Assessment of Fertilizer Quality in Horse Waste-Based Bokashi Fertilizer Formulations
Abstract
:1. Introduction
2. Materials and Methods
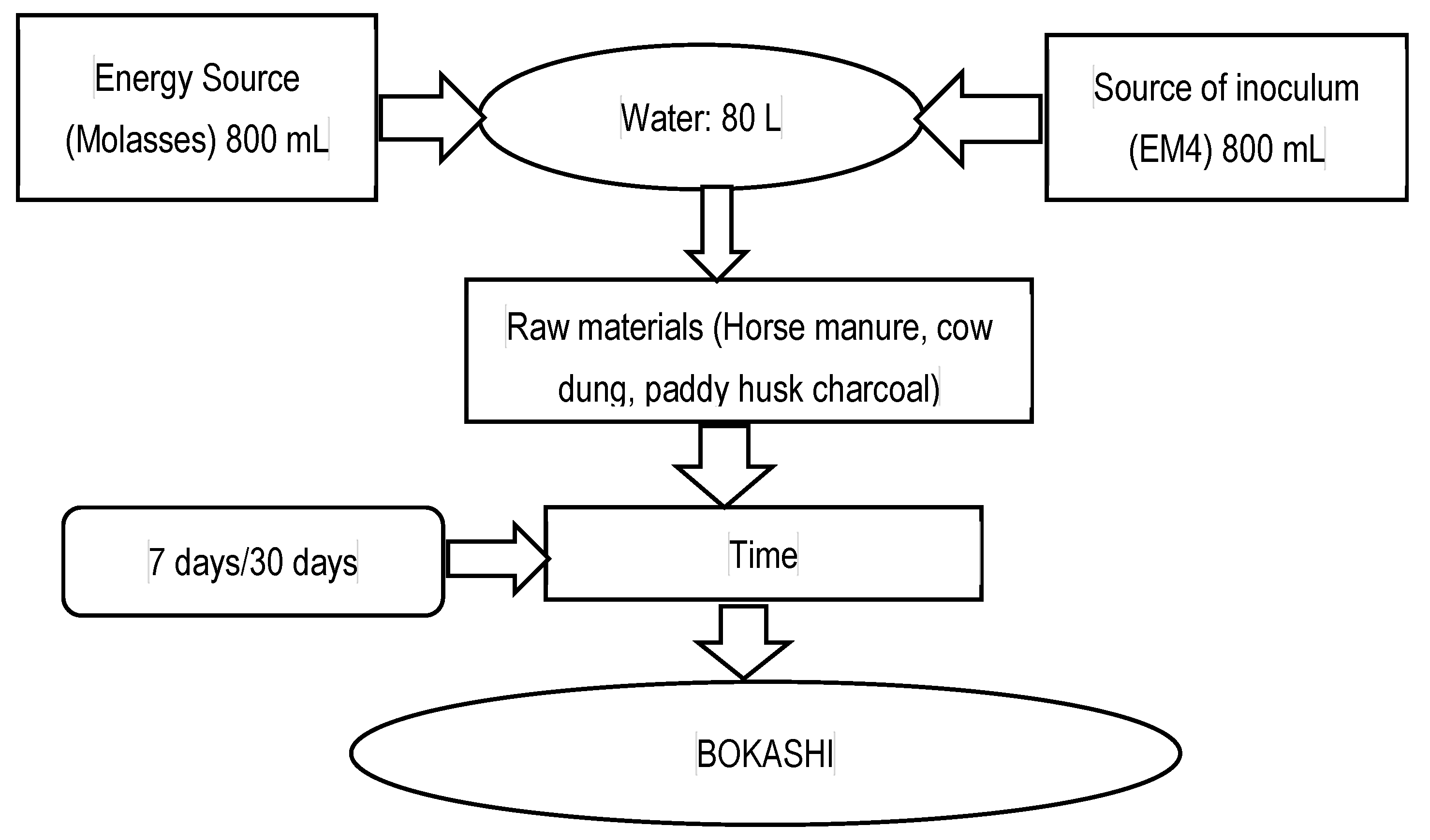
2.1. Materials Used, Their Sources, and the Bokashi Fertilizer Preparation
2.2. Bokashi Preparation
2.3. Location and Layout of the Experiment
2.4. Physical Analysis of the Bokashi Fertilizer
2.5. Chemical Analysis of the Bokashi Fertilizer
2.6. The Seed Germination Bioassay
- w/a = relative seed germination (RSG) in 50% bokashi extract,
- x/a = relative seed germination (RSG) in 75% bokashi extract,
- y/b = relative radicle growth (RRG) in 50% bokashi extract,
- z/b = relative radicle growth (RRG) in 75% bokashi extract.
