2. Materials and methods
2.1. Animals
The experiments were carried out on mitochondria isolated from the perfused liver of male Wistar rats. The permission No. 0155 was obtained from the Ethics Committee of the laboratory animals’ usage at the Lithuanian State Food and Veterinary Service. The animals were acclimated to 22 °C and a 12-h light–dark cycle (lights on at 08:00) and had free access to water and a standard chow diet.
2.2. Materials
All reagents used in this study were analytical grade obtained from Sigma–Aldrich (Taufkirchen, Germany) except for tetrabutylammonium bromide obtained from Acros Organics (New Jersey, USA); pentobarbital, from Vetoquinol (Buckingham, UK); ketamine, from Gedeon Richte (Budapest, Hungary); and HPLC-grade acetonitrile and chloroform, from Scharlau Chemie (Sentmenat, Spain).
2.3. Liver perfusion
After pre-narcosis with, CO
2 rats were anesthetized with a combination of intraperitoneal injection of sodium pentobarbital (40 mg/kg body weight) and intramuscular injection of ketamine (100 mg/kg body weight). The peritoneal cavity was opened with the cross-abdominal incision, and 1.5 IU/kg heparin was injected to the inferior vena cava. After cannulation of the portal vein of the mobilized liver, inferior vena cava and aorta were transected. The liver was perfused manually through portal vein with 20 mL of Krebs-Ringer solution with EGTA (120 mM NaCl, 24 mM NaHCO
3, 20 mM glucose, 5 mM HEPES, 4.8 mM KCl, 1.2 mM MgSO
4, 1.2 mM KH2PO4 and 0.1 mM EGTA, pH 7.4) for removal the blood and clots. Liver was removed and transferred into a thermostatic vessel and perfused with oxygenated (95% O
2, 5% CO
2) Krebs-Ringer solution with EGTA at the flow rate of 7 mL/min for 7 min. Then perfusion solution was changed to Krebs-Ringer buffer solution with Ca
2+ (120 mM NaCl, 24 mM NaHCO
3, 20 mM glucose, 5 mM HEPES, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO
4, 1.4 mM CaCl
2, pH 7.4) and perfusion continued for 8 min. The temperature of both perfusion buffers was adjusted to the same temperature value (38, 40, 42, 46, 48, 50, 52 °C) for each experiment (4–8 perfusion experiments were performed for every temperature point), thus the total duration of perfusion at certain temperature was 15 min. The temperature for the control experiments of liver perfusion in this study was 38 °C, taking into account the determined physiological temperature of rat liver [
13]. Immediately after perfusion approximately 1 g of liver tissue was excised, frozen in liquid nitrogen, weighted and subjected for the extraction of adenine nucleotides. Remaining liver tissue was placed into ice-cold 0.9% KCl and used for the isolation of mitochondria.
2.4. Isolation of mitochondria
Mitochondria were isolated as described previously [
14]. The tissue was cut into small pieces and homogenized in a glassTeflon homogenizer. Homogenization medium contained 250 mM sucrose, 10 mM Tris, 3 mM EGTA and 2 mg/mL bovine serum albumin (BSA) (pH 7.7, 2 °C). The homogenate was centrifuged at 750 ×
g for 5 min, and then the supernatant was centrifuged at 6800 ×
g for 10 min. The mitochondrial pellet was re-suspended in a suspension buffer containing 180 mM KCl, 20 mM Tris, 3 mM EGTA (pH 7.3, 2 °C) and centrifuged again. The mitochondrial pellet was again re-suspended in a suspension buffer and stored on ice. The protein concentration was determined by the biuret method using BSA as a standard [
15].
2.5. The measurement of mitochondrial respiratory rate and membrane potential
Mitochondrial respiratory rate and membrane potential (Δψ) were measured in a closed, stirred and thermostated 1.5 mL glass vessel equipped with Clark-type oxygen electrode (Rank Brothers Ltd., England) and TPP+ (tetraphenylphosphonium)sensitive electrode allowing simultaneous monitoring of Δψ and mitochondrial respiration.
