Plasticity of vagal afferent signaling in the gut
Abstract
:1. Introduction
2. Vagal sensory innervation of the gut
3. Regulation of vagal sensory neurons by gut peptides
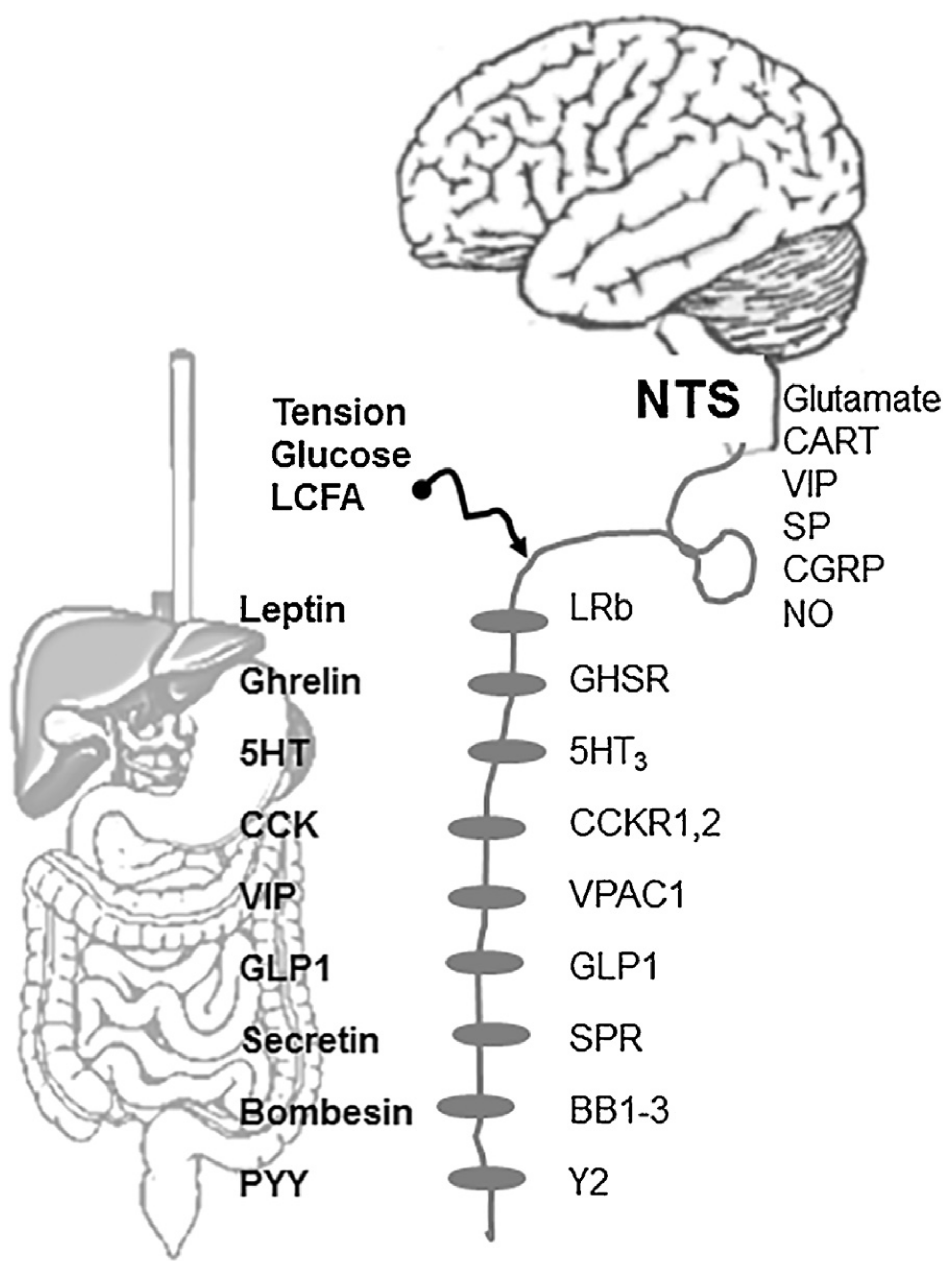
3.1. CCK
| Mediator | Source | Receptor | Vagally mediated function | References |
|---|---|---|---|---|
| CCK | Intestinal EC I-cells | CCK1 CCK2 | Contraction of gallbladder; Pancreatic stimulation; Satiety; Motility; Acid secretion. | [4,54,55,56,57,58,59,60] |
| Leptin | Adiposities; EC Parietal cells | LRb | Inhibition of food intake; mechano-sensitization | [9,16,61,62] |
| Ghrelin | EC x/A cells in the stomach | GHSR1a | Induces food intake; insulin secretion; gastric acid secretion; gastric emptying, relaxation; intestinal transit; fat tissue metabolism; regulates gene transcription and modulates neural electrical activity | [63,64,65,66,67,68,69,70,71,72] |
| Bombesin | Endocrine cells gastric mucosa | BB1 BB2 BR3 | Inhibition of gastric emptying; inhibition of food intake; stimulates gastric acid secretion and gastrin release. | [73,74,75] |
| PYY | Small intestine L-cells | Y2 | Decrease intragastric tone; inhibit gastric acid secretion | [11,76,77,78] |
| Secretin | S-cells duodenum | SPR | Stimulates pancreatic bicarbonate secretion; Inhibits gastric acid secretion and gastric motility. | [79,80] |
| GLP1 | Small intestine L-cells Pancreatic A-cells | GLP1 | Anorexic signals; Inhibits gastric secretion; reduces ghrelin plasma concentrations. | [81,82,83,84,85] |
| 5HT (serotonin) | EC cells, ENS neurons | 5HT3 | Stimulates bowel transit; induces nausea and vomiting. | [86,87,88] |
| VIP | Duodenum | VPAC1 | Inhibits gastric acid secretion. | [89] |
3.2. Leptin
3.3. Ghrelin
4. Receptor interaction
4.1. Feeding status modulates neurochemical phenotype of vagal sensory neurons
4.2. Synergistic interactions between gut neuromediators in vagal ganglia
5. Central projections of visceral afferents
| Mediator | Vagally mediated function | References |
|---|---|---|
| Glutamate | Mediates CCK-induced satiation; Modulates feeding behavior. | [159,160,161] |
| CART | Induces satiation | [13,150,162] |
| VIP | Inhibits gastric acid secretion | [89,163] |
| SP | Modulate synaptic input to NTS | [164,165,166] |
| CGRP | Induces satiety; Gastric mucosa protection | [165,167,168,169] |
| NO | Enhances mechano-sensitivity | [170,171] |
6. Vagal afferent pathways in clinical conditions
6.1. Diabetes modulates excitability of vagus
6.2. High fat feeding induces dysfunction of the vagus
6.3. Vagal CGRP mediates gastric mucosa defense
7. Concluding remarks
Conflicts of interest
Acknowledgements
R E F E R E N C E S
- Moran, TH; Baldessarini, AR; Salorio, CF; Lowery, T; Schwartz, GJ. Vagal afferent and efferent contributions to the inhibition of food intake by cholecystokinin. Am J Physiol 1997, 272, R1245–51. [Google Scholar]
- Schwartz, GJ; Moran, TH. Duodenal nutrient exposure elicits nutrient-specific gut motility and vagal afferent signals in rat. Am J Physiol 1998, 274, R1236–42. [Google Scholar]
- Lloyd, KCK; Holzer, HH; Zittel, TT; Raybould, HE. Duodenal lipid inhibits gastric acid secretion by vagal, capsaicin sensitive pathways in rats. Am J Physiol Gastrointest Liver Physiol 1993, 264, G659–63. [Google Scholar]
- Li, Y; Owyang, C. Vagal afferent pathways mediate physiological action of cholecystokinin on pancreatic secretion. J Clin Invest 1993, 92, 418–24. [Google Scholar]
- Schwartz, GJ; Berkow, G; McHugh, PR; Moran, TH. Gastric branch vagotomy blocks nutrient and cholecystokinin-induced suppression of gastric emptying. Am J Physiol 1993, 264, R630–7. [Google Scholar]
