Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of MAlH4 (M = Li and Na) via Solvent Evaporation Using Surfactants
2.2. Material Characterisation
3. Results and Discussion
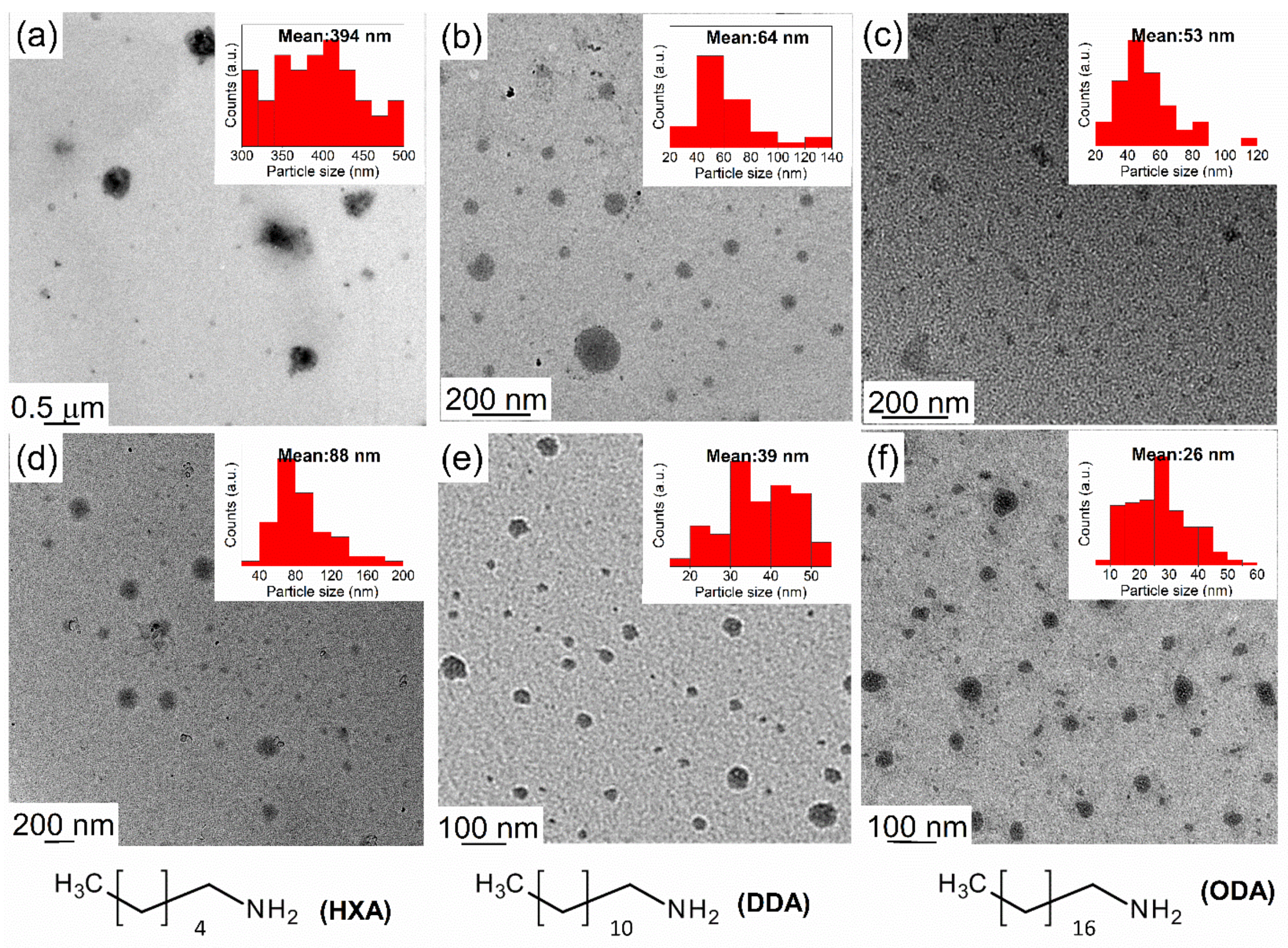
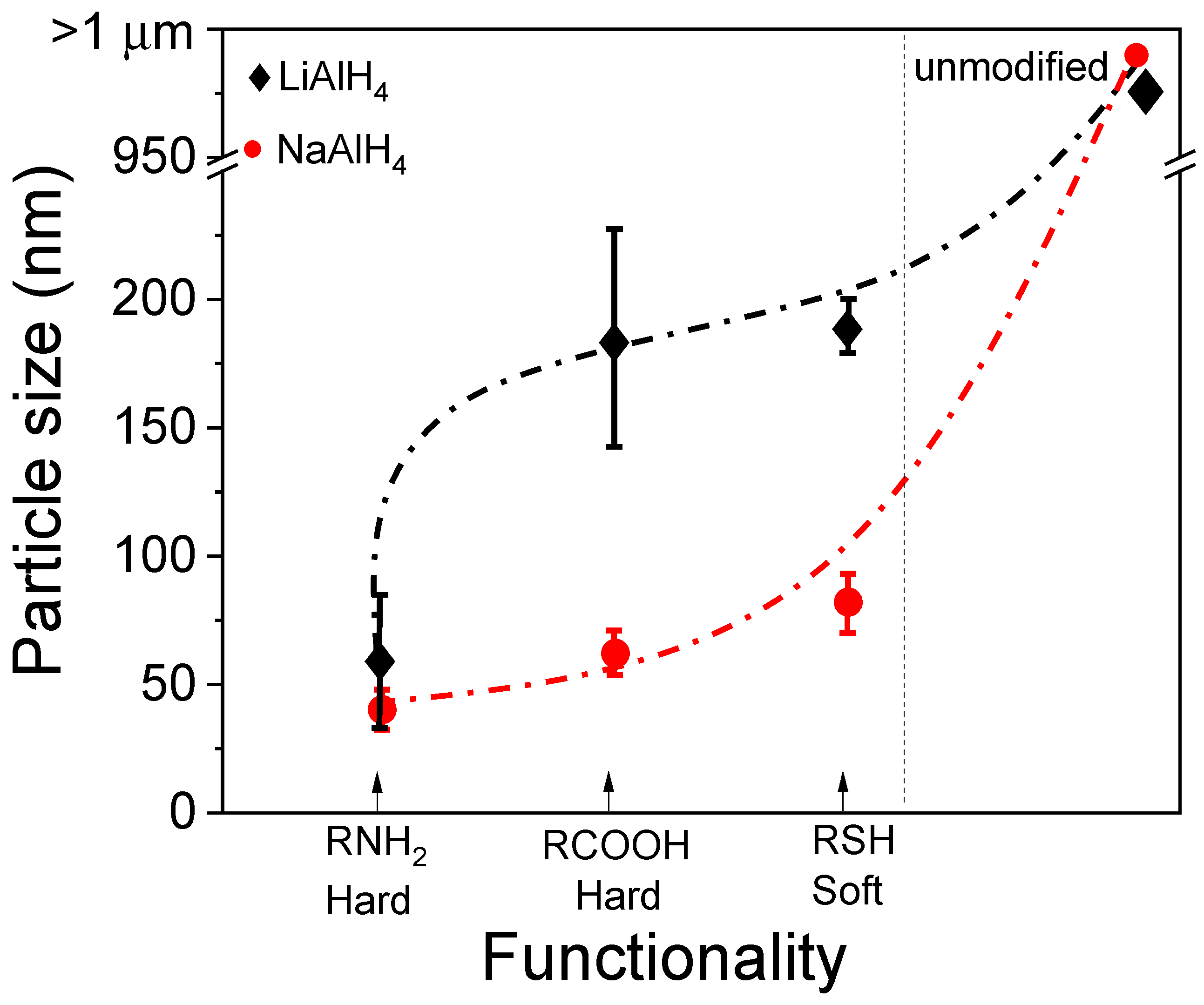
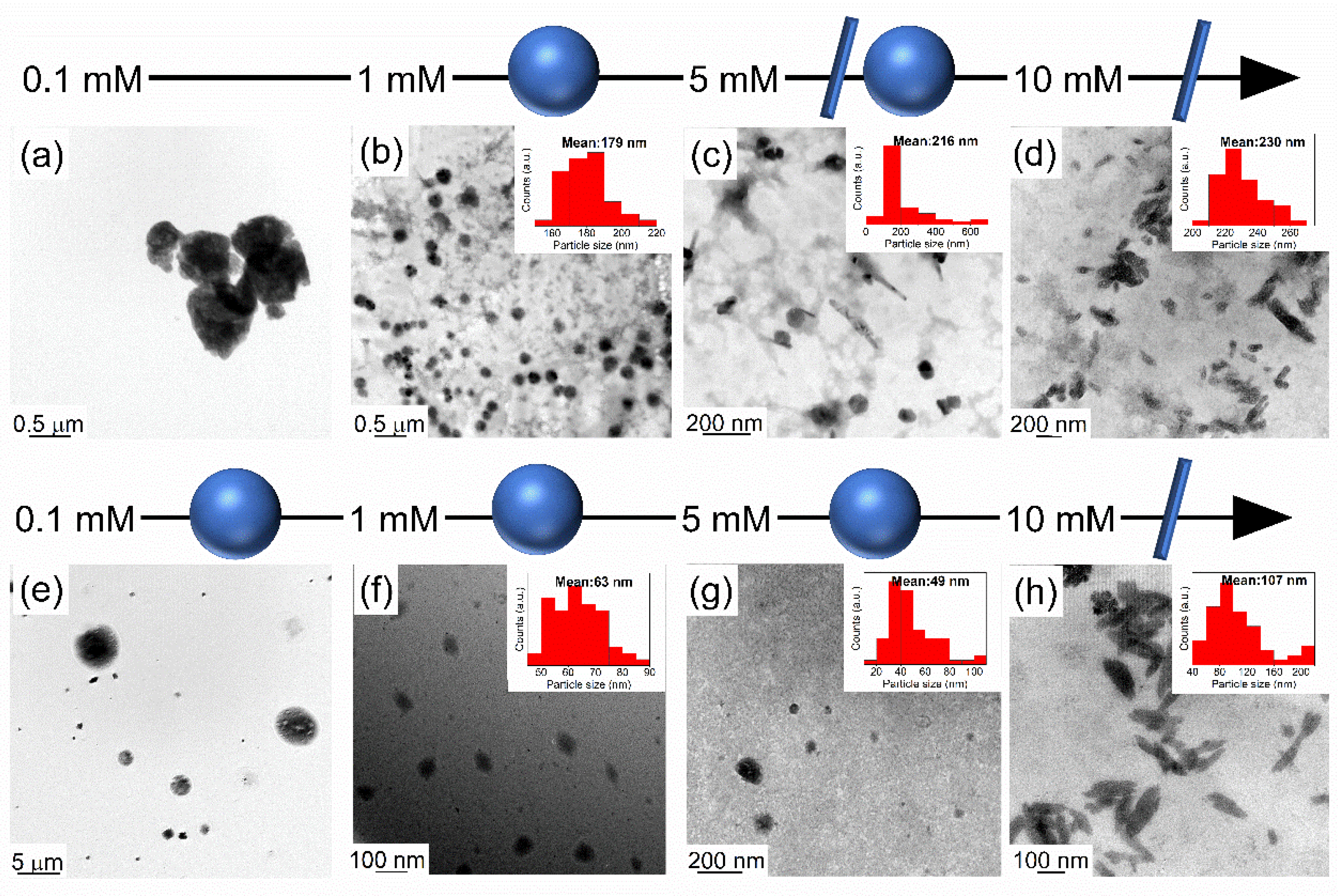
3.1. Effects of Surfactant in Directing the Size and Morphology of Alanate Nanostructures
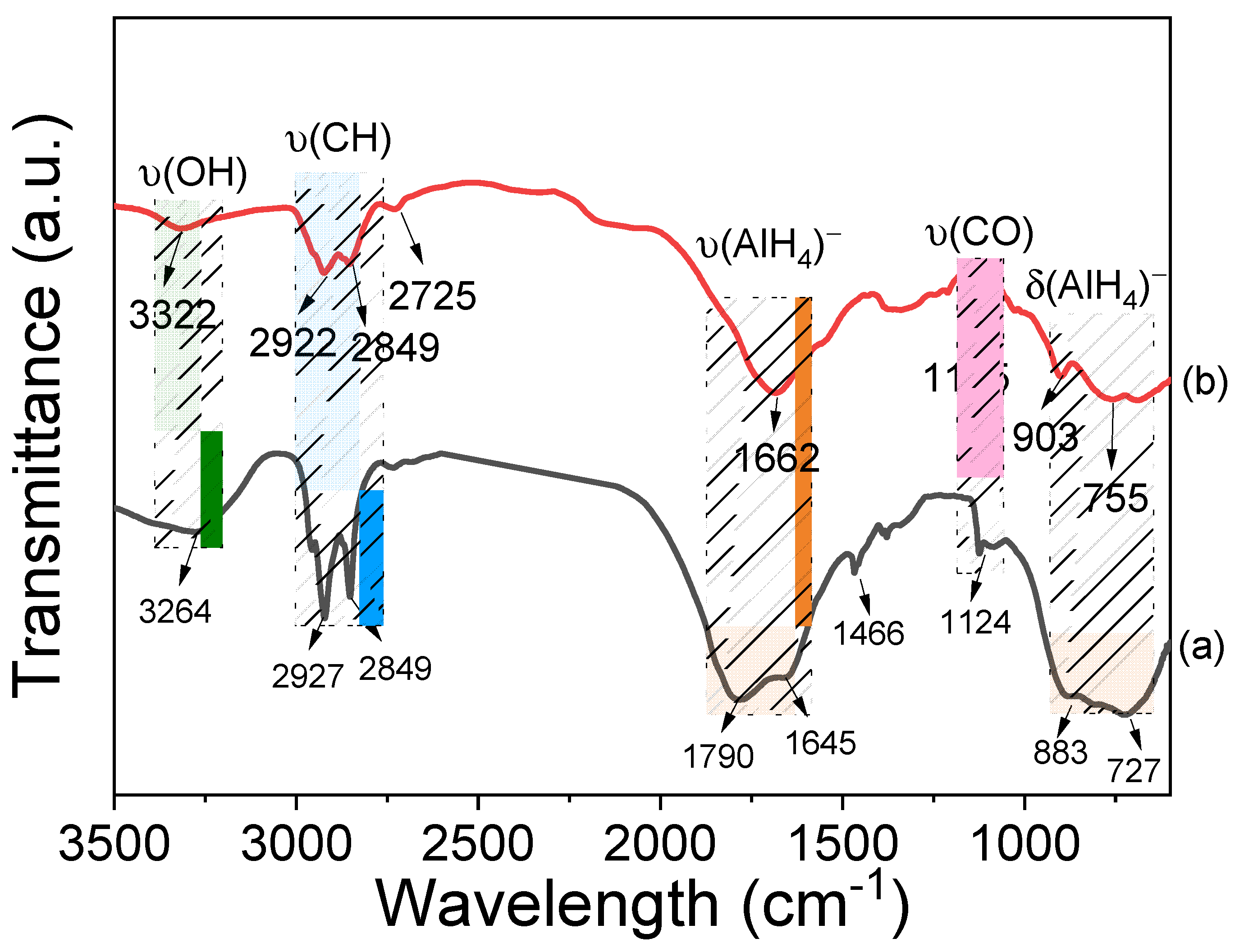
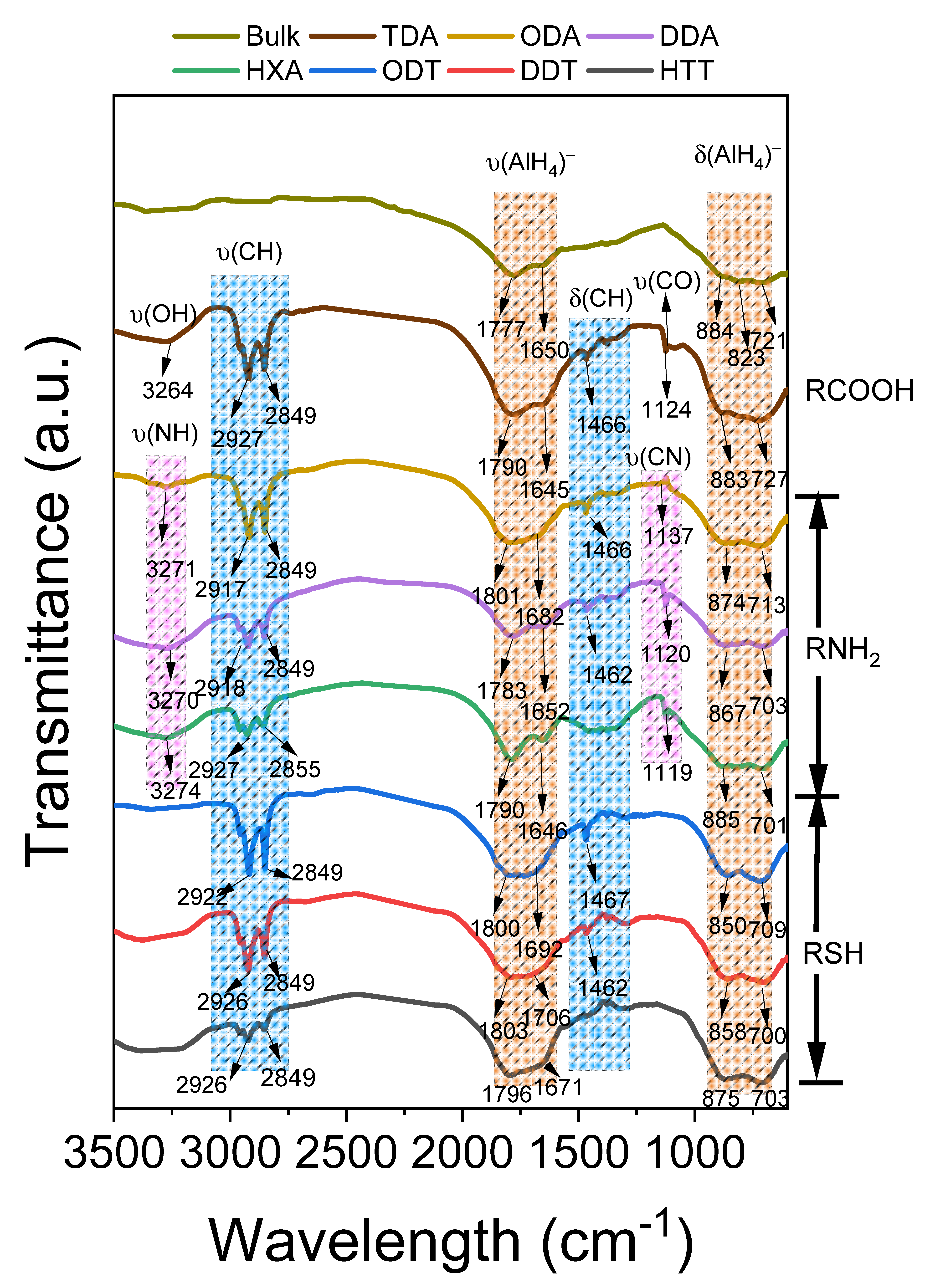
3.2. Structural Properties
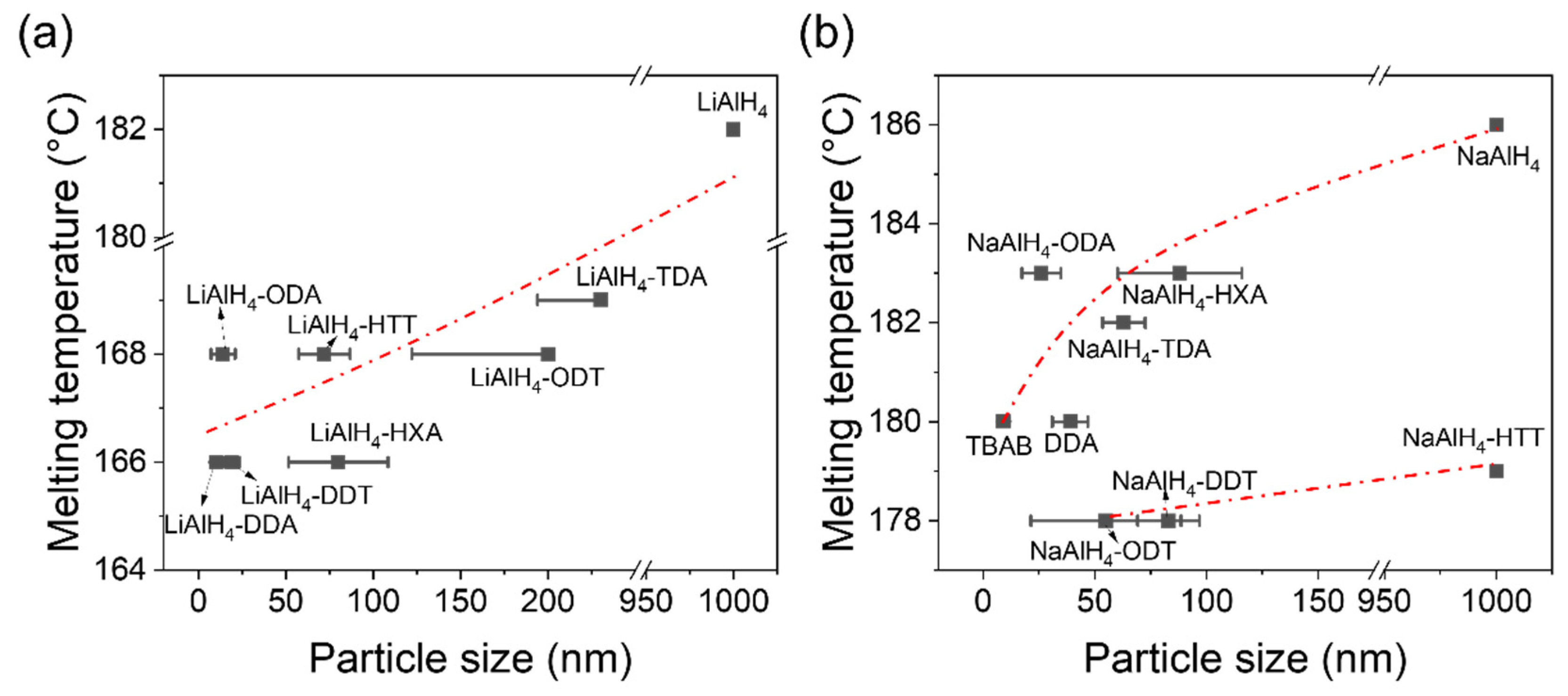
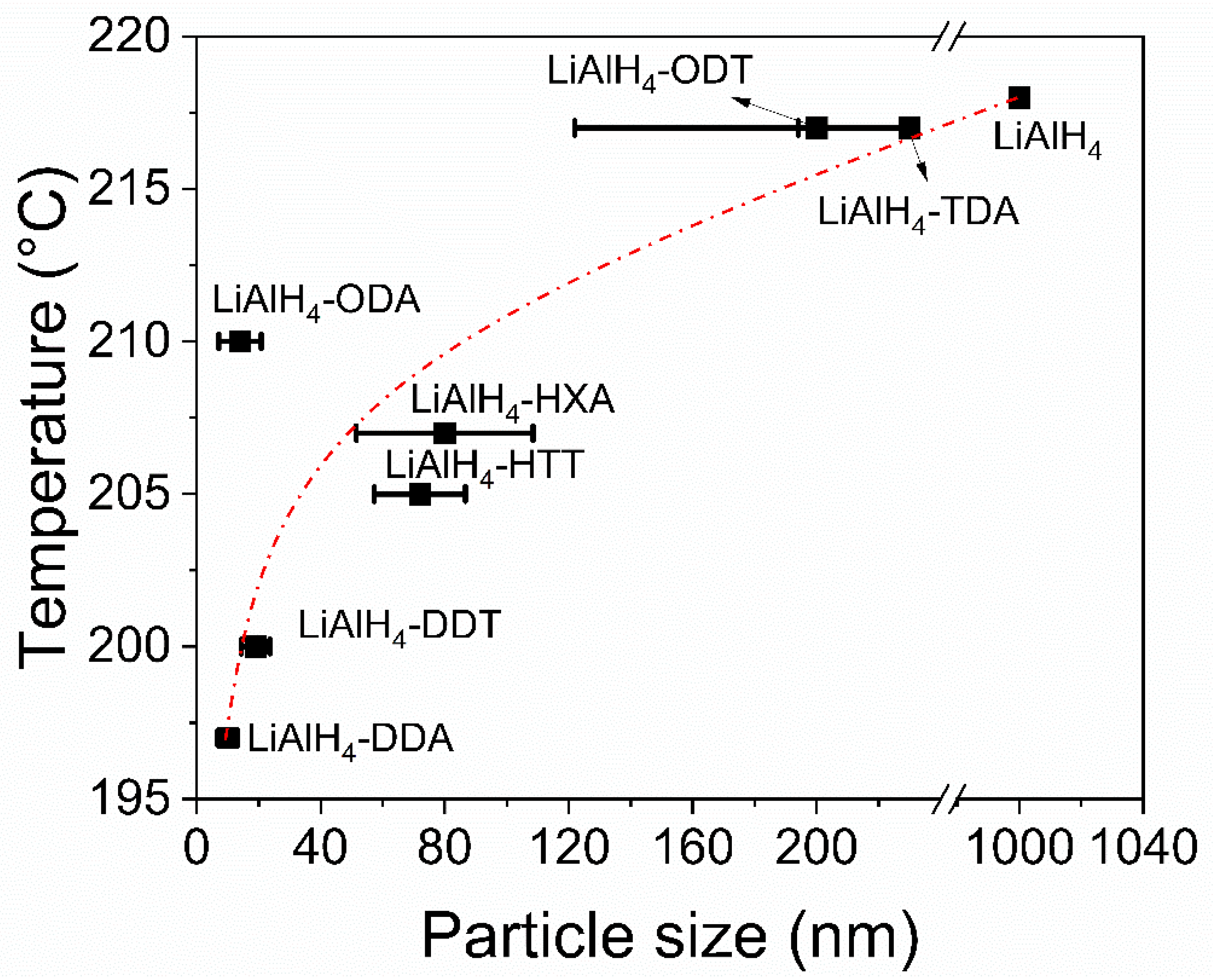
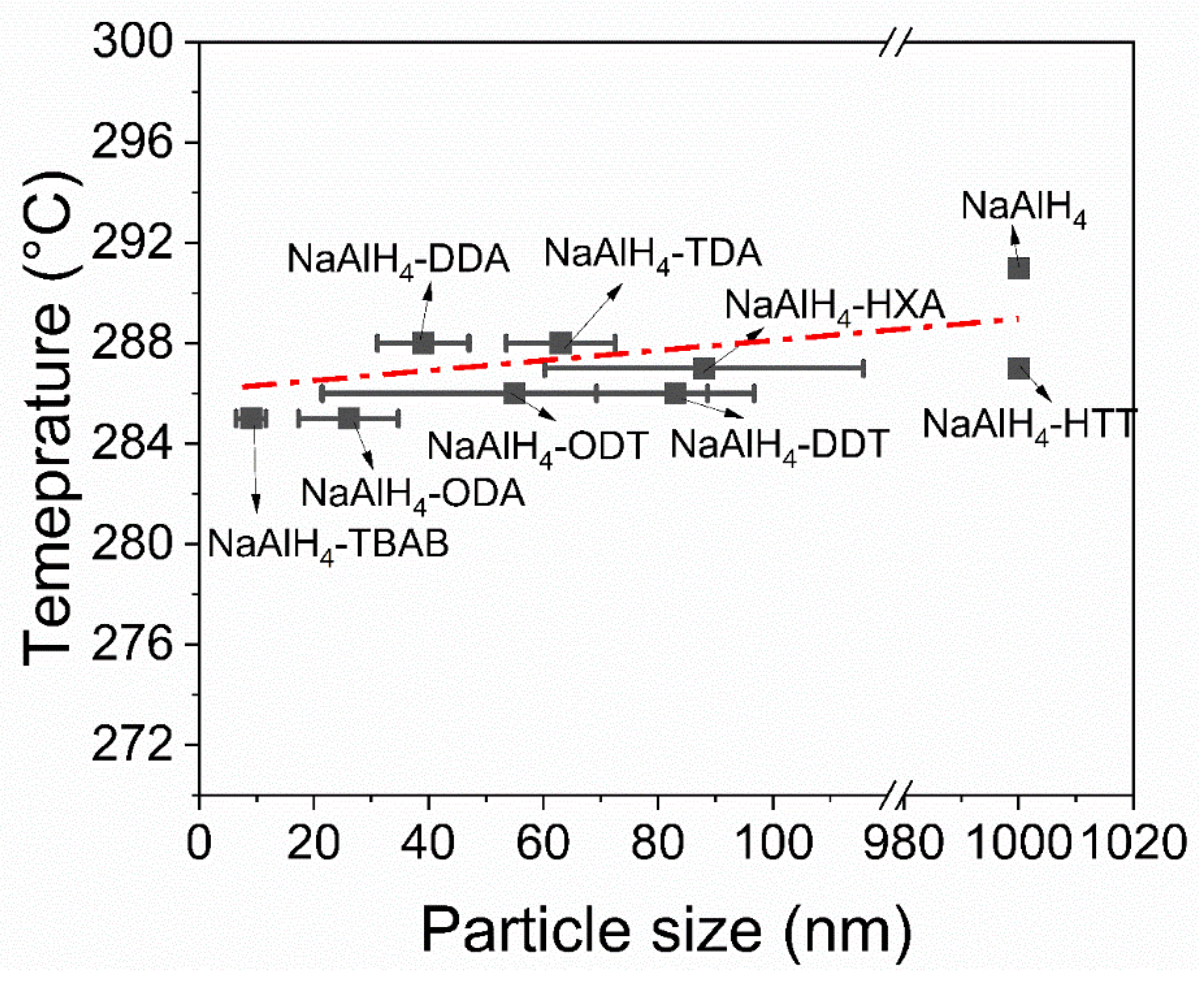
3.3. Hydrogen Desorption Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhai, F.; Li, P.; Sun, A.; Wu, S.; Wan, Q.; Zhang, W.; Li, Y.; Cui, L.; Qu, X. Significantly Improved Dehydrogenation of LiAlH4 Destabilized by MnFe2O4 Nanoparticles. J. Phys. Chem. C 2012, 116, 11939–11945. [Google Scholar] [CrossRef]
- Felderhoff, M.; Weidenthaler, C.; von Helmolt, R.; Eberle, U. Hydrogen storage: The remaining scientific and technological challenges. Phys. Chem. Chem. Phys. 2007, 9, 2643–2653. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Öztürk, Z. Lithium decoration characteristics for hydrogen storage enhancement in novel periodic porous graphene frameworks. Int. J. Hydrogen Energy 2021, 46, 11804–11814. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Ouyang, L.; Zhong, H.; Liu, J.; Wang, H.; Shao, H.; Huang, Z.; Zhu, M. Closing the loop for hydrogen storage: Facile regeneration of NaBH4 from its hydrolytic product. Angew. Chem. 2020, 132, 8701–8707. [Google Scholar] [CrossRef]
- Sazelee, N.A.; Ismail, M. Recent advances in catalyst-enhanced LiAlH4 for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2021, 46, 9123–9141. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhang, D.; Fan, G.; Chen, Y.; Fan, Y.; Liu, B. N-doped carbon coated Ti3C2 MXene as a high-efficiency catalyst for improving hydrogen storage kinetics and stability of NaAlH4. Renew. Energy 2022, 188, 778–787. [Google Scholar] [CrossRef]
- Wang, M.; Ouyang, L.; Peng, C.; Zhu, X.; Zhu, W.; Shao, H.; Zhu, M. Synthesis and hydrolysis of NaZn(BH4)3 and its ammoniates. J. Mater. Chem. A 2017, 5, 17012–17020. [Google Scholar] [CrossRef]
- Xueping, Z.; Shenglin, L. Study on hydrogen storage properties of LiAlH4. J. Alloys Compd. 2009, 481, 761–763. [Google Scholar] [CrossRef]