2.7. Statistical Analysis
3. Results
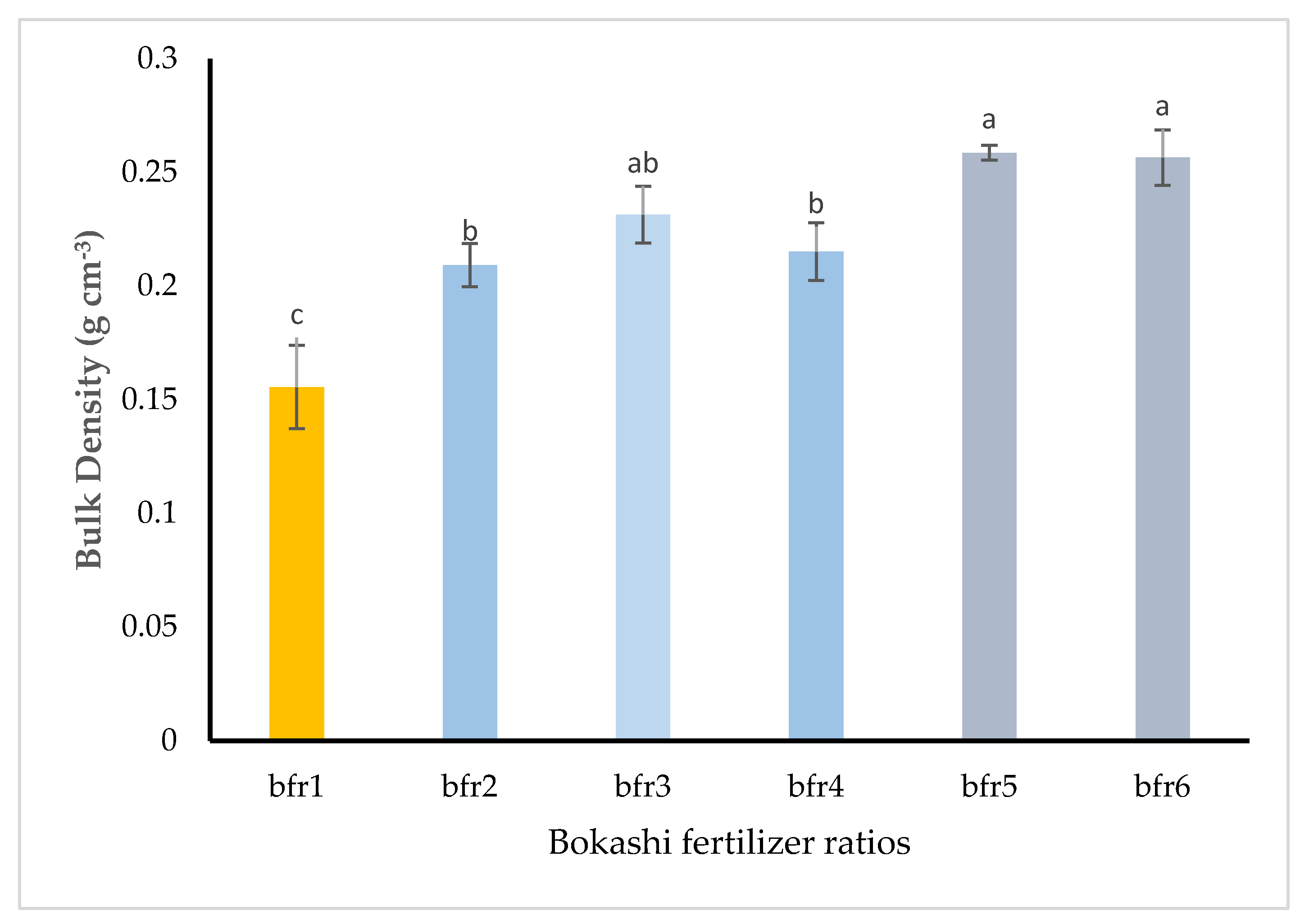
3.1. Physical Properties of Bokashi Fertilizer Ratios
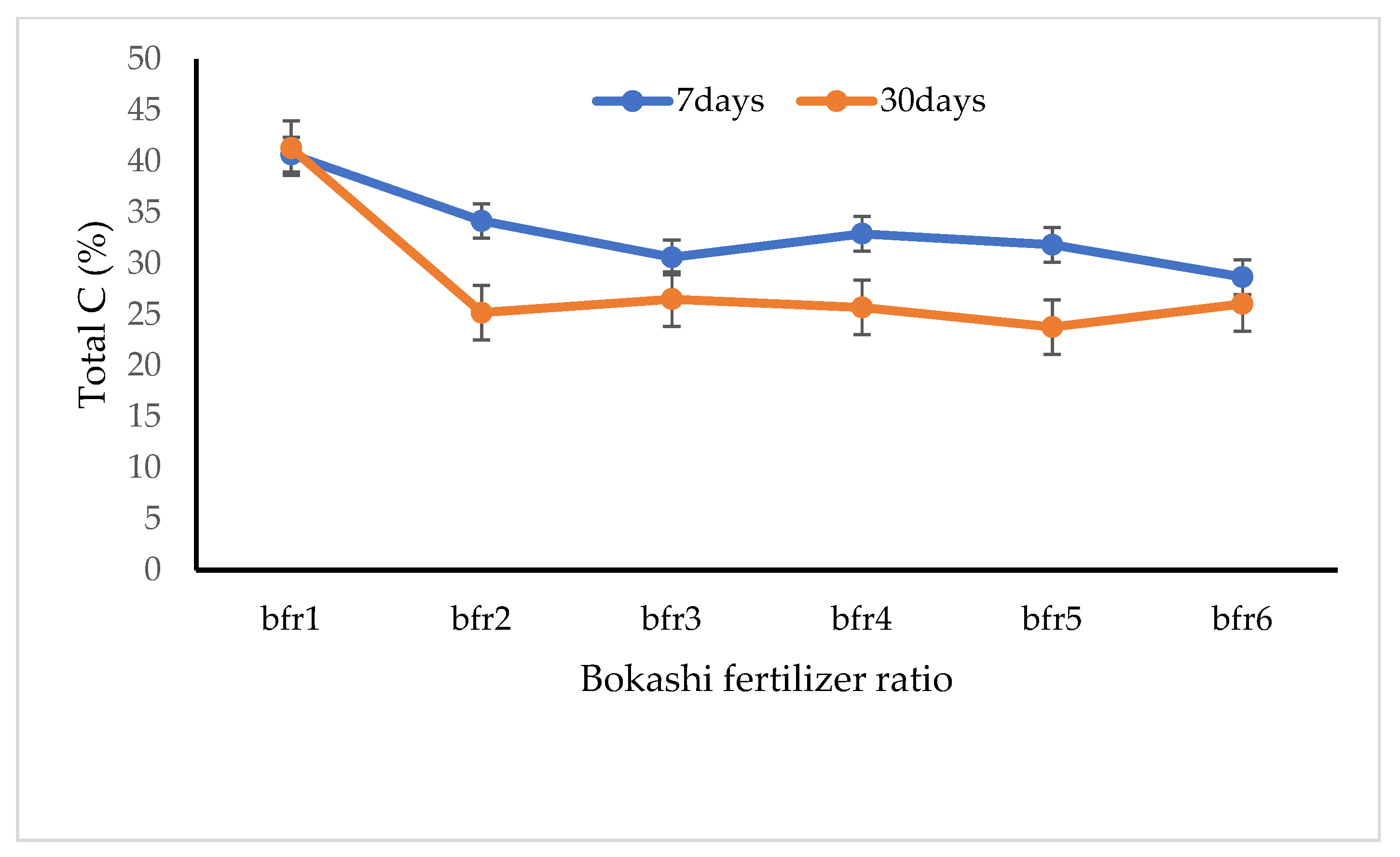
3.2. Chemical Properties of Bokashi Fertilizer
3.3. Germination Bioassay for the Bokashi Fertilizers Using Cabbage Seeds
4. Discussion
4.1. The Physical Characteristics of Horse Waste-Based Bokashi Fertilizers
4.2. The Chemical Characteristics of Horse Waste-Based Bokashi Fertilizer
4.3. Germination Bioassay for the Bokashi Fertilizers
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dinesh, R.; Srinivasan, V.; Hamza, S.; Manjusha, A. Bioresource Technology Short-term incorporation of organic manures and biofertilizers influences biochemical and microbial characteristics of soils under an annual crop [Turmeric (Curcuma longa L.)]. Bioresour. Technol. 2010, 101, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Urra, J.; Alkorta, I.; Lanzén, A.; Mijangos, I.; Garbisu, C. The application of fresh and composted horse and chicken manure affects soil quality, microbial composition and antibiotic resistance. Appl. Soil Ecol. 2019, 135, 73–84. [Google Scholar] [CrossRef]
- Zamri, N.S.; Khairul, M.; Kamarudin, A.; Armi, M.; Samah, A.; Shakir, A.; Saudi, M.; Wahab, N.A.; Hafiz, M. The environmental pollution and solid waste m anagement in Malaysia. Int. J. Acad. Res. Bus. Soc. Sci. 2019, 9, 12–23. [Google Scholar] [CrossRef]
- Ahmad, A.; Sulaiman, N.F. Save The Food for A Better Future: A Discussion on Food Wastage in Malaysia. Int. J. Law, Gov. Commun. 2018, 3, 12–21. [Google Scholar]
- Al Abboud, M.A.; Ghany, T.A.; Alawlaqi, M.M. Role of biofertilizers in agriculture: A brief review. Mycopath 2013, 11, 95–101. [Google Scholar]
- Wijayanto, T.; Zulfikar, M.; Tufaila, M.; Alam, S.M.; Zamrun, M.F. Agricultural Wastes based-Organic Fertilizers (Bokashi) Improve the Growth and Yield of Soybean (Glycine max (L.) Merrill). Int. J. Agric. Sci. 2016, 1, 27–32. [Google Scholar]
- Alam, S.; Seth, R.K. Comparative Study on Effect of Chemical and Bio- Fertilizer on Growth, Development and Yield Production of Paddy crop (Oryza sativa). Int. J. Sci. Res. ISSN 2012, 3, 2319–7064. [Google Scholar]
- Olle, M.; Williams, I.H. Effective microorganisms and their influence on vegetable production—A review. J. Hortic. Sci. Biotechnol. 2013, 88, 380–386. [Google Scholar] [CrossRef]
- Pan, I.; Dam, B.; Sen, S.K. Composting of common organic wastes using microbial inoculants. 3 Biotech 2012, 2, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Sharma, R.; Arora, A.; Shah, R.; Singh, A.; Pranaw, K.; Nain, L. Insights into rapid composting of paddy straw augmented with efficient microorganism consortium. Int. J. Recycl. Org. Waste Agric. 2014, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lasmini, S.A.; Nasir, B.; Hayati, N.; Edy, N. Improvement of soil quality using bokashi composting and NPK fertilizer to increase shallot yield on dry land. Aust. J. Crop Sci. 2018, 12, 1743–1749. [Google Scholar] [CrossRef]