- Lal, S; Kirkup, AJ; Brunsden, AM; Thompson, DG; Grundy, D. Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 2001, 281, G907–15. [Google Scholar]
- Grabauskas, G; Song, I; Zhou, S; Owyang, C. Electrophysiological identification of glucose-sensing neurons in rat nodose ganglia. J Physiol 2010, 588, 617–32. [Google Scholar]
- Grabauskas, G; Zhou, SY; Lu, Y; Song, I; Owyang, C. Essential elements for glucosensing by gastric vagal afferents: immunocytochemistry and electrophysiology studies in the rat. Endocrinology 2013, 154, 296–307. [Google Scholar]
- Kentish, SJ; O'Donnell, TA; Isaacs, NJ; Young, RL; Li, H; Harrington, AM; et al. Gastric vagal afferent modulation by leptin is influenced by food intake status. J Physiol 2013, 591, 1921–34. [Google Scholar]
- Burdyga, G; Lal, S; Varro, A; Dimaline, R; Thompson, DG; Dockray, GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons is inhibited by cholecystokinin. J Neurosci 2004, 24, 2708–15. [Google Scholar]
- Burdyga, G; de Lartigue, G; Raybould, HE; Morris, R; Dimaline, R; Varro, A; et al. Cholecystokinin regulates expression of Y2 receptors in vagal afferent neurons serving the stomach. J Neurosci 2008, 28, 11583–92. [Google Scholar]
- Burdyga, G; Varro, A; Dimaline, R; Thompson, DG; Dockray, GJ. Expression of cannabinoid CB1 receptors by vagal afferent neurons: kinetics, and role in influencing neurochemical phenotype. Am J Physiol Gastrointest Liver Physiol 2010, 299, G1514–2. [Google Scholar]
- de Lartigue, G; Barbier de la Serre, C; Espero, E; Lee, J; Raybould, HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinology and Metabolism, 2011; 30, 1, E187–95. [Google Scholar]
- de Lartigue, G; Dimaline, R; Varro, A; Dockray, GJ. Cocaine- and amphetamine-regulated transcript: stimulation of expression in rat vagal afferent neurons by cholecystokinin and suppression by ghrelin. J Neurosci 2007, 27, 2876–82. [Google Scholar]
- Daly, DM; Park, SJ; Valinsky, WC; Beyak, MJ. Impaired intestinal afferent nerve satiety signaling and vagal afferent excitability in diet induced obesity in the mouse. J Physiol 2011, 589, 2857–70. [Google Scholar]
- Kentish, S; Li, H; Philp, LK; O'Donnell, TA; Isaacs, NJ; Young, RL; et al. Diet-induced adaptation of vagal afferent function. J Physiol 2012, 590, 209–21. [Google Scholar]
- Kentish, SJ; Frisby, CL; Kennaway, DJ; Wittert, GA; Page, AJ. Circadian variation in gastric vagal afferent mechanosensitivity. J Neurosci 2013, 33, 19238–21942. [Google Scholar]
- Baker, CV; Bronner-Fraser, M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol 2001, 232, 1–61. [Google Scholar]
- Blackshaw, LA; Grundy, D. Responses of vagal efferent fibres to stimulation of gastric mechano- and chemoreceptors in the anaesthetized ferret. J Auton Nerv Syst 1989, 27, 39–45. [Google Scholar]
- Cervero, F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiol Rev 1994, 74(1), 95–138. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, JN; Gebhart, GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol 1994, 71(6), 2046–60. [Google Scholar]
- Zagorodnyuk, VP; Chen, BN; Brookes, SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol 2001, 534, 255–68. [Google Scholar]
- El Ouazzani, T; Mei, N. Electrophysiologic properties and role of the vagal thermoreceptors of lower esophagus and stomach of cat. Gastroenterology 1982, 83, 995–1001. [Google Scholar]
- Grundy, D; Hutson, D; Rudge, LJ; Scratcherd, T. Pre-pyloric mechanisms regulating gastric motor function in the conscious dog. J Exp Physiol 1989, 74, 857–65. [Google Scholar]