- Ali, N.A.; Ismail, M. Modification of NaAlH4 properties using catalysts for solid-state hydrogen storage: A review. Int. J. Hydrogen Energy 2021, 46, 766–782. [Google Scholar] [CrossRef]
- Suárez-Alcántara, K.; Tena-Garcia, J.R.; Guerrero-Ortiz, R. Alanates, a Comprehensive Review. Materials 2019, 12, 2724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Z.; Zhang, X.; Huang, Z.; Hu, J.; Li, Y.; Zheng, S.; Gao, M.; Pan, H.; Liu, Y. Controllable synthesis of 2D TiH2 nanoflakes with superior catalytic activity for low-temperature hydrogen cycling of NaAlH4. Chem. Eng. J. 2022, 427, 131546. [Google Scholar] [CrossRef]
- Ke, X.; Chen, C. Thermodynamic functions and pressure-temperature phase diagram of lithium alanates by ab initio calculations. Phys. Rev. B 2007, 76, 024112. [Google Scholar] [CrossRef]
- Jang, J.-W.; Shim, J.-H.; Cho, Y.W.; Lee, B.-J. Thermodynamic Calculation of LiH↔Li3AlH6↔LiAlH4 Reactions. J. Alloys Compd. 2006, 420, 286–290. [Google Scholar] [CrossRef]
- Gao, J.; Adelhelm, P.; Verkuijlen, M.H.W.; Rongeat, C.; Herrich, M.; van Bentum, P.J.M.; Gutfleisch, O.; Kentgens, A.P.M.; de Jong, K.P.; de Jongh, P.E. Confinement of NaAlH4 in Nanoporous Carbon: Impact on H2 Release, Reversibility, and Thermodynamics. J. Phys. Chem. C 2010, 114, 4675–4682. [Google Scholar] [CrossRef]
- Pitt, M.P.; Vullum, P.E.; Sørby, M.H.; Emerich, H.; Paskevicius, M.; Buckley, C.E.; Walmsley, J.C.; Holmestad, R.; Hauback, B.C. Hydrogen Absorption Kinetics of the Transition-Metal-Chloride-Enhanced NaAlH4 System. J. Phys. Chem. C 2012, 116, 14205–14217. [Google Scholar] [CrossRef]
- Tena-García, J.R.; Casillas-Ramírez, A.; Guerrero-Ortiz, R.; Poiré de la Cruz, D.R.; Suarez-Alcantara, K. LiAlH4–ZrCl4 mixtures for hydrogen release at near room temperature. Int. J. Hydrogen Energy 2022, 14, 98. [Google Scholar] [CrossRef]
- Lee, G.-J.; Shim, J.-H.; Whan Cho, Y.; Sub Lee, K. Reversible hydrogen storage in NaAlH4 catalyzed with lanthanide oxides. Int. J. Hydrogen Energy 2007, 32, 1911–1915. [Google Scholar] [CrossRef]
- Li, L.; Wang, Y.; Qiu, F.; Wang, Y.; Xu, Y.; An, C.; Jiao, L.; Yuan, H. Reversible hydrogen storage properties of NaAlH4 enhanced with TiN catalyst. J. Alloys Compd. 2013, 566, 137–141. [Google Scholar] [CrossRef]