- Zahrah, S. System of Rice Intensification. Encycl. Food Agric. Ethics 2019, 12, 2318. [Google Scholar] [CrossRef]
- Wakui, Y. Organic Farming Technology; The Japan Agricultural Exchange Council (JAEC): Tokyo, Japan, 2009. [Google Scholar]
- Lima, C.E.P.; Fontenelle, M.R.; Silva, L.R.B.; Soares, D.C.; Moita, A.W.; Zandonadi, D.B.; Souza, R.B.; Lopes, C.A. Short-term changes in fertility attributes and soil organic matter caused by the addition of em Bokashis in two tropical soils. Int. J. Agron. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Christel, D.M. The Use of Bokashi as a Soil Fertility Amendment in Organic Spinach Cultivation; University of Vermont: Burlington, VT, USA, 2017. [Google Scholar]
- Yamada, K.; Xu, H. Properties and Applications of an Organic Fertilizer Inoculated with Effective Microorganisms. J. Crop Prod. 2001, 3, 255–268. [Google Scholar] [CrossRef]
- Barus, W.A.; Utami, S.; Azmi, E.P. Effect of Azolla Bocachi and Liquid Organic Fertilizer of Goat Manure on the Growth and Production of Chinese Kale (Brassica oleracea L.). Indones. J. Agric. Res. 2018, 1, 78–86. [Google Scholar] [CrossRef]
- Gómez-Velasco, D.A.; Álvarez-Solís, J.D.; Ruiz-Valdiviezo, V.M.; Abud-Archila, M.; Montes-Molina, J.A.; Dendooven, L.; Gutiérrez-Miceli, F.A. Enzymatic Activities in Soil Cultivated with Coffee (Coffea arabica L. cv. ‘Bourbon’) and Amended with Organic Material. Commun. Soil Sci. Plant Anal. 2014, 45, 2529–2538. [Google Scholar] [CrossRef]
- Bocoli, F.A.; Marcon, J.A.; Izidoro, M.; Bortolon, P.D.T.; de Oliveira, S.E.R.; Spalevic, V.; de Souza, P.S. Bokashi use in the passionfruit (Passiflora edulis l.) germination and initial growth. Agric. For. 2020, 66, 101–111. [Google Scholar] [CrossRef]
- Ghanem, K.; El-Zabalawy, K.; Mustafa, A.; Elbanna, B. Impact of Using Compost Bokashi Resulting from Recycling Kitchen Waste on Head Lettuce (Lactuca sativa var. capitata L.) Grown Organically at Home. J. Soil Sci. Agric. Eng. 2017, 8, 21–27. [Google Scholar] [CrossRef]
- Quiroz, M.; Céspedes, C. Bokashi as an Amendment and Source of Nitrogen in Sustainable Agricultural Systems: A Review. J. Soil Sci. Plant Nutr. 2019, 19, 237–248. [Google Scholar] [CrossRef]
- Salisu, M.A.; Sulaiman, Z.; Rus, R.C.; Samad, M.Y.A. Water use efficiency, plant growth and vegetative traits of rubber (Hevea brasiliensis) seedlings grown using different growing media and water levels. Aust. J. Crop Sci. 2020, 14, 1497–1505. [Google Scholar] [CrossRef]