The assay medium contained 20 mM Tris, 5 mM KH
2PO
4, 110 mM KCl, 2.3 mM MgCl
2, 50 mM creatine, 0.1 mg/mL (0.2 IU/ mL) creatine phosphokinase, pH 7.2. Excess of creatine kinase (0.1 mg/mL) was added to maintain steady state respiration [
16]. The experiments were performed using 1 mM pyruvate plus 1 mM malate or 0.5 mM succinate as the respiratory substrate. Mitochondria (0.3 mg protein/mL) were incubated in the assay medium with the respiratory substrate (state 2) for 3 min at 38 ± 0.1 °C. State 3 respiration was initiated by adding 1 mM ATP. Creatine phosphokinase catalyzes the phosphorylation of creatine by using ATP, and constantly maintains high concentration of ADP in the assay medium. Therefore, such an experimental system in the stationary conditions determines the maximum rate of the mitochondrial respiration [
16]. The rate of mitochondrial respiration in state 2 (
V2), state 3 (
V3) and the respiratory control index (RCI =
V3/
V2) are defined according to the conventional terminology [
17]. The maximum activity of the mitochondrial respiratory chain was assessed by registering the oxygen consumption in the presence of 0.34 μM uncoupler carbonyl cyanide
m-chloro-phenyl hydrazone (CCCP). The concentration of molecular oxygen dissolved in the assay medium at 37 °C was determined polarographically using glucose oxidase reaction as described previously [
8] and was 394.6 μmol O/L. The mitochondrial respiratory rate is expressed in nmol O/min per 1 mg of mitochondrial protein.
Before each measurement of the membrane potential the TPP+-sensitive electrode was calibrated by small additions of TTPCl solution to a final concentration of 267 nM. Δ
ψ was calculated from the distribution of TPP+ using Nernst equation and TPP+ binding correction factor of 0.162 μL/mg protein [
18]. The effects of hyperthermia on mitochondrial respiratory chain and inner membrane proton permeability were distinguished by partial modular kinetic analysis (described in detail in [
8,
18]), namely, by measuring the kinetic dependence of proton leak on Δ
ψ and titrating the flux through the respiratory module with rotenone (0–0.08 nmol/mg mitochondrial protein) when the phosphorylation was fully blocked by addition of oligomycin excess (2 μg/mg mitochondrial protein).
2.6. Isolation of hepatocytes
Hepatocytes were isolated using two-step collagenase perfusion [
19]. The animals were killed and the liver was quickly removed and placed in the thermostatic vessel. The liver was perfused through
vena cava with oxygenated (95% O
2, 5% CO
2) Krebs-Ringer solution with EGTA (120 mM NaCl, 24 mM NaHCO
3, 20 mM glucose, 5 mM HEPES, 4.8 mM KCl, 1.2 mM MgSO
4, 1.2 mM KH
2PO
4 and 0.1 mM EGTA, pH 7.4) in order to remove the blood and clots. Afterwards liver was perfused with Krebs-Ringer buffer solution with Ca
2+ and collagenase IV (120 mM NaCl, 24 mM NaHCO
3, 20 mM glucose, 5 mM HEPES, 4.8 mM KCl, 1.2 mM MgSO
4, 1.2 mM KH
2PO
4, 1.4 mM CaCl
2, 156 IU/mL collagenase IV, pH 7.4) for 15 min. After perfusion the liver was placed in a beaker with 20 mL of Krebs-Ringer buffer (4 °C), the capsule was cut and cells were dispersed by aspirating with a large-bore pipet. Suspension was filtered through a 70 μm cell strainer into a centrifuge tube with 25-mL Krebs-Ringer buffer with 2% BSA (4 °C) and centrifuged at 50 ×
g for 5 min (4 °C). Cell pellet was resuspended in 30 mL Krebs-Ringer buffer with 2% BSA by inverting tube and centrifuged again. This procedure was repeated 3 times. After final centrifugation cells were resuspended in 10-mL Krebs-Ringer buffer with 2% BSA. Initial cell suspension viability determined by trypan blue was more than 80%.
2.7. Incubation of hepatocytes
After isolation and cell counting hepatocyte suspension in Krebs-Ringer buffer was aliquoted into separate preheated tubes and incubated for 15 min at different temperatures (38, 40, 42, 45, 46, 50, 52 °C) in water bath. Immediately after incubation cells were subjected for cell counting and isolation procedure of adenine nucleotides.