- Chowarot, C.; Siriwongrungsona, V.; Hongrapipat, J.; Pang, S.; Messner, M. Characterization of deposited Ti-doped lithium aluminium hydride thin film using dip coating method. J. Phys. Conf. Ser. 2022, 2175, 012015. [Google Scholar] [CrossRef]
- Berseth, P.A.; Harter, A.G.; Zidan, R.; Blomqvist, A.; Araújo, C.M.; Scheicher, R.H.; Ahuja, R.; Jena, P. Carbon Nanomaterials as Catalysts for Hydrogen Uptake and Release in NaAlH4. Nano Lett. 2009, 9, 1501–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; You, L.; Xia, G.; Yu, X. A balance between catalysis and nanoconfinement towards enhanced hydrogen storage performance of NaAlH4. J. Mater. Sci. Technol. 2021, 79, 205–211. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art. ChemSusChem 2015, 8, 2789–2825. [Google Scholar] [CrossRef]
- Schneemann, A.; White, J.L.; Kang, S.; Jeong, S.; Wan, L.F.; Cho, E.S.; Heo, T.W.; Prendergast, D.; Urban, J.J.; Wood, B.C.; et al. Nanostructured Metal Hydrides for Hydrogen Storage. Chem. Rev. 2018, 118, 10775–10839. [Google Scholar] [CrossRef]
- Jeong, U.; Kim, H.; Ramesh, S.; Dogan, N.A.; Wongwilawan, S.; Kang, S.; Park, J.; Cho, E.S.; Yavuz, C.T. Rapid access to ordered mesoporous carbons for chemical hydrogen storage. Angew. Chem. 2021, 133, 22652–22660. [Google Scholar] [CrossRef]
- Baldé, C.P.; Hereijgers, B.P.; Bitter, J.H.; de Jong, K.P. Facilitated hydrogen storage in NaAlH4 supported on carbon nanofibers. Angew. Chem. 2006, 118, 3581–3583. [Google Scholar] [CrossRef] [Green Version]
- Niemann, M.U.; Srinivasan, S.S.; Phani, A.R.; Kumar, A.; Goswami, D.Y.; Stefanakos, E.K. Nanomaterials for Hydrogen Storage Applications: A Review. J. Nanomater. 2008, 2008, 950967. [Google Scholar] [CrossRef] [Green Version]
- Lai, Q.; Pratthana, C.; Yang, Y.; Rawal, A.; Aguey-Zinsou, K.-F. Nanoconfinement of Complex Borohydrides for Hydrogen Storage. ACS Appl. Nano Mater. 2021, 4, 973–978. [Google Scholar] [CrossRef]
- Wang, L.; Rawal, A.; Quadir, M.Z.; Aguey-Zinsou, K.-F. Nanoconfined lithium aluminium hydride (LiAlH4) and hydrogen reversibility. Int. J. Hydrogen Energy 2017, 42, 14144–14153. [Google Scholar] [CrossRef]