- Salisu, M.A.; Sulaiman, Z.; Samad, M.Y.A. Growth and biomass yield of rubber seedlings grown on soilless and soil-based media. Bangladesh J. Bot. 2020, 49, 229–236. [Google Scholar] [CrossRef]
- Abo-Sido, N. Analysis of the Nutrient Composition, Efficacy, and Sustainability of Bokashi Fertilizers; Wellesley College: Wellesley, MA, USA, 2018. [Google Scholar]
- Stevanus, C.T.; Bintarti, A.F.; Setiawan, N. The effect of oil palm frond-based compost as growing media amendment for rubber (Hevea Brasiliensis, Müll. Arg.) planting material. Malaysian J. Soil Sci. 2016, 20, 195–209. [Google Scholar]
- Amasuomo, E.; Baird, J. The Concept of Waste and Waste Management. J. Manag. Sustain. 2016, 6, 88. [Google Scholar] [CrossRef]
- Talukdar, P.; Baruah, M.; Saikia, P. Waste management for sustainable agricultural production—A brief review. Asian J. Home Sci. 2018, 13, 657–659. [Google Scholar] [CrossRef]
- Kebrom, T.H.; Woldesenbet, S.; Bayabil, H.K.; Garcia, M.; Gao, M.; Ampim, P.; Awal, R.; Fares, A. Evaluation of phytotoxicity of three organic amendments to collard greens using the seed germination bioassay. Environ. Sci. Pollut. Res. 2019, 26, 5454–5462. [Google Scholar] [CrossRef] [Green Version]
- Oktiawan, W.; Zaman, B. Use of a germination bioassay to test compost maturity in Tekelan Village. E3S Web Conf. 2018, 31, 05012. [Google Scholar] [CrossRef] [Green Version]
- Arora, M.; Kiran, B.; Rani, S.; Rani, A.; Kaur, B.; Mittal, N. Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem. 2008, 111, 811–815. [Google Scholar] [CrossRef]
- Mohammed, N.K.; Khamis, F.O. Assessment of heavy metal contamination in vegetables consumed in Zanzibars. Nat. Sci. 2012, 4, 588–594. [Google Scholar] [CrossRef] [Green Version]
- Hata, F.; Ventura, M.; Fregonezi, G.; Lima, R. Bokashi, boiled manure and penergetic applications increased agronomic production variables and may enhance powdery mildew severity of organic tomato plants. Horticulturae 2021, 7, 27. [Google Scholar] [CrossRef]
- Teh, C.B.S.; Talib, J. Soil and Plant Analyses Vol. I Soil Physics Analyses; UPM Press: Serdang, Malaysia, 2006; Volume 1, p. 42. [Google Scholar]
- Karanja, A.W.; Njeru, E.M.; Maingi, J.M. Assessment of physicochemical changes during composting rice straw with chicken and donkey manure. Int. J. Recycl. Org. Waste Agric. 2019, 8, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Chapman, H.D. Cation-exchange capacity. In Methods of Soil Analysis. Agronomy Monograph; Black, C.A., Ed.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 891–901. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An examination of the Degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Jones, J.J.B. Plants. In Official Methods of Analysis of the Association of Official Analytical Chemists; Williams, S., Ed.; Association of Official Analytical Chemists: Arlington, VA, USA, 1984; pp. 38–64. [Google Scholar]