- Page, AJ; Blackshaw, LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol 1998, 512, 907–16. [Google Scholar]
- Page, AJ; Martin, CM; Blackshaw, LA. Vagal mechanoreceptors and chemoreceptors in mouse stomach and esophagus. J Neurophysiol 2002, 87, 2095–103. [Google Scholar]
- Su, X; Gebhart, GF. Mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat are polymodal in character. J Neurophysiol 1998, 80, 2632–44. [Google Scholar]
- Ness, TJ; Gebhart, GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol 1991, 66, 20–8. [Google Scholar]
- Ness, TJ; Gebhart, GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol 1991, 66, 29–39. [Google Scholar]
- Yu, X; Hu, Y; Ru, F; Kollarik, M; Undem, BJ; Yu, S. TRPM8 function and expression in vagal sensory neurons and afferent nerves innervating guinea pig esophagus. Am J Physiol Gastrointest Liver Physiol 2015, 308, G489–96. [Google Scholar]
- Yu, S; Undem, BJ; Kollarik, M. Vagal afferent nerves with nociceptive properties in guinea pig oesophagus. J Physiol 2005, 563, 831–42. [Google Scholar]
- Page, AJ; Blackshaw, LA. Roles of gastro-oesophageal afferents in the mechanisms and symptoms of reflux disease. Hand Exp Pharmacol 2009, 194, 227–57. [Google Scholar]
- Sengupta, JN; Kauvar, D; Goyal, RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. J Neurophysiol 1989, 61, 1001–10. [Google Scholar]
- Undem, BJ; Weinreich, D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. J Auton Nerv Syst 1993, 44, 17–33. [Google Scholar]
- Berthoud, HR; Kressel, M; Raybould, HE; Neuhuber, WL. Vagal sensors in the rat duodenal mucosa: distribution and structure as revealed by in vivo DiI-tracing. Anat Embryol (Berl) 1995, 191, 203–12. [Google Scholar]
- Williams, RM; Berthoud, HR; Stead, RH. Vagal afferent nerve fibres contact mast cells in rat small intestinal mucosa. Neuroimmunomodulation 1997, 4, 266–70. [Google Scholar]
- Clarke, GD; Davison, JS. Mucosal receptors in the gastric antrum and small intestine of the rat with afferent fibres in the cervical vagus. J Physiol 1978, 284, 55–67. [Google Scholar]
- Ozaki, N; Sengupta, JN; Gebhart, GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. J Neurophysiol 1999, 82, 2210–20. [Google Scholar]
- Ozaki, N; Gebhart, GF. Characterization of mechanosensitive splanchnic nerve afferent fibers innervating the rat stomach. Am J Physiol Gastrointest Liver Physiol 2001, 281, G1449–5. [Google Scholar]
- Blackshaw, LA; Grundy, D. Effect of cholecystokinin on two classes of gastroduodenal vagal afferent fibers. J Auton Nerv Syst 1990, 31, 191–201. [Google Scholar]
- Mei, N; Garnier, L. Osmosensitive vagal receptors in the small intestine of the cat. J Auton Nerv Syst 1986, 16, 159–70. [Google Scholar]
- Kobashi, M; Mizutani, M; Adachi, A. Facilitation of gastric motility induced by portal infusion of hyper- and hypotonic solution in rats. J Auton Nerv Syst 1998, 73(November), 156–62. [Google Scholar] [CrossRef]
- Zhou, JX; Zhu, XY; Owyang, C; Li, Y. Intestinal serotonin acts as a paracrine substance to mediate vagal signal transmission evoked by luminal factors in the rat. J Physiol 2001, 530, 431–42. [Google Scholar]
- Mei, N. Vagal glucoreceptors in the small intestine of the cat. J Physiol 1978, 282, 485–506. [Google Scholar] [CrossRef] [PubMed]
- Berthoud, HR; Lynn, PA; Blackshaw, LA. Vagal and spinal mechanosensors in the rat stomach and colon have multiple receptive fields. Am J Physiol Regul Integr Comp Physiol 2001, 280, R1371–8. [Google Scholar]
- Powley, TL; Wang, XY; Fox, EA; Phillips, RJ; Liu, LW; Huizinga, JD. Ultrastructural evidence for communication between intramuscular vagal mechanoreceptors and interstitial cells of Cajal in the rat fundus. Neurogastroenterol Motil 2008, 20, 69–79. [Google Scholar]