2.8. Extraction of adenine nucleotides from hepatocytes
Immediately after incubation of hepatocyte suspension at different temperatures (38, 40, 42, 45, 46, 50, 52 °C) for 15 min, the suspension was aliquoted to 10
6 cells per tube and centrifuged at 1000 ×
g for 3 min (4 °C). The supernatant was discarded and the extraction procedure was performed as described previously [
20] with minor changes. Briefly, cells were lysed with 1 mL of ice cold 0.5 M KOH. Samples were incubated on ice for 3 min, then 840 μL of sample was mixed with 170 μL of 8.5% H
3PO
4 and incubated on ice for 3 min. After incubation the extract was cleared by 3-min centrifugation at 1000 ×
g (4 °C). Supernatant was filtered through the syringe filter with 0.2 μm pore diameter PVDF membrane. Prepared samples were kept at −80 °C and analyzed within 48 h.
2.9. The extraction of adenine nucleotides from the perfused liver tissue
Immediately after perfusion approximately 1 g of the liver tissue was excised, frozen in liquid nitrogen and weighted. The extraction procedure was performed as described previously [
21]. A first 120-s homogenization was performed with 7 mL of ice-cold precipitating solution composed of CH
3CN + 10 mM KH
2PO
4, pH 7.4 (3:1; v:v), using a Ultra-Turrax set at 24 000 rpm/ min (Janke & Kunkel, Staufen, Germany). The precipitating solution was prepared weekly and its pH (7.4) was checked immediately before use. After centrifugation at 20 690 ×
g, for 10 min at 4 °C, the clear supernatant was saved; pellets were supplemented with 3 mL of the precipitating solution and homogenized again as described above. A second centrifugation was performed (20 690 ×
g, for 10 min at 4 °C), pellets were saved, and supernatant combined with that previously obtained, extracted by vigorous agitation with a double volume of CHCl
3, and centrifuged as above. The upper aqueous phases (containing water-soluble low-molecular-weight compounds) were collected, subjected to CHCl
3 washings for two more times (this procedure allowed the removal of all the organic solvent and any lipid soluble compound from the buffered tissue extracts) and stored at −80 °C.
2.10. HPLC analysis of adenine nucleotides
Tissue extracts were filtered through a 0.2-μm PVDF filter and then assayed by ion pairing HPLC for the simultaneous separation of AMP, ADP and ATP. HPLC analysis was performed using Agilent 1200 Series system, consisting of diode array detector, manual injector with 50-μL loop, vacuum degasser, quaternary pump, symmetry 3.5 μm, 4.6 × 100 mm column with precolumn (Waters Corporation, USA) and ChemStation software for data processing (Agilent Technologies, USA). The conditions of chromatographic analysis were column temperature of 25 °C and injection volume of 50 μL. There were the following eluents: A, 10 mM KPi (pH 5), 2 mM TBA (tetrabutylammonium bromide), 3% acetonitrile; and B, 10 mM KPi (pH 7.5), 2 mM TBA, 30% acetonitrile. The following gradient elution was used: B 0 min, 1%; 30 min, 95%; and 32 min, 1%. Detection wavelength was 259 nm. AMP, ADP, and ATP in the samples were identified by comparison with retention times of the standards. The concentrations of analytes were determined from peak area using the external standard (0–20 μM) method.
2.11. Statistical analysis
Data are presented as means of 3–8 independent experiments ± standard error. The means of each individual experiment with the same mitochondrial preparation were obtained repeating each measurement for 3 times. Statistical significance of the temperature effects was evaluated using the Student t test (unpaired). The differences were assumed to be statistically significant at P < 0.05.