- Gariglio, N.F.; Buyatti, M.A.; Pilatti, R.A.; Gonzalez, D.E.; Acosta, M.R.; Fe, S. New Zealand Journal of Crop and Horticultural Science Use of a germination bioassay to test compost maturity of willow (Salix sp.) sawdust. N. Z. J. Crop. Hortic. Sci. 2002, 30, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Khater, E.S.G. Some Physical and Chemical Properties of Compost. Int. J. Waste Resour. 2015, 5, 172. [Google Scholar] [CrossRef]
- Troy, S.M.; Nolan, T.; Kwapinski, W.; Leahy, J.J.; Healy, M.G.; Lawlor, P.G. Effect of sawdust addition on composting of separated raw and anaerobically digested pig manure. J. Environ. Manag. 2012, 111, 70–77. [Google Scholar] [CrossRef] [Green Version]
- Zaha, C.; Dumitrescu, L.; Manciulea, I. Correlations Between Composting Conditions and Characteristics of Compost As Biofertilizer. Bull. Transilv. Univ. Brasov. Eng. Sciences. Ser. I 2013, 6, 51. [Google Scholar]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.M.; Roh, A.S.; Choi, S.C.; Kim, E.J.; Choi, M.T.; Ahn, B.K.; Weon, H.Y. Soil pH and electrical conductivity are key edaphic factors shaping bacterial communities of greenhouse soils in Korea. J. Microbiol. 2016, 54, 838–845. [Google Scholar] [CrossRef]
- Noguera, P.; Abad, M.; Puchades, R.; Maquieira, A.; Noguera, V. Influence of particle size on physical and chemical properties of coconut coir dust as container medium. Commun. Soil Sci. Plant Anal. 2003, 34, 593–605. [Google Scholar] [CrossRef]
- Saharinen, M.H. Evaluation of changes in CEC during composting. Compost Sci. Util. 1998, 6, 29–37. [Google Scholar] [CrossRef]
- Hazelton, P.; Murphy, B. Interpreting Soil Test Results; CSIRO: Collingwood, Australia, 2016. [Google Scholar]
- Kala, D.R.; Rosenani, A.B.; Fauziah, C.I.; Thohirah, L.A. Composting oil palm wastes and sewage sludge for use in potting media of ornamental plants. Malaysian J. Soil Sci. 2009, 13, 77–91. [Google Scholar]
- Chinwuba, A.J.; Alisa, C.O.; Okoye, U.G. Comparative analysis of macro and micro nutrients of bio waste, Compost and Verm icompost (using indigenous Lumbricus terrestris) of Solanum melongena (fruit) waste. J. Sci. Eng. Res. 2017, 4, 102–104. [Google Scholar]
- Sullivan, D.M.; Bary, A.I.; Miller, R.O.; Brewer, L.J. Interpreting Compost Analyses; Oregon State University Extension: Corvallis, OR, USA, 2018. [Google Scholar]
- Manohara, B.; Belagali, S.L. Characterization of Essential Nutrients and Heavy Metals during Municipal Solid Waste. Int. J. Innov. Res. Sci. 2014, 3, 9664–9672. [Google Scholar]
- Raviv, M.; Tarre, S.; Geler, Z.; Shelef, G. Changes in some physical and chemical properties of fibrous solids from cow manure and digested cow manure during composting. Biol. Wastes 1987, 19, 309–318. [Google Scholar] [CrossRef]