- Phillips, RJ; Powley, TL. Tension and stretch receptors in gastrointestinal smooth muscle: re-evaluating vagal mechanoreceptor electrophysiology. Brain Res Brain Res Rev 2000, 34, 1–26. [Google Scholar]
- Zhang, X; Renehan, WE; Fogel, R. Neurons in the vagal complex of the rat respond to mechanical and chemical stimulation of the GI tract. Am J Physiol 1998, 274, G331–41. [Google Scholar]
- Travagli, RA; Hermann, GE; Browning, KN; Rogers, RC. Brainstem circuits regulating gastric function. Annu Rev Physiol 2006, 68, 279–305. [Google Scholar]
- Dockray, GJ. Gastrointestinal hormones and the dialogue between gut and brain. J Physiol 2014, 592, 2927–41. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A. Tension receptors in the stomach and the urinary bladder. J Physiol 1955, 128, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Cervero, F; Sharkey, KA. An electrophysiological and anatomical study of intestinal afferent fibres in the rat. J Physiol 1988, 401, 381–97. [Google Scholar]
- Wang, GJ; Tomasi, D; Backus, W; Wang, R; Telang, F; Geliebter, A; et al. Gastric distention activates satiety circuitry in the human brain. Neuroimage 2008, 39, 1824–31. [Google Scholar]
- Ivy, AC; Oldberg, EA. A hormone mechanism for gallbladder contraction and evacuation. Am J Physiol 1928, 86, 599–613. [Google Scholar]
- Cameron, AJ; Phillips, SF; Summerskill, WH. Effect of cholecystokinin, gastrin, and glucagon on human gallbladder muscle in vitro. Proc Soc Exp Biol Med 1969, 131, 149–54. [Google Scholar]
- Harper, AA; Raper, HS. Pancreozymin, a stimulant of the secretion of pancreatic enzymes in extracts of the small intestine. J Physiol 1943, 102, 115–25. [Google Scholar]
- Leroy, J; Morisset, JA; Webster, PD. Dose-related response of pancreatic synthesis and secretion to cholecystokinin-pancreazymin. J Lab Clin Med 1971, 78, 149–57. [Google Scholar]
- Gibbs, J; Young, RC; Smith, GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973, 84, 488–95. [Google Scholar]
- Kissileff, HR; Pi-Sunyer, FX; Thornton, J; Smith, GP. C-terminal octapeptide of cholecystokinin decreases food intake in man. Am J Clin Nutr 1981, 34, 154–60. [Google Scholar]
- Li, Y; Owyang, C. Pancreatic secretion evoked by cholecystokinin and non-cholecystokinin-dependent duodenal stimuli via vagal afferent fibres in the rat. J Physiol 1996, 494, 773–82. [Google Scholar]
- Barrachina, MD; Martinez, V; Wang, L; Wei, JY; Tache, Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci USA 1997, 94, 10455–60. [Google Scholar]
- Sachot, C; Rummel, C; Bristow, AF; Luheshi, GN. The role of the vagus nerve in mediating the long-term anorectic effects of leptin. J Neuroendocrinol 2007, 19, 250–61. [Google Scholar]
- Masuda, Y; Tanaka, T; Inomata, N; Ohnuma, N; Tanaka, S; Itoh, Z; et al. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem Biophys Res Commun 2000, 276, 905–8. [Google Scholar]
- Asakawa, A; Inui, A; Kaga, T; Yuzuriha, H; Nagata, T; Ueno, N; et al. Ghrelin is an appetite-stimulatory signal from stomach with structural resemblance to motilin. Gastroenterology 2001, 120, 337–45. [Google Scholar]
- Broglio, F; Arvat, E; Benso, A; Gottero, C; Muccioli, G; Papotti, M; et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab 2001, 86, 5083–6. [Google Scholar]
- Tschop, M; Smiley, DL; Heiman, ML. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–13. [Google Scholar]
- Wortley, KE; del Rincon, JP; Murray, JD; Garcia, K; Iida, K; Thorner, MO; et al. Absence of ghrelin protects against early-onset obesity. J Clin Invest 2005, 115, 3573–8. [Google Scholar]
