujwalla, Z.M. Effect of malignant transformation on lactate levels of human mammary epithelial cells. Adv. Enzym. Regul. 2001, 41, 251–260. [Google Scholar] [CrossRef]
- Koukourakis, M.I.; Giatromanolaki, A.; Sivridis, E. Lactate dehydrogenase isoenzymes 1 and 5: Differential expression by neoplastic and stromal cells in non-small cell lung cancer and other epithelial malignant tumors. Tumour Biol. J. Int. Soc. Oncodevelopmental. Biol. Med. 2003, 24, 199–202. [Google Scholar] [CrossRef]
- Payen, V.L.; Mina, E.; Van Hée, V.F.; Porporato, P.E.; Sonveaux, P. Monocarboxylate transporters in cancer. Mol. Metab. 2019, 33, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Price, N.T. The proton-linked monocarboxylate transporter (MCT) family: Structure, function and regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef]
- Pérez-Escuredo, J.; Van Hée, V.F.; Sboarina, M.; Falces, J.; Payen, V.L.; Pellerin, L.; Sonveaux, P. Monocarboxylate transporters in the brain and in cancer. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2016, 1863, 2481–2497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, T.; Eliyahu, G.; Frydman, L.; Degani, H. Kinetics of hyperpolarized 13C1-pyruvate transport and metabolism in living human breast cancer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 18131–18136. [Google Scholar] [CrossRef] [Green Version]
- Lodi, A.; Woods, S.M.; Ronen, S.M. Treatment with the MEK inhibitor U0126 induces decreased hyperpolarized pyruvate to lactate conversion in breast, but not prostate, cancer cells. NMR Biomed. 2013, 26, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Hussien, R.; Brooks, G.A. Mitochondrial and Plasma Membrane Lactate Transporter and Lactate Dehydrogenase Isoform Expression in Breast Cancer Cell Lines. Physiol. Genom. 2011, 43, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, C.; Longatto-Filho, A.; Azevedo-Silva, J.; Casal, M.; Schmitt, F.; Baltazar, F. Role of monocarboxylate transporters in human cancers: State of the art. J. Bioenerg. Biomembr. 2012, 44, 127–139. [Google Scholar] [CrossRef]
- Contreras-Baeza, Y.; Sandoval, P.; Alarcón, R.; Galaz, A.; Cortés-Molina, F.; Alegría, K.; Baeza-Lehnert, F.; Arce-Molina, R.; Guequen, A.; Flores, C.; et al. Monocarboxylate transporter 4 (MCT4) is a high affinity transporter capable of exporting lactate in high-lactate microenvironments. J. Biol. Chem. 2019, 294, 20135–20147. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S.M.; Castorino, J.J.; Wang, D.; Philp, N.J. Monocarboxylate transporter 4 regulates maturation and trafficking of CD147 to the plasma membrane in the metastatic breast cancer cell line MDA-MB-231. Cancer Res. 2007, 67, 4182–4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baenke, F.; Dubuis, S.; Brault, C.; Weigelt, B.; Dankworth, B.; Griffiths, B.; Jiang, M.; Mackay, A.; Saunders, B.; Spencer-Dene, B.; et al. Functional screening identifies MCT4 as a key regulator of breast cancer cell metabolism and survival. J. Pathol. 2015, 237, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Rao, Y.; Gammon, S.; Zacharias, N.M.; Liu, T.; Salzillo, T.; Xi, Y.; Wang, J.; Bhattacharya, P.; Piwnica-Worms, D. Hyperpolarized [1-13C]pyruvate-to-[1-13C]lactate conversion is rate-limited by monocarboxylate transporter-1 in the plasma membrane. Proc. Natl. Acad. Sci. USA 2020, 117, 22378–22389. [Google Scholar] [CrossRef]
- Elia, I.; Haigis, M.C. Metabolites and the tumour microenvironment: From cellular mechanisms to systemic metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [CrossRef]