- Lew, P.S.; Nik Ibrahim, N.N.L.; Kamarudin, S.; Thamrin, N.M.; Misnan, M.F. Optimization of bokashi-composting process using effective microorganisms-1 in smart composting bin. Sensors 2021, 21, 2847. [Google Scholar] [CrossRef]
- Sarkar, P.; Chourasia, R. Bioconversion of organic solid wastes into biofortified compost using a microbial consortium. Int. J. Recycl. Org. Waste Agric. 2017, 6, 321–334. [Google Scholar] [CrossRef] [Green Version]
- Compost Compost Report Interpretation Guide. Available online: https://umaine.edu/soiltestinglab/wp-content/uploads/sites/227/2016/07/Compost-Report-Interpretation-Guide.pdf (accessed on 22 July 2021).
- Abu-zahra, T.R.; Ta’any, R.A.; Arabiyyat, A.R. Changes in compost physical and chemical properties during aerobic decomposition. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 479–486. [Google Scholar]
- Álvarez-Solís, J.D.; Mendoza-Núñez, J.A.; León-Martínez, N.S.; Castellanos-Albores, J.; Gutiérrez-Miceli, F.A. Effect of bokashi and vermicompost leachate on yield and quality of pepper (Capsicum annuum) and onion (Allium cepa) under monoculture and intercropping cultures. Cienc. E Investig. Agrar. 2016, 43, 243–252. [Google Scholar] [CrossRef] [Green Version]
- Tratsch, M.V.M.; Ceretta, C.A.; da Silva, L.S.; Ferreira, P.A.A.; Brunetto, G. Composition and mineralization of organic compost derived from composting of fruit and vegetable waste. Rev. Ceres 2019, 66, 307–315. [Google Scholar] [CrossRef]
- Gohardoust, M.R.; Bar-Tal, A.; Effati, M.; Tuller, M. Characterization of physicochemical and hydraulic properties of organic and mineral soilless culture substrates and mixtures. Agronomy 2020, 10, 1403. [Google Scholar] [CrossRef]
- Bernal, M.P.; Alburquerque, J.A.; Moral, R. Composting of animal manures and chemical criteria for compost maturity assessment. A review. Bioresour. Technol. 2009, 100, 5444–5453. [Google Scholar] [CrossRef]
- Tiquia, S.M.; Tam, N.F.Y.; Hodgkiss, I.J. Effects of composting on phytotoxicity of spent pig-manure sawdust litter. Environ. Pollut. 1996, 93, 249–256. [Google Scholar] [CrossRef]
- Brinton, W.F. Compost Quality Standards & Guidelines; Woods End Research Laboratory, Inc.: New York, NY, USA, 2000. [Google Scholar]
- França, F.C.T.; Pedrosa, M.W.; Carlos, L.D.A.; Maciel, G.M.; Da Silva, E.C. Tomato yield and quality under various combinations of organic compost. Biosci. J. 2016, 32, 1147–1154. [Google Scholar] [CrossRef] [Green Version]
- Amlinger, D.F.; Pollak, D.M.; Favoino, E. Heavy Metals and Organic Compounds from Wastes Used as Organic Fertilisers; Compost-Legislation and Standard: Vienna, Austria, 2004. [Google Scholar]