- Mano-Otagiri, A; Iwasaki-Sekino, T; Nemoto, H; Ohata, Y; Shuto, H; Nakabayashi; et al. Genetic suppression of ghrelin receptors activates brown adipocyte function and decreases fat storage in rats. Regul Pept 2010, 160, 81–90. [Google Scholar]
- Date, Y; Murakami, N; Toshinai, K; Matsukura, S; Niijima, A; Matsuo, H; et al. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002, 123, 1120–8. [Google Scholar]
- Burdyga, G; Varro, A; Dimaline, R; Thompson, DG; Dockray, GJ. Ghrelin receptors in rat and human nodose ganglia: putative role in regulating CB-1 and MCH receptor abundance. Am J Physiol 2006, 290, G1289–97. [Google Scholar]
- Fukuda, H; Mizuta, Y; Isomoto, H; Takeshima, F; Ohnita, K; Ohba, K; et al. Ghrelin enhances gastric motility through direct stimulation of intrinsic neural pathways and capsaicin-sensitive afferent neurons in rats. Scand J Gastroenterol 2004, 39, 1209–14. [Google Scholar]
- Kobashi, M; Yanagihara, M; Fujita, M; Mitoh, Y; Matsuo, R. Fourth ventricular administration of ghrelin induces relaxation of the proximal stomach in the rat. Am J Physiol Regul Integr Comp Physiol 2009, 296, R217–23. [Google Scholar]
- Sayegh, AI. The role of bombesin and bombesin-related peptides in the short-term control of food intake. Prog Mol Biol Transl Sci 2013, 114, 343–70. [Google Scholar] [PubMed]
- Polak, JM; Bloom, SR; Hobbs, S; Solcia, E; Pearse, AG. Distribution of a bombesin-like peptide in human gastrointestinal tract. Lancet 1976, 1(7969), 1109–10. [Google Scholar] [CrossRef]
- Ladenheim, EE; Knipp, S. Capsaicin treatment differentially affects feeding suppression by bombesin-like peptides. Physiol Behav 2007, 91, 36–41. [Google Scholar]
- Janssen, P; Verschueren, S; Rotondo, A; Tack, J. Role of Y(2) receptors in the regulation of gastric tone in rats. Am J Physiol Gastrointest Liver Physiol 2012, 302, G732–9. [Google Scholar]
- Neary, NM; Small, CJ; Druce, MR; Park, AJ; Ellis, SM; Semjonous, NM; et al. Peptide YY3-36 and glucagon-like peptide-17-36 inhibit food intake additively. Endocrinology 2005, 146, 5120–7. [Google Scholar]
- Eissele, R; Koop, H; Arnold, R. Effect of peptide YY on gastric acid secretion, gastrin and somatostatin release in the rat. Z Gastroenterol 1990, 28, 129–31. [Google Scholar]
- Grabauskas, G; Wu, X; Lu, Y; Heldsinger, A; Song, I; Zhou, SY; et al. KATP channels in the nodose ganglia mediate the orexigenic actions of ghrelin. J Physiol 2015, 593, 3973–89. [Google Scholar]
- Li, P; Chang, TM; Chey, WY. Secretin inhibits gastric acid secretion via a vagal afferent pathway in rats. Am J Physiol 1998, 275, G22–8. [Google Scholar]
- Bucinskaite, V; Tolessa, T; Pedersen, J; Rydqvist, B; Zerihun, L; Holst, JJ; et al. Receptor-mediated activation of gastric vagal afferents by glucagon-like peptide-1 in the rat. Neurogastroenterol Motil 2009, 21, 978–1078. [Google Scholar]
- Panaro, BL; Tough, IR; Engelstoft, MS; Matthews, RT; Digby, GJ; Møller, CL; et al. The melanocortin-4 receptor is expressed in enteroendocrine L cells and regulates the release of peptide YY and glucagon-like peptide 1 in vivo. Cell Metab 2014, 20, 1018–29. [Google Scholar]
- Ronveaux, CC; Tomé, D; Raybould, HE. Glucagon-like peptide 1 interacts with ghrelin and leptin to regulate glucose metabolism and food intake through vagal afferent neuron signaling. J Nutr 2015, 145, 672–80. [Google Scholar]
- Poleni, PE; Akieda-Asai, S; Koda, S; Sakurai, M; Bae, CR; Senba, K; et al. Possible involvement of melanocortin-4-receptor and AMP-activated protein kinase in the interaction of glucagon-like peptide-1 and leptin on feeding in rats. Biochem Biophys Res Commun 2012, 420, 36–41. [Google Scholar]
