Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia
Abstract
:1. Introduction
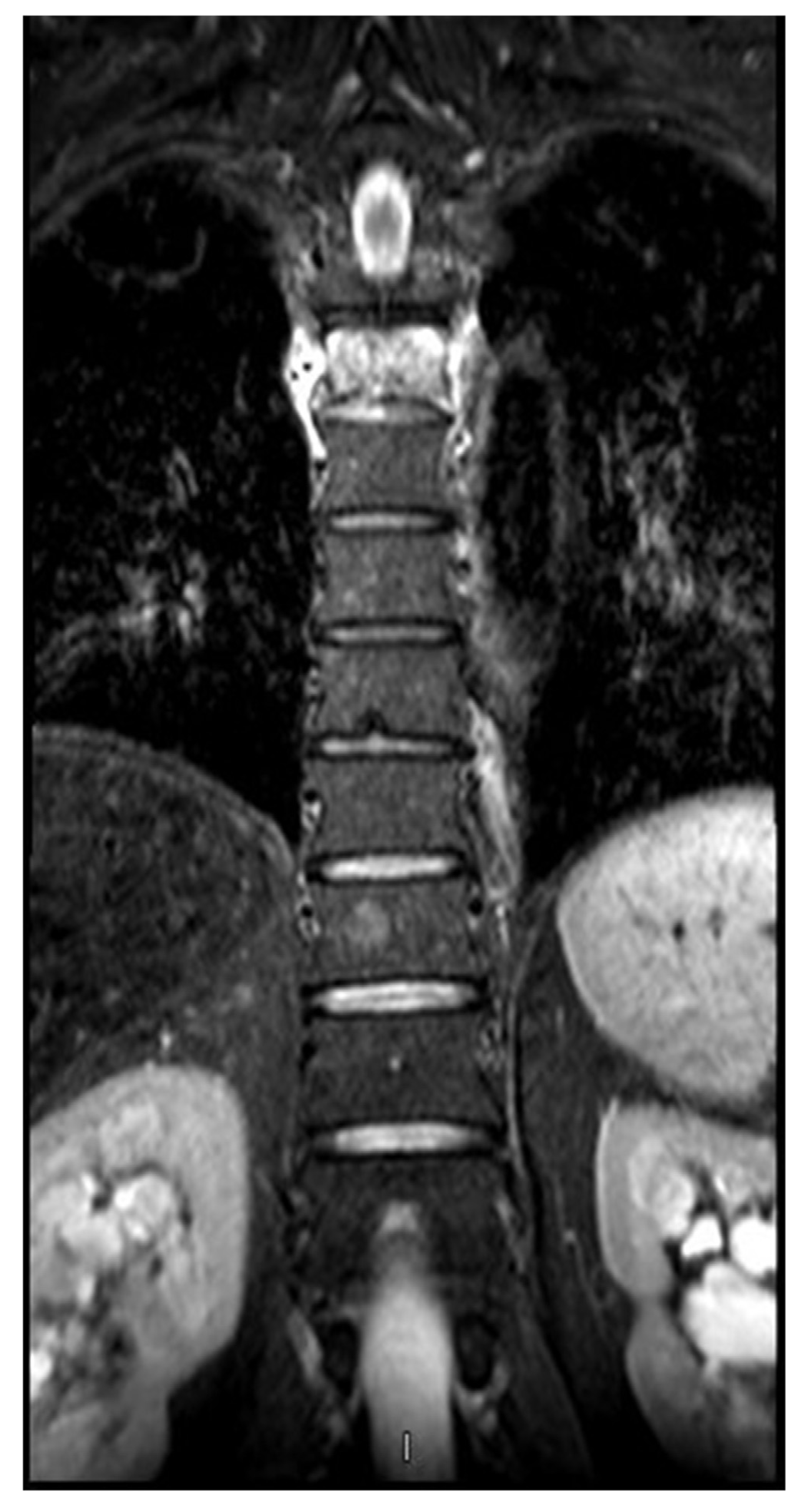
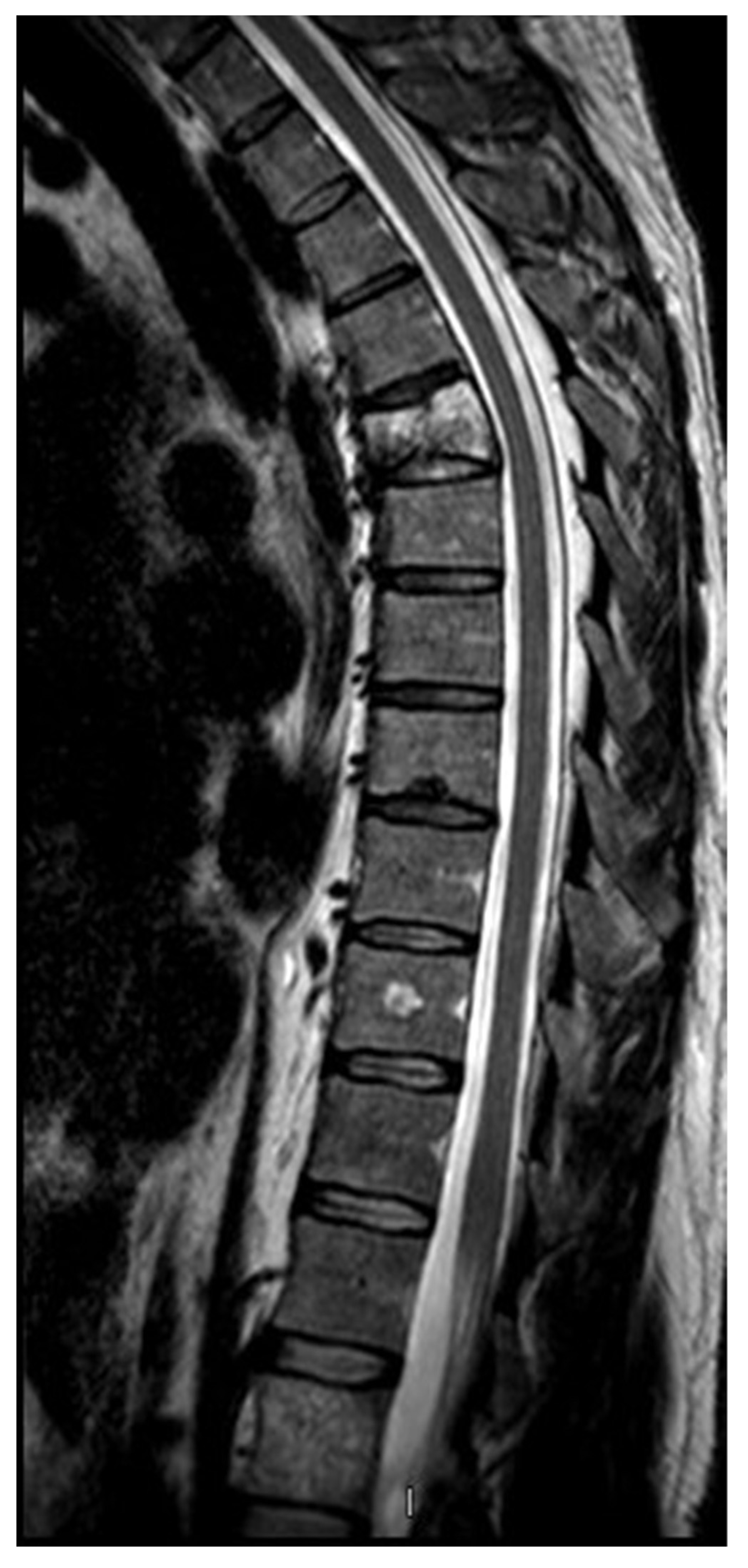
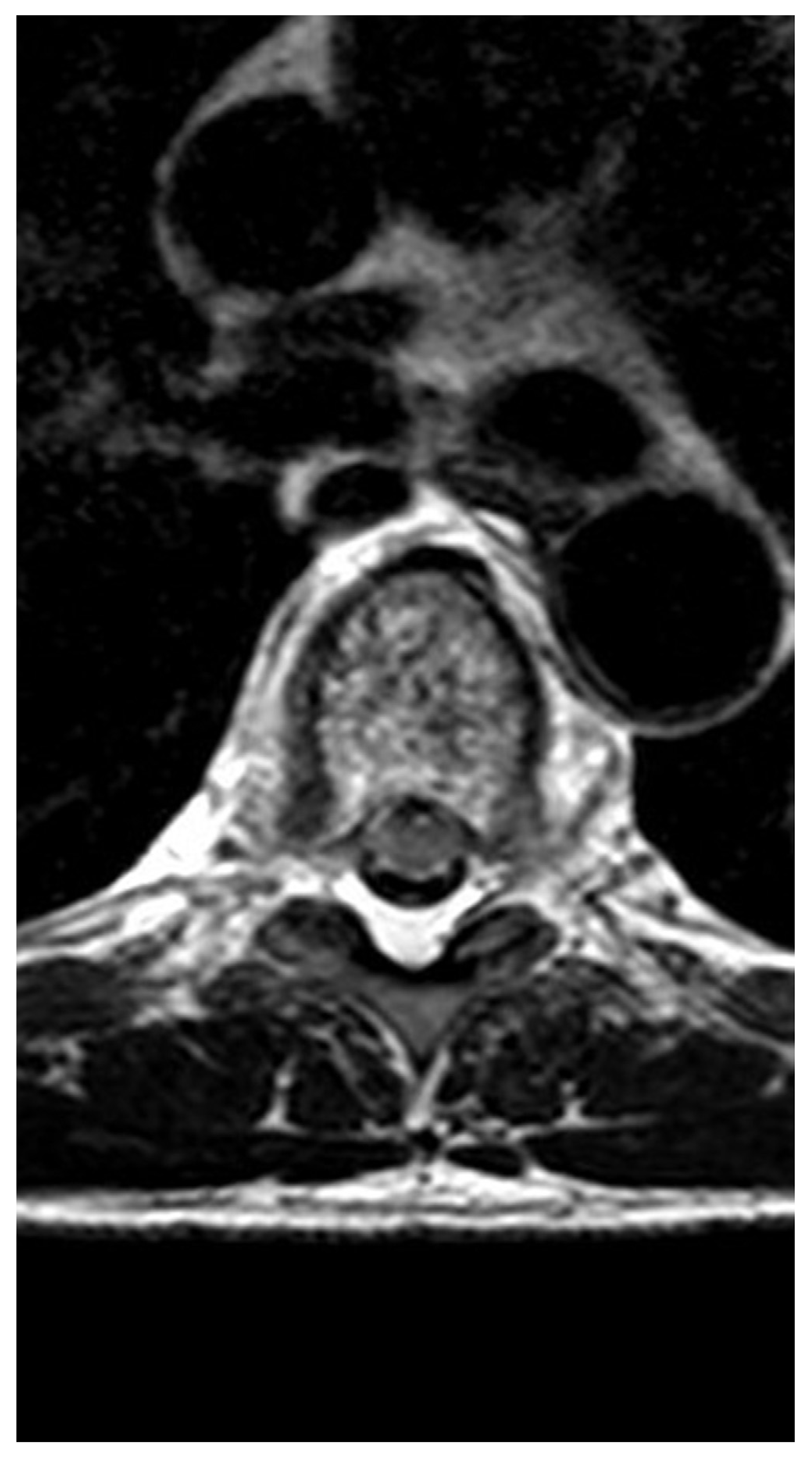
2. Case presentation
3. Discussion
3.1. Vertebral hemangiomas and potential symptoms
3.2. Master athlete and osteopenia
3.3. Osteopenia and sex hormones
3.4. Osteopenia and age
4. Conclusions
Competing interests
Author Contributions
Consent to publish
Funding
References
- Sweet, C; Silbergleit, R; Mehta, B. Primary intraosseous hemangioma of the orbit: CT and MR appearance. Am J Neuroradiol 1997, 18(2), 379–81. [Google Scholar]
- Murphey, MD; Fairbairn, KJ; Parman, LM. Musculoskeletal angiomatous lesions: radiologic-pathologic correlation. Radiographic 1995, 15(4), 893–917. [Google Scholar] [CrossRef] [PubMed]
- Gaudino, S; Martucci, M; Colantonio, R; Lozupone, E; Visconti, E; Leone, A; et al. A systematic approach to vertebral hemangioma. Skelet Radiol 2015, 44(1), 25–36. [Google Scholar]
- Slon, V; Peled, N; Abbas, J; Stein, D; Cohen, H; Hershkovitz, I. Vertebral hemangiomas and their correlation with other pathologies. Spine 2016, 41(8), E481–8. [Google Scholar]
- Fox, MW; Onofrio, BM. The natural history and management of symptomatic and asymptomatic vertebral hemangiomas. J Neurosurg 1993, 78, 36–45. [Google Scholar]
- Hao, J; Hu, Z. Percutaneous cement vertebroplasty in the treatment of symptomatic vertebral hemangiomas. Pain Physician 2012, 15(1), 43–9. [Google Scholar]
- Jones, J; Bruel, BM; Vattam, SR. Management of painful vertebral hemangiomas with kyphoplasty: a report of two cases and a literature review. Pain Physician 2009, 12(4), E297–303. [Google Scholar]
- Slon, V; Stein, D; Cohen, H; Sella-Tunis, T; May, H; Hershkovitz, I. Vertebral hemangiomas: their demographical characteristics, location along the spine and position within the vertebral body. Eur Spine J 2015, 24(10), 2189–95. [Google Scholar]