- Cho, Y.; Li, S.; Snider, J.L.; Marple, M.A.T.; Strange, N.A.; Sugar, J.D.; El Gabaly, F.; Schneemann, A.; Kang, S.; Kang, M.-h.; et al. Reversing the Irreversible: Thermodynamic Stabilization of LiAlH4 Nanoconfined Within a Nitrogen-Doped Carbon Host. ACS Nano 2021, 15, 10163–10174. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Xia, G.; Yu, X. Confined NaAlH4 nanoparticles inside CeO2 hollow nanotubes towards enhanced hydrogen storage. Nanoscale 2017, 9, 14612–14619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Zhou, G.; Fang, F.; Yu, X.; Zhang, Q.; Ouyang, L.; Zhu, M.; Sun, D. De-/re-hydrogenation features of NaAlH4 confined exclusively in nanopores. Acta Mater. 2011, 59, 1829–1838. [Google Scholar] [CrossRef]
- Wang, L.; Aguey-Zinsou, K.-F. Synthesis of LiAlH4 Nanoparticles Leading to a Single Hydrogen Release Step upon Ti Coating. Inorganics 2017, 5, 38. [Google Scholar] [CrossRef] [Green Version]
- Christian, M.L.; Aguey-Zinsou, K.-F. Core–Shell Strategy Leading to High Reversible Hydrogen Storage Capacity for NaBH4. ACS Nano 2012, 6, 7739–7751. [Google Scholar] [CrossRef]
- Aguey-Zinsou, F. (Invited) Core-Shell Hydride Nanoarchitectures for Reversible Hydrogen Storage. ECS Meet. Abstr. 2016, 38, 2471. [Google Scholar] [CrossRef]
- Salman, M.S.; Rawal, A.; Aguey-Zinsou, K.-F. Tunable NaBH4 Nanostructures Revealing Structure-Dependent Hydrogen Release. Adv. Energy Sustain. Res. 2021, 2, 2100063. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef]
- Wagner, L.K.; Majzoub, E.H.; Allendorf, M.D.; Grossman, J.C. Tuning metal hydride thermodynamics via size and composition: Li–H, Mg–H, Al–H, and Mg–Al–H nanoclusters for hydrogen storage. Phys. Chem. Chem. Phys. 2012, 14, 6611–6616. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.-F. Controlling the Growth of LiBH4 Nanoparticles for Hydrogen Storage. Energy Technol. 2019, 7, 1801159. [Google Scholar] [CrossRef]
- Wang, T.; Aguey-Zinsou, K.-F. Controlling the growth of NaBH4 nanoparticles for hydrogen storage. Int. J. Hydrogen Energy 2020, 45, 2054–2067. [Google Scholar] [CrossRef]
- Dirmyer, M.R.; Martin, J.; Nolas, G.S.; Sen, A.; Badding, J.V. Thermal and Electrical Conductivity of Size-Tuned Bismuth Telluride Nanoparticles. Small 2009, 5, 933–937. [Google Scholar] [CrossRef] [PubMed]