- Eissele, R; Koop, H; Arnold, R. Effect of glucagon-like peptide-1 on gastric somatostatin and gastrin secretion in the rat. Scand J Gastroenterol 1990, 25, 449–54. [Google Scholar]
- Li, Y; Wu, XY; Owyang, C. Serotonin and cholecystokinin synergistically stimulate rat vagal primary afferent neurons. J Physiol 2004, 559, 651–62. [Google Scholar]
- Li, Y; Hao, YB; Zhu, JX; Owyang, C. Serotonin released from intestinal enterochromaffin cells mediates luminal non-CCK-stimulated pancreatic secretion in rats. Gastroenterology 2000, 118, 1197–207. [Google Scholar]
- Gershon, MD. Review article: serotonin receptors and transporters – roles in normal and abnormal gastrointestinal motility. Aliment Pharmacol Ther 2004, 20 (Suppl. 7), 3–14. [Google Scholar] [CrossRef] [PubMed]
- Nassar, CF; Abdallah, LE; Barada, KA; Atweh, SF; Saadé, NE. Effects of intravenous vasoactive intestinal peptide injection on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept 1995, 55(3), 261–7. [Google Scholar]
- Buchan, AM; Polak, JM; Solcia, E; Capella, C; Hudson, D; Pearse, AG. Electron immunohistochemical evidence for the human intestinal I cell as the source of CCK. Gut 1978, 19, 403–7. [Google Scholar]
- Polak, JM; Bloom, SR; Rayford, PL; Pearse, AG; Buchan, AM; Thompson, JC. Identification of cholecystokinin-secreting cells. Lancet 1975, 2, 1016–8. [Google Scholar]
- Liou, AP; Lu, X; Sei, Y; Zhao, X; Pechhold, S; Carrero, RJ; et al. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology 2011, 140, 903–12. [Google Scholar]
- Moran, TH; Norgren, R; Crosby, RJ; McHugh, PR. Central and peripheral vagal transport of cholecystokinin binding sites occurs in afferent fibers. Brain Res 1990, 526, 95–102. [Google Scholar]
- Moriarty, P; Dimaline, R; Thompson, DG; Dockray, GJ. Characterization of cholecystokinin A and cholecystokinin B receptors expressed by vagal afferent neurons. Neuroscience 1997, 79, 905–13. [Google Scholar]
- Ito, M; Matsui, T; Taniguchi, T; Tsukamoto, T; Murayama, T; Arima, N; et al. Functional characterization of a human brain cholecystokinin-B receptor. A trophic effect of cholecystokinin and gastrin. J Biol Chem 1993, 268, 18300–5. [Google Scholar]
- Broberger, C; Holmberg, K; Shi, TJ; Dockray, G; Hokfelt, T. Expression and regulation of cholecystokinin and cholecystokinin receptors in rat nodose and dorsal root ganglia. Brain Res 2001, 903, 128–40. [Google Scholar]
- Lin, CW; Miller, TR. Both CCK-A and CCK-B/gastrin receptors are present on rabbit vagus nerve. Am J Physiol 1992, 263, R591–5. [Google Scholar]
- Sternini, C; Wong, H; Pham, T; De Giorgio, R; Miller, LJ; Kuntz, SM; et al. Expression of cholecystokinin A receptors in neurons innervating the rat stomach and intestine. Gastroenterology 1999, 117, 1136–46. [Google Scholar]
- Berthoud, HR; Patterson, LM. Anatomical relationship between vagal afferent fibers and CCK-immunoreactive entero-endocrine cells in the rat small intestinal mucosa. Acta Anat (Basel) 1996, 156, 123–31. [Google Scholar]
- Patterson, LM; Zheng, H; Berthoud, HR. Vagal afferents innervating the gastrointestinal tract and CCKA-receptor immunoreactivity. Anat Rec 2002, 266, 10–20. [Google Scholar]
- Schwartz, GJ; McHugh, PR; Moran, TH. Integration of vagal afferent responses to gastric loads and cholecystokinin in rats. Am J Physiol 1991, 261, R64–9. [Google Scholar]
- Li, Y; Zhang, XC; Wang, LM; Renenan, EW; Fogel, R; Owyang, C. Vagal afferent pathway mediates physiological action of CCK on pancreatic enzyme secretion: pancreatic secretion, neurophysiological and receptor autoradiographic studies. Gastroenterology 1993, 104, A837. [Google Scholar]