- Schaefer, C.; Mayer, W.K.; Kruger, W.; Vaupel, P. Microregional distributions of glucose, lactate, ATP and tissue pH in experimental tumours upon local hyperthermia and/or hyperglycaemia. J. Cancer Res. Clin. Oncol. 1993, 119, 599–608. [Google Scholar] [CrossRef]
- Kallinowski, F.; Runkel, S.; Fortmeyer, H.P.; Forster, H.; Vaupel, P. L-glutamine: A major substrate for tumor cells in vivo? J. Cancer Res. Clin. Oncol. 1987, 113, 209–215. [Google Scholar] [CrossRef]
- Otto, A.M.; Hintermair, J.; Janzon, C. NADH-Linked Metabolic Plasticity of MCF-7 Breast Cancer Cells Surviving in a Nutrient-Deprived Microenvironment. J. Cell Biochem. 2015, 116, 822–835. [Google Scholar] [CrossRef]
- Gkiouli, M.; Biechl, P.; Eisenreich, W.; Otto, A.M. Diverse Roads Taken by 13C-Glucose-Derived Metabolites in Breast Cancer Cells Exposed to Limiting Glucose and Glutamine Conditions. Cells 2019, 8, 1113. [Google Scholar] [CrossRef] [Green Version]
- Otto, A.M. Metabolic Constants and Plasticity of Cancer Cells in a Limiting Glucose and Glutamine Microenvironment—A Pyruvate Perspective. Front. Oncol. 2020, 10, 596197. [Google Scholar] [CrossRef]
- Butler, W.B. Preparing nuclei from cells in monolayer cultures suitable for counting and for following synchronized cells through the cell cycle. Anal. Biochem. 1984, 141, 70–73. [Google Scholar] [CrossRef]
- Maughan, R.J. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-µL blood sample. Clin. Chim. Acta 1982, 122, 231–240. [Google Scholar] [CrossRef]
- Hundshammer, C.; Braeuer, M.; Müller, C.A.; Hansen, A.E.; Schillmaier, M.; Düwel, S.; Feuerecker, B.; Glaser, S.J.; Haase, A.; Weichert, W.; et al. Simultaneous characterization of tumor cellularity and the Warburg effect with PET, MRI and hyperpolarized 13C-MRSI. Theranostics 2018, 8, 4765–4780. [Google Scholar] [CrossRef] [PubMed]
- Schilling, F.; Duwel, S.; Kollisch, U.; Durst, M.; Schulte, R.F.; Glaser, S.J.; Haase, A.; Otto, A.M.; Menzel, M.I. Diffusion of hyperpolarized 13C-metabolites in tumor cell spheroids using real-time NMR spectroscopy. NMR Biomed. 2013, 26, 557–568. [Google Scholar] [CrossRef]
- Chen, F.; Rydzewski, K.; Kutzner, E.; Häuslein, I.; Schunder, E.; Wang, X.; Meighen-Berger, K.; Grunow, R.; Eisenreich, W.; Heuner, K. Differential substrate usage and metabolic fluxes in Francisella tularensis subspecies holoarctica and Francisella novocida. Front. Cell Infect. Microbiol. 2017, 7, 275. [Google Scholar] [CrossRef]
- Ahmed, Z.; Zeeshan, S.; Huber, C.; Hensel, M.; Schomburg, D.; Münch, R.; Eylert, E.; Eisenreich, W.; Dandekar, T. ‘Isotopo’ a database application for facile analysis and management of mass isotopomer data. Database J. Biol. Databases Curation 2014, 2014, bau077. [Google Scholar] [CrossRef] [Green Version]
- Vaux, D.L. Know when your numbers are significant. Nature 2012, 492, 180. [Google Scholar] [CrossRef]
- Düwel, S.; Hundshammer, C.; Gersch, M.; Feuerecker, B.; Steiger, K.; Buck, A.; Walch, A.; Haase, A.; Glaser, S.J.; Schwaiger, M.; et al. Imaging of pH in vivo using hyperpolarized 13C-labelled zymonic acid. Nat. Commun. 2017, 8, 15126. [Google Scholar] [CrossRef] [Green Version]
- Talaiezadeh, A.; Shahriari, A.; Tabandeh, M.R.; Fathizadeh, P.; Mansouri, S. Kinetic characterization of lactate dehydrogenase in normal and malignant human breast tissues. Cancer Cell Int. 2015, 15, 19. [Google Scholar] [CrossRef] [Green Version]
- Goto, T.; Sugawara, K.; Nakamura, S.; Kidokoro, S.I.; Wakui, H.; Nunomura, W. Enzymatic and thermodynamic profiles of a heterotetramer lactate dehydrogenase isozyme in swine. Biochem. Biophys. Res. Commun. 2016, 479, 860–867. [Google Scholar] [CrossRef]