- Slon, V; Peled, N; Abbas, J; Stein, D; Cohen, H; Hershkovitz, I. Vertebral hemangiomas and their correlation with other pathologies. Spine (Phila Pa 1976) 2016, 41(8), E481–8. [Google Scholar]
- Guarnieri, G; Ambrosanio, G; Vassallo, P; Pezzullo, MG; Galasso, R; Lavanga, A; et al. Vertebroplasty as treatment of aggressive and symptomatic vertebral hemangiomas: up to 4 years of follow-up. Neuroradiology 2009, 51(7), 471–6. [Google Scholar]
- Rand, T; Lomoschitz, F; Cejna, M; Grohs, A; Kettenbach, J. Percutaneous radiologically-guided vertebroplasty in the treatment of osteoporotic and tumorous spinal body lesions. Radiologe 2003, 43(9), 723–8. [Google Scholar]
- Zhang, X; Lin, J; Yang, X; Fei, Q; Wang, B; Yang, Y; et al. Investigation of osteoporosis prevalence and osteoporosis- related clinical risk factors among healthy elderly male. Zhonghua Yi Xue Za Zhi 2015, 95(41), 3366–9. [Google Scholar]
- Cauley, JA; Zmuda, JM; Wisniewski, SR; Krishnaswami, S; Palermo, L; Stone, KL; et al. Bone mineral density and prevalent vertebral fractures in men and women. Osteoporos Int 2004, 15(1), 32–7. [Google Scholar]
- Jones, G; White, C; Nguyen, T; Sambrook, PN; Kelly, PJ; Eisman, JA. Prevalent vertebral deformities: relationship to bone mineral density and spinal osteophytosis in elderly men and women. Osteoporos Int 1996, 6(3), 233–9. [Google Scholar]
- Legrand, E; Chappard, D; Pascaretti, C; Duquenne, M; Rondeau, C; Simon, Y; et al. Bone mineral density and vertebral fractures in men. Osteoporos Int 1999, 10(4), 265–70. [Google Scholar]
- Hongsdusit, N; von Mühlen, D; Barrett-Connor, E. A comparison between peripheral BMD and central BMD measurements in the prediction of spine fractures in men. Osteoporos Int 2006, 17(6), 872–7. [Google Scholar]