- Visaveliya, N.; Köhler, J.M. Control of Shape and Size of Polymer Nanoparticles Aggregates in a Single-Step Microcontinuous Flow Process: A Case of Flower and Spherical Shapes. Langmuir 2014, 30, 12180–12189. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; He, R.; Gu, H.-C. Oleic acid coating on the monodisperse magnetite nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Ming, H.; Ming, J.; Oh, S.-M.; Tian, S.; Zhou, Q.; Huang, H.; Sun, Y.-K.; Zheng, J. Surfactant-Assisted Synthesis of Fe2O3 Nanoparticles and F-Doped Carbon Modification toward an Improved Fe3O4@CFx/LiNi0.5Mn1.5O4 Battery. ACS Appl. Mater. Interfaces 2014, 6, 15499–15509. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, N.; Reifsnyder, D.; Xie, R.; Aldana, J.; Peng, X. Surface Ligand Dynamics in Growth of Nanocrystals. J. Am. Chem. Soc. 2007, 129, 9500–9509. [Google Scholar] [CrossRef]
- Chaudhary, S.; Sharma, P.; Kumar, R.; Mehta, S.K. Nanoscale surface designing of Cerium oxide nanoparticles for controlling growth, stability, optical and thermal properties. Ceram. Int. 2015, 41, 10995–11003. [Google Scholar] [CrossRef]
- Olmos-Asar, J.A.; Ludueña, M.; Mariscal, M.M. Monolayer protected gold nanoparticles: The effect of the headgroup–Au interaction. Phys. Chem. Chem. Phys. 2014, 16, 15979–15987. [Google Scholar] [CrossRef]
- Chattaraj, P.K.; Maiti, B. HSAB Principle Applied to the Time Evolution of Chemical Reactions. J. Am. Chem. Soc. 2003, 125, 2705–2710. [Google Scholar] [CrossRef]
- Simek, J.W.; Tuck, T.; Bush, K.C. Reduction of Carboxylic Acids with Sodium Borohydride and an Electrophile. J. Chem. Educ. 1997, 74, 107. [Google Scholar] [CrossRef]
- Cha, J.S.; Brown, H.C. Reaction of sodium aluminum hydride with selected organic compounds containing representative functional groups. Comparison of the reducing characteristics of lithium and sodium aluminum hydrides. J. Org. Chem. 1993, 58, 4727–4731. [Google Scholar] [CrossRef]
- Rao, A.; Mohan, H.; Burke, S.; Danheiser, R. Handbook of Reagentsfor Organic Synthesis, Oxidizing and Reducing Agents; Wiley: New York, NY, USA, 1999. [Google Scholar]
- Wang, J.; Ju, M.-Y.; Wang, X.; Ma, Y.-N.; Wei, D.; Chen, X. Hydroboration Reaction and Mechanism of Carboxylic Acids using NaNH2(BH3)2, a Hydroboration Reagent with Reducing Capability between NaBH4 and LiAlH4. J. Org. Chem. 2021, 86, 5305–5316. [Google Scholar] [CrossRef] [PubMed]
- Conversion of Carboxylic Acids to Alcohols Using LiAlH4. 2020. Available online: https://chem.libretexts.org/@go/page/5452 (accessed on 6 April 2022).