- Raybould, HE; Tache, Y. Cholecystokinin inhibits gastric motility and emptying via a capsaicin-sensitive vagal pathway in rats. Am J Physiol 1988, 255, G242–6. [Google Scholar]
- Rogers, RC; Hermann, GE. Mechanisms of action of CCK to activate central vagal afferent terminals. Peptides 2008, 29, 1716–25. [Google Scholar]
- Berthoud, HR; Blackshaw, LA; Brookes, SJ; Grundy, D. Neuroanatomy of extrinsic afferents supplying the gastrointestinal tract. Neurogastroenterol Motil 2004, 16, 28–33. [Google Scholar]
- Li, Y; Zhu, J; Owyang, C. Electrical physiological evidence for high- and low-affinity CCK-A receptors. Am J Physiol 1999, 277, G469–77. [Google Scholar]
- Li, Y; Owyang, C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology 1994, 107, 525–31. [Google Scholar]
- Schwartz, GJ; Tougas, G; Moran, TH. Integration of vagal afferent responses to duodenal loads and exogenous CCK in rats. Peptides 1995, 16, 707–11. [Google Scholar]
- Ritter, RC; Ladenheim, EE. Capsaicin pretreatment attenuates suppression of food intake by cholecystokinin. Am J Physiol 1985, 248, R501–4. [Google Scholar]
- South, EH; Ritter, RC. Capsaicin application to central or peripheral vagal fibers attenuates CCK satiety. Peptides 1988, 9, 601–12. [Google Scholar]
- Joyner, K; Smith, GP; Gibbs, J. Abdominal vagotomy decreases the satiating potency of CCK-8 in sham and real feeding. Am J Physiol 1993, 264, R912–6. [Google Scholar]
- Eisen, S; Phillips, RJ; Geary, N; Baronowsky, EA; Powley, TL; Smith, GP. Inhibitory effects on intake of cholecystokinin-8 and cholecystokinin-33 in rats with hepatic proper or common hepatic branch vagal innervation. Am J Physiol Regul Integr Comp Physiol 2005, 289, R456–62. [Google Scholar]
- Kurosawa, M; Bucinskaite, V; Taniguchi, T; Miyasaka, K; Funakoshi, A; Lundeberg, T. Response of the gastric vagal afferent activity to cholecystokinin in rats lacking type A cholecystokinin receptors. J Auton Nerv Syst 1999, 75, 51–9. [Google Scholar]
- Wang, DQ; Schmitz, F; Kopin, AS; Carey, MC. Targeted disruption of the murine cholecystokinin-1 receptor promotes intestinal cholesterol absorption and susceptibility to cholesterol cholelithiasis. J Clin Invest 2004, 114, 521–8. [Google Scholar]
- Owyang, C; Logsdon, CD. New insights into neurohormonal regulation of pancreatic secretion. Gastroenterology 2004, 127, 957–69. [Google Scholar]
- Browning, KN; Babic, T; Holmes, GM; Swartz, E; Travagli, RA. A critical re-evaluation of the specificity of action of perivagal capsaicin. J Physiol 2013, 591, 1563–80. [Google Scholar]
- Babic, T; Browning, KN; Kawaguchi, Y; Tang, X; Travagli, RA. Pancreatic insulin and exocrine secretion are under the modulatory control of distinct subpopulations of vagal motoneurones in the rat. J Physiol 2012, 590, 3611–22. [Google Scholar]
- Bado, A; Levasseur, S; Attoub, S; Kermorgant, S; Laigneau, JP; Bortoluzzi, MN; et al. The stomach is a source of leptin. Nature 1998, 394, 790–3. [Google Scholar]
- Mix, H; Widjaja, A; Jandl, O; Cornberg, M; Kaul, A; Göke, M; et al. Expression of leptin and leptin receptor isoforms in the human stomach. Gut 2000, 47, 481–6. [Google Scholar]
- Friedman, JM; Halaas, JL. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–70. [Google Scholar]
- Schwartz, MW; Seeley, RJ; Campfield, LA; Burn, P; Baskin, DG. Identification of targets of leptin action in rat hypothalamus. J Clin Invest 1996, 98, 1101–6. [Google Scholar]