- Selim, S.M.; Zayed, M.S.; Houssam, M.A. Evaluation of phytotoxicity of compost during composting process. Nat. Sci. 2012, 10, 69–77. [Google Scholar]
- Luo, Y.; Liang, J.; Zeng, G.; Chen, M.; Mo, D.; Li, G.; Zhang, D. Seed germination test for toxicity evaluation of compost: Its roles, problems and prospects. Waste Manag. 2018, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]





| Total Plate Count | Cells/mL |
|---|---|
| Phosphate solubilizing bacteria | 3.4 × 106 |
| Lactobacillus bacteria | 3.0 × 105 |
| Yeast | 1.95 × 103 |
| Actinomycetes | + |
| Photosynthetic bacteria | + |
| Escherichia coli | 0 |
| Salmonella | 0 |
| Factors | pH | EC (mS cm−1) | CEC (Cmol kg−1) |
|---|---|---|---|
| BFR | |||
| bfr1 | 7.30 ± 0.05 | 0.27 ± 0.02 d | 15.34 ± 0.86 c |
| bfr2 | 7.28 ± 0.18 | 0.69 ± 0.07 c | 15.46 ± 1.69 bc |
| bfr3 | 7.35 ± 0.10 | 1.09 ± 0.10 b | 18.42 ± 1.03 ab |
| bfr4 | 7.55 ± 0.09 | 0.69 ± 0.07 c | 18.01 ± 0.93 abc |
| bfr5 | 7.39 ± 0.19 | 1.18 ± 0.11 b | 20.59 ± 0.60 a |
| bfr6 | 7.50 ± 0.16 | 1.51 ± 0.09 a | 19.66 ± 1.16 a |
| p-value | 0.5848 | <0.0001 | <0.0001 |
| TMBF (days) | |||
| 7 | 7.55 ± 0.05 a | 0.79 ± 0.10 b | 16.09 ± 0.70 b |
| 30 | 7.24 ± 0.08 b | 1.02 ± 0.11 a | 19.73 ± 0.53 a |
| p-value | 0.0061 | 0.0003 | <0.0001 |
| BFR × TMBF | 0.3629 | 0.6872 | 0.0562 |
| Factors | Ca (Cmol kg−1) | Mg (Cmol kg−1) | K (Cmol kg−1) | Na (Cmol kg−1) |
|---|---|---|---|---|
| BFR | ||||
| bfr1 | 8.28 ± 0.35 c | 4.23 ± 0.30 c | 6.98 ± 0.36 c | 1.32 ± 0.15 d |
| bfr2 | 14.01 ± 0.58 b | 7.15 ± 0.59 b | 9.73 ± 0.54 bc | 3.35 ± 0.23 c |
| bfr3 | 16.93 ± 1.01 a | 8.86 ± 0.32 a | 22.21 ± 0.79 a | 5.25 ± 0.11 ab |
| bfr4 | 14.15 ± 0.74 b | 7.39 ± 0.69 b | 10.51 ± 0.71 b | 3.18 ± 0.24 c |
| bfr5 | 15.52 ±0.26 ab | 8.58 ± 0.50 a | 20.26 ± 2.38 a | 4.79 ± 0.23 b |
| bfr6 | 15.29 ± 0.80 ab | 8.67 ± 0.55 a | 22.69 ± 1.11 a | 5.48 ± 0.33 a |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| TMBF (days) | ||||
| 7 | 14.20 ± 0.89 | 6.54 ± 0.41 b | 14.23 ± 1.65 b | 3.57 ± 0.37 b |
| 30 | 13.86 ± 0.61 | 8.42 ± 0.43 a | 16.56 ± 1.69 a | 4.22 ± 0.36 a |
| BFR × TMBF | 0.1264 | 0.3939 | 0.8285 | 0.8996 |
| Factors | Total N (%) | Total P (%) | OC (%) | OM (%) |
|---|---|---|---|---|
| BFR | ||||
| bfr1 | 0.59 ± 0.02 d | 0.055 ± 0.01d | 38.32 ± 1.54 a | 66.28 ± 2.66 a |
| bfr2 | 0.96 ± 0.07 c | 0.109 ± 0.01 c | 33.95 ± 0.98 ab | 58. 74 ± 1.70 ab |
| bfr3 | 1.25 ± 0.09 ab | 0.151 ± 0.01 ab | 32.95 ± 0.65 bc | 55. 98 ± 1.13 bc |
| bfr4 | 1.10 ± 0.07 c | 0.127 ± 0.01 bc | 30.87 ± 1.48 bc | 53.39 ± 2.57 bc |
| bfr5 | 1.33 ± 0.02 a | 0.161 ± 0.01 a | 30.45 ± 2.26 bc | 52.68 ± 3.92 bc |
| bfr6 | 1.38 ± 0.07 a | 0.179 ± 0.02 a | 28.15 ± 2.48 c | 48.69 ± 4.30 c |
| p-value | <0.0001 | <0.0001 | 0.0001 | 0.0001 |
| SE | 0.038 | 0.0076 | 1.193 | 2.064 |