- Kherad, M; Mellström, D; Rosengren, BE; Hasserius, R; Nilsson, JÅ; Redlund-Johnell, I; et al. The number and characteristics of prevalent vertebral fractures in elderly men are associated with low bone mass and osteoporosis. Bone Jt J 2015, 97B(8), 1106–10. [Google Scholar]
- Geesmann, B; Gibbs, JC; Mester, J; Koehler, K. Association between energy balance and metabolic hormone suppression during ultra-endurance exercise. Int J Sports Physiol Perform 2016, 1–20, [Epub ahead of print]. [Google Scholar]
- Guglielmini, C; Paolini, AR; Conconi, F. Variations of serum testosterone concentrations after physical exercises of different duration. Int J Sports Med 1984, 5(5), 246–9. [Google Scholar]
- MacKelvie, KJ; Taunton, JE; McKay, HA; Khan, KM. Bone mineral density and serum testosterone in chronically trained, high mileage 40–55 year old male runners. Br J Sports Med 2000, 34(4), 273–8. [Google Scholar]
- Lucas, SJE; Helge, JW; Schütz, UHW; Goldman, RF; Cotter, JD. Moving in extreme environments: extreme loading; carriage versus distance. Extrem Physiol Med 2016, 5(6). [Google Scholar]
- Voss, LA; Fadale, PD; Hulstyn, MJ. Exercise-induced loss of bone density in athletes. J Am Acad Orthop Surg 1998, 6(6), 349–57. [Google Scholar]
- Auchus, RJ. Steroid assays and endocrinology: best practices for basic scientists. Endocrinology 2014, 155(6), 2049–51. [Google Scholar]
- Scofield, KL; Hecht, S. Bone health in endurance athletes: runners, cyclists, and swimmers. Curr Sports Med Rep 2012, 11(6), 328–34. [Google Scholar]
- Skarda, ST; Burge, MR. Prospective evaluation of risk factors for exercise-induced hypogonadism in male runners. West J Med 1998, 169(1), 9–12. [Google Scholar]
- Ackerman, KE; Skrinar, GS; Medvedova, E; Misra, M; Miller, KK. Estradiol levels predict bone mineral density in male collegiate athletes: a pilot study. Clin Endocrinol (Oxf) 2012, 76(3), 339–45. [Google Scholar]