4. Discussion
The choice of the range of liver perfusion temperatures used in this study was based on temperature fluctuations determined in the transition zone of the liver tissue during radiofrequency ablation [
5,
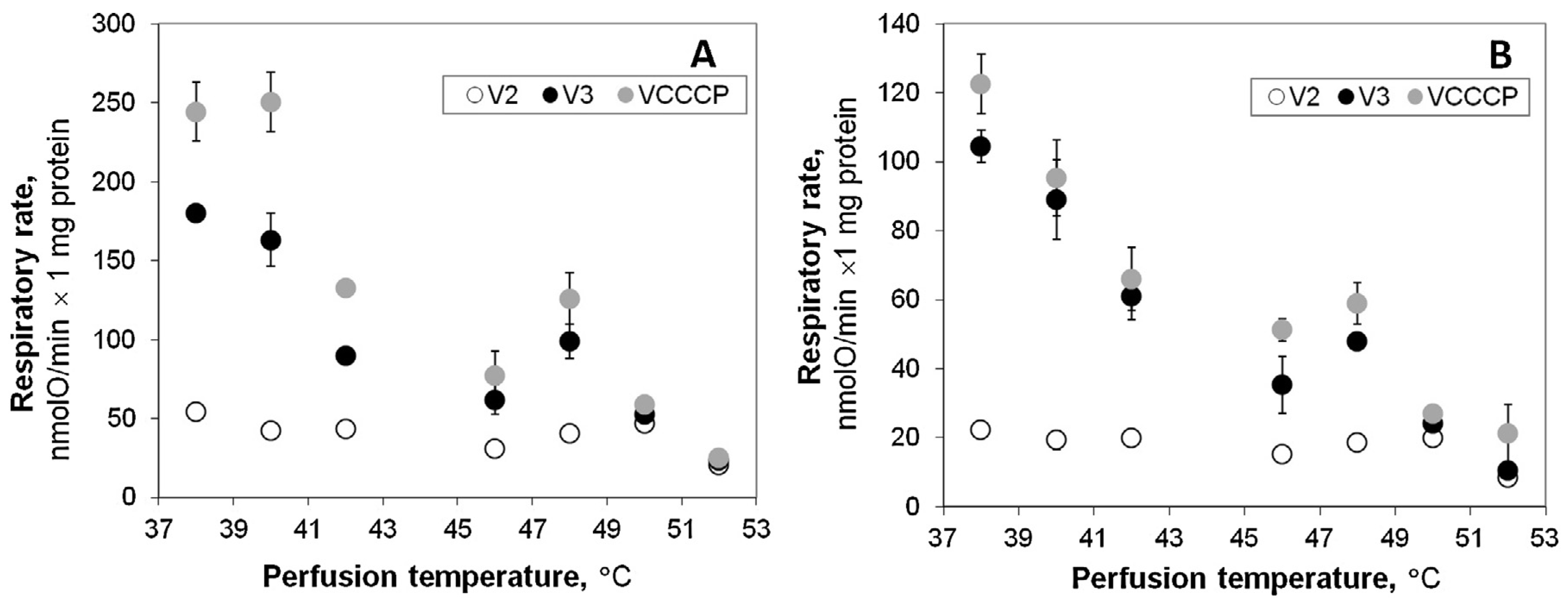
22] – temperatures were shown to increase up to 44–50 °C in the transition zone, which is considered as potentially responsible for the local tumor recurrence. In this study we used experimental model of hyperthermic liver perfusion to estimate how such changes in temperature may affect function of mitochondria and energy status of the healthy tissue. We compared respiration rates registered in mitochondria isolated both from liver subjected to relatively short (15 min) normothermic (38 °C) and hyperthermic (40– 52 °C) perfusion. The obtained results show the absence of differences in respiration of mitochondria isolated from liver after perfusion at 40 and at 38 °C (control). However, increase of the perfusion temperature to 42 °C caused obvious irreversible injury of oxidative phosphorylation: the activity of the respiration in isolated mitochondria respiring with pyruvate + malate or with succinate was significantly inhibited (
Fig. 1). The observed decrease in
VCCCP clearly shows the impaired function of the respiratory chain, while the stable value in membrane potential in state 3 (
Fig. 2) can also indicate possible inhibition of the phosphorylation subsystem components. Liver perfusion at 42 °C did not affect permeability of the inner mitochondrial membrane (
Fig. 3). Such effect became evident only after liver perfusion at 46 °C together with the progressive inhibition of the respiratory subsystem – both these effects led to the substantial drop (about 20 mV) in Δ
ψ (
Fig. 2) both in state 2 and state 3. Further increase in perfusion temperature to 48 °C had strong uncoupling effect (
Fig. 3), and severely compromised mitochondrial respiration (
Fig. 1). At 50–52 °C liver mitochondria completely lost their energetic functions (RCI values close to 1), and may even hydrolyze ATP leading to the depletion of cellular energetic resources.