- Corral, I.; Mó, O.; Yáñez, M. Cu+ association to some Ph–X (X=OH, NH2, CHO, COOH, CF3) phenyl derivatives.: A comparison with Li+ complexes. Int. J. Mass Spectrom. 2006, 255–256, 20–27. [Google Scholar] [CrossRef]
- Inoue, T.; Yamakawa, H. Micelle formation of nonionic surfactants in a room temperature ionic liquid, 1-butyl-3-methylimidazolium tetrafluoroborate: Surfactant chain length dependence of the critical micelle concentration. J. Colloid Interface Sci. 2011, 356, 798–802. [Google Scholar] [CrossRef]
- Buckingham, S.A.; Garvey, C.J.; Warr, G.G. Effect of head-group size on micellization and phase behavior in quaternary ammonium surfactant systems. J. Phys. Chem. 1993, 97, 10236–10244. [Google Scholar] [CrossRef]
- Di Michele, A.; Brinchi, L.; Di Profio, P.; Germani, R.; Savelli, G.; Onori, G. Effect of head group size, temperature and counterion specificity on cationic micelles. J. Colloid Interface Sci. 2011, 358, 160–166. [Google Scholar] [CrossRef]
- Wang, J.; Ebner, A.D.; Ritter, J.A. Synthesis of Metal Complex Hydrides for Hydrogen Storage. J. Phys. Chem. C 2007, 111, 14917–14924. [Google Scholar] [CrossRef]
- Dong, T.; Knoshaug, E.P.; Pienkos, P.T.; Laurens, L.M.L. Lipid recovery from wet oleaginous microbial biomass for biofuel production: A critical review. Appl. Energy 2016, 177, 879–895. [Google Scholar] [CrossRef] [Green Version]
- Jarusuwannapoom, T.; Hongrojjanawiwat, W.; Jitjaicham, S.; Wannatong, L.; Nithitanakul, M.; Pattamaprom, C.; Koombhongse, P.; Rangkupan, R.; Supaphol, P. Effect of solvents on electro-spinnability of polystyrene solutions and morphological appearance of resulting electrospun polystyrene fibers. Eur. Polym. J. 2005, 41, 409–421. [Google Scholar] [CrossRef]
- González-Pérez, A.; Varela, L.M.; García, M.; Rodríguez, J.R. Sphere to rod transitions in homologous alkylpyridinium salts: A Stauff-Klevens-type equation for the second critical micelle concentration. J. Colloid Interface Sci. 2006, 293, 213–221. [Google Scholar] [CrossRef]
- Sau, T.K.; Murphy, C.J. Self-Assembly Patterns Formed upon Solvent Evaporation of Aqueous Cetyltrimethylammonium Bromide-Coated Gold Nanoparticles of Various Shapes. Langmuir 2005, 21, 2923–2929. [Google Scholar] [CrossRef] [PubMed]