- Mellström, D; Vandenput, L; Mallmin, H; Holmberg, AH; Lorentzon, M; Odén, A; et al. Older men with low serum estradiol and high serum SHBG have an increased risk of fractures. J Bone Miner Res 2008, 23(10), 1552–60. [Google Scholar]
- Vandenput, L; Mellström, D; Kindmark, A; Johansson, H; Lorentzon, M; Ohlsson, C. High serum SHBG predicts incident vertebral fractures in elderly men. J Bone Miner Res 2016, 31(3), 683–9. [Google Scholar]
- Cawthon, PM; Schousboe, JT; Harrison, SL; Ensrud, KE; Black, D; Cauley, JA; et al. Osteoporotic Fractures in Men (MrOS) Study Research Group. Sex hormones, sex hormone binding globulin, and vertebral fractures in older men. Bone 2016, 84, 271–8. [Google Scholar]
- Hoppé, E; Bouvard, B; Royer, M; Audran, M; Legrand, E. Sex hormone-binding globulin in osteoporosis. Jt Bone Spine 2010, 77(4), 306–12. [Google Scholar]
- Legrand, E; Hedde, C; Gallois, Y; Degasne, I; Boux de Casson, F; Mathieu, E; et al. Osteoporosis in men: a potential role for the sex hormone binding globulin. Bone 2001, 29(1), 90–5. [Google Scholar]
- Lormeau, C; Soudan, B; d’Herbomez, M; Pigny, P; Duquesnoy, B; Cortet, B. Sex hormone-binding globulin, estradiol, and bone turnover markers in male osteoporosis. Bone 2004, 34(6), 933–9. [Google Scholar]
- Gillberg, P; Johansson, AG; Ljunghall, S. Decreased estradiol levels and free androgen index and elevated sex hormone-binding globulin levels in male idiopathic osteoporosis. Calcif Tissue Int 1999, 64(3), 209–13. [Google Scholar]
- Pietschmann P; Kudlacek, S; Grisar, J; Spitzauer, S; Woloszczuk, W; Willvonseder, R; et al. Bone turnover markers and sex hormones in men with idiopathic osteoporosis. Eur J Clin Investig 2001, 31(5), 444–51. [Google Scholar]