The results indicating uncoupling of oxidative phosphorylation by direct hyperthermia (when heating was applied in mitochondrial respiration chamber) were reported earlier by us and other authors [
9,
10,
11]. There was only some discrepancy concerning the lowest range of uncoupling temperature – there was no change in ADP/O ratio detected in liver mitochondria respiring on b-hydroxybutyrate and succinate at 40 °C by the most early study [
23], whereas small but statistically significant
V2 increase was reported at this temperature in liver mitochondria oxidizing pyruvate + malate [
11]. The obtained results indicate that even if direct hyperthermia might lead to some uncoupling at 40 °C [
11], such effect is reversible as it is not observed in our experimental model (
Fig. 1). Obvious uncoupling effect (increase in
V2) in the higher range of direct hyperthermia (42 and 43 °C) was reported [
10,
11], however we did not register
V2 increase in liver mitochondria isolated after hyperthermic liver perfusion at 42 °C (
Fig. 1). Instead, this model provided evidence for twice stronger and irreversible suppression of the respiratory chain activity as compared to the direct mitochondrial heating model [
11], and this difference at least partially may be explained by a longer duration of hyperthermic treatment (15 min in this study versus 7 min [
11]). Thus, the experimental model used in this study allowed to demonstrate that inhibition of the respiratory chain by hyperthermia is irreversible and that contribution of this effect to the loss of the efficiency of oxidative phosphorylation in liver upon mild hyperthermic condition (42 °C) can be far more important than impact of the mostly possibly reversible increase in the membrane permeability.
We have shown previously, that isolated heart mitochondria responded with increased
V2 to heating of their respiration medium in the range from 42 to 48 °C, indicating an increased membrane permeability [
8]. In line with these observations, strong uncoupling of oxidative phosphorylation with ATP synthase reversal was reported in mitochondria isolated from the heart subjected to hyperthermic perfusion at 43 °C [
24]. Uncoupling was also significant but much less pronounced in isolated rat liver mitochondria, but statistically significant
V2 increase was observed at lower temperature – 40 °C [
11].
The response of mitochondria isolated from the liver subjected to hyperthermic perfusion was different in comparison to direct response of isolated mitochondria [
11], namely, the perfusion did not cause increase in
V2 (
Fig. 1). This difference may be explained by three possible reasons: (1) mitochondrial membrane permeability increases only when mitochondria are exposed to hyperthermic treatment in vitro (‘‘direct’’ hyperthermia), but not in the perfused liver; (2) hyperthermia-induced changes in
V2 are reversible, therefore they are observable only upon direct hyperthermic treatment in the polarographic chamber but not in mitochondria isolated from the liver subjected to hyperthermic perfusion; (3) hyperthermia causes strong inhibition of the respiratory chain, evidenced by the substantial drop both in
V3 and Δ
ψ, and the latter effect is masking increase in the membrane permeability – at lower Δ
ψ values of
V2 are much lower despite increased membrane leak. The data presented in
Fig. 3 provide evidence that mitochondrial membrane permeability was irreversibly increased starting from perfusion at 46 °C, implying that inhibition of the respiratory chain is prevailing effect of hyperthermia at 42 °C, but in the higher range of temperatures this effect is supplemented by the substantial increase in the membrane permeability.
The published data indicates that the components of the oxidative phosphorylation system are quite resistant to heat denaturation [
25,
26,
27,
28,
29,
30]. In the environment of lipids the thermodenaturation of complex III was observed at 64 °C [
25]; complex IV, at 63 °C [
26,
27]; cytochrome C, at 80 °C [
28]; and ATP synthase, at 70 °C [
29] or 60 °C [
30]. Mitochondrial respiration with succinate and pyruvate + malate in the uncoupled state was inhibited to similar extent by hyperthermic perfusion, indicating that thermal sensitivity of the respiratory chain Complex I is not very important for the observed effects. Therefore, it is unlikely that all mentioned proteins may start melting at 45 °C, subsequently leading to a loss of the respiratory chain activity. However, the activity of the enzymes imbedded in the mitochondrial membrane is highly dependent on the adjacent environment of the membrane lipids. The membrane structures formed from the lipids are most sensitive to hyperthermia compared to other cellular structures, i.e. even mild hyperthermia initiates changes in physical properties of cellular membranes [
31]. Complex phase transition in isolated rat liver mitochondria starting from physiological and going up to higher temperatures was reported [
14]. The changes of lipid–protein interactions may explain non-specific effects of the relatively short hyperthermia on biological functions of various proteins. In addition, hyperthermia at 45 °C results in significant increase in the permeability to ions of plasma and intracellular membranes in mammalian cells, leading to a substantial ion imbalance and Ca
2+ overload [
32,
33]. It might have additional adverse effects on the activity of various enzymes. The results of Δ
ψ measurements (
Fig. 3) indicate that reversal of ATP synthase in mitochondria isolated from the perfused liver requires higher perfusion temperatures (50–52 °C) in comparison to the similar situation in the perfused heart (43 °C) [
24].