- Ozeki, S.; Ikeda, S. The sphere—Rod transition of micelles and the two-step micellization of dodecyltrimethylammonium bromide in aqueous NaBr solutions. J. Colloid Interface Sci. 1982, 87, 424–435. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Q.; Xu, D.; Sun, R.; Zhang, P.; Bai, B.; Kang, W. SiO2 nanoparticle-assisted low-concentration viscoelastic cationic surfactant fracturing fluid. J. Mol. Liq. 2018, 266, 864–869. [Google Scholar] [CrossRef]
- Dreiss, C.A. Wormlike micelles: Where do we stand? Recent developments, linear rheology and scattering techniques. Soft Matter 2007, 3, 956–970. [Google Scholar] [CrossRef] [PubMed]
- Nanda, K.; Maisels, A.; Kruis, F.; Fissan, H.; Stappert, S. Higher surface energy of free nanoparticles. Phys. Rev. Lett. 2003, 91, 106102. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xu, Y.; Wang, Y.; Wang, Y.; Qiu, F.; An, C.; Jiao, L.; Yuan, H. NbN nanoparticles as additive for the high dehydrogenation properties of LiAlH4. Dalton Trans. 2014, 43, 1806–1813. [Google Scholar] [CrossRef]
- Silvestri, L.; Farina, L.; Meggiolaro, D.; Panero, S.; Padella, F.; Brutti, S.; Reale, P. Reactivity of Sodium Alanates in Lithium Batteries. J. Phys. Chem. C 2015, 119, 28766–28775. [Google Scholar] [CrossRef]
- Smith, M.B.; Wolinsky, J. Reduction of thiols with LiAlD4: A mechanistic study. J. Chem. Soc. Perkin Trans. 1998, 2, 1434. [Google Scholar] [CrossRef]
- Gupta, R.D.; Raghav, N. Differential effect of surfactants tetra-n-butyl ammonium bromide and N-Cetyl-N, N, N-trimethyl ammonium bromide bound to nano-cellulose on binding and sustained release of some non-steroidal anti-inflammatory drugs. Int. J. Biol. Macromol. 2020, 164, 2745–2752. [Google Scholar] [CrossRef]
- Lin-Vien, D.; Colthup, N.B.; Fateley, W.G.; Grasselli, J.G. The Handbook of Infrared and Raman Characteristic Frequencies of Organic Molecules; Elsevier: Amsterdam, The Netherlands, 1991. [Google Scholar]
- Hostetler, M.J.; Stokes, J.J.; Murray, R.W. Infrared Spectroscopy of Three-Dimensional Self-Assembled Monolayers: N-Alkanethiolate Monolayers on Gold Cluster Compounds. Langmuir 1996, 12, 3604–3612. [Google Scholar] [CrossRef]
- MacPhail, R.A.; Strauss, H.L.; Snyder, R.G.; Elliger, C.A. Carbon-hydrogen stretching modes and the structure of n-alkyl chains. 2. Long, all-trans chains. J. Phys. Chem. 1984, 88, 334–341. [Google Scholar] [CrossRef]
- Dicko, A.; Bourque, H.; Pézolet, M. Study by infrared spectroscopy of the conformation of dipalmitoylphosphatidylglycerol monolayers at the air–water interface and transferred on solid substrates. Chem. Phys. Lipids 1998, 96, 125–139. [Google Scholar] [CrossRef]