- Di Luigi, L; Sgrò, P; Fierro, V; Bianchini, S; Battistini, G; Magini, V; et al. Prevalence of undiagnosed testosterone deficiency in aging athletes: does exercise training influence the symptoms of male hypogonadism? J Sex Med 2010, 7(7), 2591–601. [Google Scholar]
- Liu, CC; Wu, WJ; Lee, YC; Wang, CJ; Ke, HL; Li, WM; et al. The prevalence of and risk factors for androgen deficiency in aging Taiwanese men. J Sex Med 2009, 6(4), 936–46. [Google Scholar]
- Vega, E; Ghiringhelli, G; Mautalen, C; Rey Valzacchi, G; Scaglia, H; Zylberstein, C. Bone mineral density and bone size in men with primary osteoporosis and vertebral fractures. Calcif Tissue Int 1998, 62(5), 465–9. [Google Scholar]
- Kanis, JA; Johansson, H; Oden, A; Johnell, O; De Laet, C; Eisman, JA; et al. A family history of fracture and fracture risk: a meta-analysis. Bone 2004, 35(5), 1029–37. [Google Scholar]


© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Knechtle, B.; Nikolaidis, P.T.; Lutz, B.; Rosemann, T.; Baerlocher, C.B. Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia. Medicina 2017, 53, 131-137. https://doi.org/10.1016/j.medici.2017.02.003
Knechtle B, Nikolaidis PT, Lutz B, Rosemann T, Baerlocher CB. Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia. Medicina. 2017; 53(2):131-137. https://doi.org/10.1016/j.medici.2017.02.003
Chicago/Turabian StyleKnechtle, Beat, Pantelis T. Nikolaidis, Bruno Lutz, Thomas Rosemann, and Christian B. Baerlocher. 2017. "Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia" Medicina 53, no. 2: 131-137. https://doi.org/10.1016/j.medici.2017.02.003
APA StyleKnechtle, B., Nikolaidis, P. T., Lutz, B., Rosemann, T., & Baerlocher, C. B. (2017). Pathologic fracture of the thoracic spine in a male master ultra-marathoner due to the combination of a vertebral hemangioma and osteopenia. Medicina, 53(2), 131-137. https://doi.org/10.1016/j.medici.2017.02.003