- Halestrap, A.P. Monocarboxylic Acid Transport. Compr. Physiol. 2013, 3, 1611–1643. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, L.; Halestrap, A.P. The kinetics, substrate and inhibitor specificity of the lactate transporter of Ehrlich-Lettre tumour cells studied with the intracellular pH indicator BCECF. Biochem. J. 1994, 304, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edlund, G.L.; Halestrap, A.P. The kinetics of transport of lactate and pyruvate into rat hepatocytes. Evidence for the presence of a specific carrier similar to that in erythrocytes. Biochem. J. 1988, 249, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaglio, D.; Metallo, C.M.; Gameiro, P.A.; Hiller, K.; Danna, L.S.; Balestrieri, C.; Alberghina, L.; Stephanopoulos, G.; Chiaradonna, F. Oncogenic K-Ras decouples glucose and glutamine metabolism to support cancer cell growth. Mol. Syst. Biol. 2011, 7, 523. [Google Scholar] [CrossRef]
- Neermann, J.; Wagner, R. Comparative analysis of glucose and glutamine metabolism in transformed mammalian cell lines, insect and primary liver cells. J. Cell. Physiol. 1996, 166, 152–169. [Google Scholar] [CrossRef]
- Schwartz, J.P.; Passonne, J.V.; Johnson, G.S.; Pastan, I. Effect of growth-conditions on NAD+ and NADH concentrations and NAD+:NADH ratio in normal and transformed fibroblasts. J. Biol. Chem. 1974, 249, 4138–4143. [Google Scholar] [CrossRef]
- Otto, A.M.; Müller, C.S.G.; Huff, T.; Hannappel, E. Chemotherapeutic drugs change actin skeleton organization and the expression of beta-thymosins in human breast cancer cells. J. Cancer Res. Clin. Oncol. 2002, 128, 247–256. [Google Scholar] [CrossRef]
- Phannasil, P.; Ansari, I.-u.H.; El Azzouny, M.; Longacre, M.J.; Rattanapornsompong, K.; Burant, C.F.; MacDonald, M.J.; Jitrapakdee, S. Mass spectrometry analysis shows the biosynthetic pathways supported by pyruvate carboxylase in highly invasive breast cancer cells. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2017, 1863, 537–551. [Google Scholar] [CrossRef]
- Xu, H.N.; Kadlececk, S.; Profka, H.; Glickson, J.D.; Rizi, R.; Li, L.Z. Is higher lactate an indicator of tumor metastatic risk? A pilot MRS study using hyperpolarized 13C-pyruvate. Acad. Radiol. 2014, 21, 223–231. [Google Scholar] [CrossRef] [Green Version]
- Macdonald, E.B.; Begovatz, P.; Barton, G.P.; Erickson-Bhatt, S.; Inman, D.R.; Cox, B.L.; Eliceiri, K.W.; Strigel, R.M.; Ponik, S.M.; Fain, S.B. Hyperpolarized 13C Magnetic Resonance Spectroscopic Imaging of Pyruvate Metabolism in Murine Breast Cancer Models of Different Metastatic Potential. Metabolites 2021, 11, 274. [Google Scholar] [CrossRef]
- Witney, T.H.; Kettunen, M.I.; Hu, D.E.; Gallagher, F.A.; Bohndiek, S.E.; Napolitano, R.; Brindle, K.M. Detecting treatment response in a model of human breast adenocarcinoma using hyperpolarised [1-13C]pyruvate and [1,4-13C2]fumarate. Br. J. Cancer 2010, 103, 1400–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalezic, A.; Udicki, M.; Srdic Galic, B.; Aleksic, M.; Korac, A.; Jankovic, A.; Korac, B. Lactate Metabolism in Breast Cancer Microenvironment: Contribution Focused on Associated Adipose Tissue and Obesity. Int. J. Mol. Sci. 2020, 21, 9676. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, F.A.; Woitek, R.; McLean, M.A.; Gill, A.B.; Manzano Garcia, R.; Provenzano, E.; Riemer, F.; Kaggie, J.; Chhabra, A.; Ursprung, S.; et al. Imaging breast cancer using hyperpolarized carbon-13 MRI. Proc. Natl. Acad. Sci. USA 2020, 117, 2092–2098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prochownik, E.V.; Wang, H. The Metabolic Fates of Pyruvate in Normal and Neoplastic Cells. Cells 2021, 10, 762. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, E.; Fahimi, H.D.; Stich, A.; Völkl, A. L-lactate dehydrogenase A4- and A3B isoforms are bona fide peroxisomal enzymes in rat liver. Evidence for involvement in intraperoxisomal NADH reoxidation. J. Biol. Chem. 1996, 271, 3846–3855. [Google Scholar] [CrossRef] [Green Version]