Experiments for the estimation of effects of hyperthermic incubation on viability of isolated hepatocytes (
Fig. 4) were performed aiming to evaluate potential importance of mitochondrial injury for hepatocyte survival. The results revealed that viability of hepatocytes is not affected by hyperthermic incubation up to 45 °C, despite quite substantial impairment of mitochondrial respiratory function at 42 °C (
Fig. 1). Low ATP/ ADP ratio detected in isolated hepatocytes (
Table 1) strongly favors stimulation of glycolytic energy production [
34], therefore partial decrease in mitochondrial energy production may be not crucial for energy supply in these cells. Only more severe hyperthermia (46 °C and above) causing both uncoupling (
Fig. 3) and progressive inhibition of the respiratory chain (
Fig. 1) correlated with substantially compromised energy status (
Table 1) and reduced viability of hepatocytes (
Fig. 4). Furthermore, both energy status (
Table 1) and viability of hepatocytes was strongly compromised at 50–52 °C, under conditions when the reversal of mitochondrial ATP synthase activity may cause depletion of all cellular energy resources, including ATP obtained from glycolysis.
Thus, the obtained results on mitochondrial response to hyperthermia are consistent with changes in viability and energy resources of hepatocytes. However, it was more complicated to connect the observed changes in mitochondrial functions with changes in the amounts of adenine nucleotides detected in the perfused liver tissue (
Table 2). Only several tendencies characteristic for isolated hepatocytes were kept in the liver tissue as well, e.g., high AMP amount and low ATP/ADP ratio after perfusion at 38 °C, and this may reflect situation in the tissue relying mostly on glycolytic energy supply [
34,
35]. Our results (
Table 2) demonstrate that increase in perfusion temperature to 46–50 °C leads to the substantially increased total amount of adenine nucleotides in the liver tissue as compared to the controls (perfusion at 38 °C), and this effect was opposite to the tendency observed in experiments on isolated hepatocytes (
Table 1). It is important to note, that adenine nucleotide amounts presented in
Table 2 are averaged values for all tissue parts and hardly reflect energy status inside hepatocytes. The increased amount of ATP to some extent might be associated with the inhibition of ATP consumption processes in liver cells. On the other hand, these unexpected observations may be explained by very complex interaction of various cell types, enzymes and events occurring at the same time inside cells, within the intercellular matrix, in vascular interface and in the interior of blood vessels. The discovery of the regulated release of different nucleotides from various cell types has been met very skeptically; however, these phenomena were proved as universal and now are finally accepted [
36,
37,
38]. Cellular nucleotides are released to the extracellular milieu in a regulated manner to accomplish extracellular signaling and under stress conditions such release is massively stimulated. The transporters mediating reabsorption of nucleotides back to the cells were also described [
37]. The question how hyperthermia affects enzymes and transporters involved in the release (or reabsorption) of nucleotides from various cell types (or extracellular milieu) and how nucleotides are distributed among cells and tissue compartments deserves future investigations. Our results indicate that stress caused by hyperthermia interferes with multiple processes in liver tissue and leads to substantial changes in the amounts of adenine nucleotides in the liver tissue. However, we do not have sufficient information on the nature of these changes; therefore, it is not possible to directly associate the observed impairment of mitochondrial functions to changes in the tissue adenine nucleotide levels and energy status. The remarkable increase in ATP amount in tissue and inside liver cells may be in favor for self-destruction process (apoptosis) requiring energy resources. Decreased ATP consumption and inhibition of ATP synthesis at higher temperatures can be mark of the beginning of cell death. The above events are substantiated by our recent report, demonstrating that apoptosis is activated in the majority of cells in the transition zone, where temperature ranged from 42.8 to 48.9 °C [
5]. The comparative investigations of the response of normal hepatocytes and transformed cancerogenic cells in this range of hyperthermia could be helpful for evaluating the recurrence potential in the transition zone.