| TMBF (days) | ||||
| 7 | 0.99 ± 0.06 b | 0.112 ± 0.01 b | 34.78 ± 0.74 a | 60.16 ± 1.28 a |
| 30 | 1.20 ± 0.07 a | 0.149 ± 0.01 a | 29.92 ± 1.29 b | 51.76 ± 2.23 b |
| p-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| BFR × TMBF | 0.0791 | 0.4286 | 0.3039 | 0.3041 |
| Factor | Cd (mg/kg) | Cr (mg/kg) | Cu (mg/kg) | Ni (mg/kg) | Pb (mg/kg) |
|---|---|---|---|---|---|
| BFR | |||||
| bfr1 | 0.08 ± 0.03 c | 7.63 ± 2.91 b | 9.53 ± 0.97 b | 1.05 ± 0.26 b | 7.83 ± 1.43 |
| bfr2 | 0.42 ± 0.04 b | 17.40 ± 1.37 ab | 14.95 ± 0.62 a | 2.95 ± 0.17 ab | 10.70 ± 1.98 |
| bfr3 | 0.57 ± 0.06 ab | 18.27 ± 2.64 ab | 16.30 ± 1.13 a | 3.82 ± 0.78 a | 8.90 ± 1.06 |
| bfr4 | 0.68 ± 0.08 a | 20.78 ± 4.26 a | 17.18 ± 1.39 a | 3.67 ± 0.44 a | 9.47 ± 0.94 |
| bfr5 | 0.60 ± 0.08 ab | 15.93 ± 1.94 ab | 16.78 ± 0.94 a | 4.20 ± 0.45 a | 10.20 ± 1.01 |
| bfr6 | 0.70 ± 0.09 a | 16.10 ± 1.16 ab | 19.08 ± 1.07 a | 4.22 ± 0.13 a | 8.73 ± 1.13 |
| p-value | <0.0001 | 0.0462 | <0.0001 | 0.0003 | 0.5703 |
| TMBF (days) | |||||
| 7 | 0.46 ± 0.05 b | 15.78 ± 1.49 | 14.68 ± 0.73 b | 3.45 ± 0.36 | 8.62 ± 0.53 |
| 30 | 0.56 ± 0.07 a | 16.26 ± 1.93 | 16.59 ± 1.03 a | 3.18 ± 0.35 | 9.99 ± 0.88 |
| p-value | 0.0239 | 0.8329 | 0.0268 | 0.4695 | 0.1650 |
| BFR × TMBF | 0.0102 | 0.4242 | 0.3781 | 0.5756 | 0.0581 |
| Number of Seed Germinated in Bokashi Extract | Average Radicle Length (mm) in Bokashi Extract | GI (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dish No | No. seed | SGo | RLo | 25% | 50% | 75% | 100% | 25% | 50% | 75% | 100% | 90.1 |
| 1 | 10 | 8 | 2 | 10 | 6 | 9 | 6 | 10.6 | 5.2 | 2.8 | 2 | |
| 2 | 10 | 6 | 12.3 | 9 | 7 | 8 | 8 | 1.8 | 7.4 | 2.9 | 2.1 | |
| 3 | 10 | 6 | 3 | 8 | 6 | 8 | 4 | 2.4 | 7.8 | 2.6 | 2 | |
| 4 | 10 | 6 | 3.7 | 7 | 5 | 5 | 8 | 2.1 | 5.4 | 1.7 | 1.7 | |
| 5 | 10 | 6 | 5 | 7 | 7 | 6 | 6 | 2.4 | 8.4 | 2.2 | 2.1 | |
| Total | 50 | 32 (a) | 26 (b) | 43 | 31 (w) | 36 (x) | 32 | 19.3 | 34.2 (y) | 12.2 (z) | 9.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gashua, A.G.; Sulaiman, Z.; Yusoff, M.M.; Samad, M.Y.A.; Ramlan, M.F.; Salisu, M.A. Assessment of Fertilizer Quality in Horse Waste-Based Bokashi Fertilizer Formulations. Agronomy 2022, 12, 937. https://doi.org/10.3390/agronomy12040937
Gashua AG, Sulaiman Z, Yusoff MM, Samad MYA, Ramlan MF, Salisu MA. Assessment of Fertilizer Quality in Horse Waste-Based Bokashi Fertilizer Formulations. Agronomy. 2022; 12(4):937. https://doi.org/10.3390/agronomy12040937
Chicago/Turabian StyleGashua, Auwalu Garba, Zulkefly Sulaiman, Martini Mohammad Yusoff, Mohd Yusoff Abd Samad, Mohd Fauzi Ramlan, and Monsuru Adekunle Salisu. 2022. "Assessment of Fertilizer Quality in Horse Waste-Based Bokashi Fertilizer Formulations" Agronomy 12, no. 4: 937. https://doi.org/10.3390/agronomy12040937