- McClelland, G.B.; Khanna, S.; González, G.F.; Butz, C.E.; Brooks, G.A. Peroxisomal membrane monocarboxylate transporters: Evidence for a redox shuttle system? Biochem. Biophys. Res. Commun. 2003, 304, 130–135. [Google Scholar] [CrossRef]
- Pizzuto, R.; Paventi, G.; Porcile, C.; Sarnataro, D.; Daniele, A.; Passarella, S. L-Lactate metabolism in HEP G2 cell mitochondria due to the L-lactate dehydrogenase determines the occurrence of the lactate/pyruvate shuttle and the appearance of oxaloacetate, malate and citrate outside mitochondria. Biochim. Biophys. Acta (BBA) Bioenerg. 2012, 1817, 1679–1690. [Google Scholar] [CrossRef] [Green Version]
- Brooks, G.A. Cell-cell and intracellular lactate shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef]
- Pienta, K.J.; Hoover, C.N. Coupling of cell structure to cell metabolism and function. J. Cell Biochem. 1994, 55, 16–21. [Google Scholar] [CrossRef]
- Kohnhorst, C.L.; Kyoung, M.; Jeon, M.; Schmitt, D.L.; Kennedy, E.L.; Ramirez, J.; Bracey, S.M.; Luu, B.T.; Russell, S.J.; An, S. Identification of a multienzyme complex for glucose metabolism in living cells. J. Biol. Chem. 2017, 292, 9191–9203. [Google Scholar] [CrossRef] [Green Version]
- Ovadi, J.; Saks, V. On the origin of intracellular compartmentation and organized metabolic systems. Mol. Cell. Biochem. 2004, 256, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Serrao, E.M.; Rodrigues, T.B.; Gallagher, F.A.; Kettunen, M.I.; Kennedy, B.W.C.; Vowler, S.L.; Burling, K.A.; Brindle, K.M. Effects of fasting on serial measurements of hyperpolarized [1-13C]pyruvate metabolism in tumors. NMR Biomed. 2016, 29, 1048–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topping, G.J.; Heid, I.; Trajkovic-Arsic, M.; Kritzner, L.; Grashei, M.; Hundshammer, C.; Aigner, M.; Skinner, J.G.; Braren, R.; Schilling, F. Hyperpolarized 13C Spectroscopy with Simple Slice-and-Frequency-Selective Excitation. Biomedicines 2021, 9, 121. [Google Scholar] [CrossRef] [PubMed]
- Feuerecker, B.; Durst, M.; Michalik, M.; Schneider, G.; Saur, D.; Menzel, M.; Schwaiger, M.; Schilling, F. Hyperpolarized 13C Diffusion MRS of Co-Polarized Pyruvate and Fumarate to Measure Lactate Export and Necrosis. J. Cancer 2017, 8, 3078–3085. [Google Scholar] [CrossRef] [Green Version]
- Qin, H.; Tang, S.; Riselli, A.M.; Bok, R.A.; Delos Santos, R.; van Criekinge, M.; Gordon, J.W.; Aggarwal, R.; Chen, R.; Goddard, G.; et al. Clinical translation of hyperpolarized 13C pyruvate and urea MRI for simultaneous metabolic and perfusion imaging. Magn. Reson. Med. 2022, 87, 138–149. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grashei, M.; Biechl, P.; Schilling, F.; Otto, A.M. Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment. Cancers 2022, 14, 1845. https://doi.org/10.3390/cancers14071845
Grashei M, Biechl P, Schilling F, Otto AM. Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment. Cancers. 2022; 14(7):1845. https://doi.org/10.3390/cancers14071845
Chicago/Turabian StyleGrashei, Martin, Philipp Biechl, Franz Schilling, and Angela M. Otto. 2022. "Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment" Cancers 14, no. 7: 1845. https://doi.org/10.3390/cancers14071845
APA StyleGrashei, M., Biechl, P., Schilling, F., & Otto, A. M. (2022). Conversion of Hyperpolarized [1-13C]Pyruvate in Breast Cancer Cells Depends on Their Malignancy, Metabolic Program and Nutrient Microenvironment. Cancers, 14(7), 1845. https://doi.org/10.3390/cancers14071845