- Puri, P.; Yang, V. Effect of Particle Size on Melting of Aluminum at Nano Scales. J. Phys. Chem. C 2007, 111, 11776–11783. [Google Scholar] [CrossRef]
- Wronski, C. The size dependence of the melting point of small particles of tin. Br. J. Appl. Phys. 1967, 18, 1731. [Google Scholar] [CrossRef]
- Jiang, H.; Moon, K.-s.; Dong, H.; Hua, F.; Wong, C.P. Size-dependent melting properties of tin nanoparticles. Chem. Phys. Lett. 2006, 429, 492–496. [Google Scholar] [CrossRef]
- Bhakta, R.K.; Maharrey, S.; Stavila, V.; Highley, A.; Alam, T.; Majzoub, E.; Allendorf, M. Thermodynamics and kinetics of NaAlH4 nanocluster decomposition. Phys. Chem. Chem. Phys. 2012, 14, 8160–8169. [Google Scholar] [CrossRef]
- de Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, J.; Wu, G.; Xiong, Z.; Chen, P. Large Amount of Hydrogen Desorption from the Mixture of Mg(NH2)2 and LiAlH4. J. Phys. Chem. C 2007, 111, 19161–19164. [Google Scholar] [CrossRef]
- Chua, Y.S.; Xiong, Z.; Wu, G.; Chen, P. Ternary amide-hydride system: A study on LiAl(NH2)4-LiAlH4 interaction. J. Alloys Compd. 2019, 790, 597–601. [Google Scholar] [CrossRef]
- Chen, P.; Xiong, Z.; Luo, J.; Lin, J.; Tan, K.L. Interaction between Lithium Amide and Lithium Hydride. J. Phys. Chem. B 2003, 107, 10967–10970. [Google Scholar] [CrossRef]
- Haubenstock, H.; Mester, T., Jr. On the reaction of lithium aluminum hydride with alcohols. J. Org. Chem. 1983, 48, 945–948. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pratthana, C.; Aguey-Zinsou, K.-F. Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage. Appl. Sci. 2022, 12, 4742. https://doi.org/10.3390/app12094742
Pratthana C, Aguey-Zinsou K-F. Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage. Applied Sciences. 2022; 12(9):4742. https://doi.org/10.3390/app12094742
Chicago/Turabian StylePratthana, Chulaluck, and Kondo-Francois Aguey-Zinsou. 2022. "Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage" Applied Sciences 12, no. 9: 4742. https://doi.org/10.3390/app12094742
APA StylePratthana, C., & Aguey-Zinsou, K.-F. (2022). Surfactant Induced Synthesis of LiAlH4 and NaAlH4 Nanoparticles for Hydrogen Storage. Applied Sciences, 12(9), 4742. https://doi.org/10.3390/app12094742