- Buyse, M; Ovesjo, ML; Goiot, H; Guilmeau, S; Peranzi, G; Moizo, L; et al. Expression and regulation of leptin receptor proteins in afferent and efferent neurons of the vagus nerve. Eur J Neurosci 2001, 14, 64–72. [Google Scholar]
- Peters, JH; McKay, BM; Simasko, SM; Ritter, RC. Leptin-induced satiation mediated by abdominal vagal afferents. Am J Physiol Regul Integr Comp Physiol 2005, 288, R879–84. [Google Scholar]
- Peters, JH; Ritter, RC; Simasko, SM. Leptin and CCK modulate complementary background conductances to depolarize cultured nodose neurons. Am J Physiol Cell Physiol 2006, 290, C427–32. [Google Scholar]
- Peters, JH; Ritter, RC; Simasko, SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol 2006, 290(6), R1544–9. [Google Scholar]
- Patel, JD; Ebenezer, IS. The effect of intraperitoneal administration of leptin on short-term food intake in rats. Eur J Pharmacol 2008, 580(1–2), 143–52. [Google Scholar] [CrossRef] [PubMed]
- de Lartigue, G; Ronveaux, CC; Raybould, HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Molecular metabolism 2014, 3, 595–607. [Google Scholar]
- Peters, JH; Simasko, SM; Ritter, RC. Modulation of vagal afferent excitation and reduction of food intake by leptin and cholecystokinin. Physiol Behav 2006, 89, 477–85. [Google Scholar]
- Kojima, M; Hosoda, H; Date, Y; Nakazato, M; Matsuo, H; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–60. [Google Scholar]
- Hosoda, H; Kojima, M; Matsuo, H; Kangawa, K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem Biophys Res Commun 2000, 279, 909–13. [Google Scholar]
- Date, Y; Kojima, M; Hosoda, H; Sawaguchi, A; Mondal, MS; Suganuma, T; et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology 2000, 141, 4255–61. [Google Scholar]
- Gnanapavan, S; Kola, B; Bustin, SA; Morris, DG; McGee, P; Fairclough, P; et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 2002, 87, 2988. [Google Scholar]
- Cowley, MA; Smith, RG; Diano, S; Tschop, M; Pronchuk, N; Grove, KL; et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron 2003, 7, 649–61. [Google Scholar]
- Xu, L; Depoortere, I; Tomasetto, C; Zandecki, M; Tang, M; Timmermans, JP; et al. Evidence for the presence of motilin, ghrelin, and the motilin and ghrelin receptor in neurons of the myenteric plexus. Regul Pept 2005, 124, 119–25. [Google Scholar]
- Page, AJ; Slattery, JA; Milte, C; Laker, R; O'Donnell, T; Dorian, C; et al. Ghrelin selectively reduces mechanosensitivity of upper gastrointestinal vagal afferents. Am J Physiol Gastrointest Liver Physiol 2007, 292, G1376–84. [Google Scholar]
- Date, Y; Toshinai, K; Koda, S; Miyazato, M; Shimbara, T; Tsuruta, T; et al. Peripheral interaction of ghrelin with cholecystokinin on feeding regulation. Endocrinology 2005, 146, 3518–25. [Google Scholar]
- Arnold, M; Mura, A; Langhans, W; Geary, N. Gut vagal afferents are not necessary for the eating-stimulatory effect of intraperitoneally injected ghrelin in the rat. J Neurosci 2006, 26, 11052–60. [Google Scholar]
- Gomez, R; Navarro, M; Ferrer, B; Trigo, JM; Bilbao, A; Del, AI; et al. A peripheral mechanism for CB1 cannabinoid receptor-dependent modulation of feeding. J Neurosci 2002, 22, 9612–7. [Google Scholar]
- Burdyga, G; Varro, A; Dimaline, R; Thompson, DG; Dockray, GJ. Feeding-dependent depression of melanin-concentrating hormone and melanin-concentrating hormone receptor-1 expression in vagal afferent neurons. Neuroscience 2006, 137, 1405–15. [Google Scholar]
- Emond, M; Schwartz, GJ; Ladenheim, EE; Moran, TH. Central leptin modulates behavioral and neural responsivity to CCK. Am J Physiol 1999, 276, R1545–9. [Google Scholar]
- Matson, CA; Reid, DF; Cannon, TA; Ritter, RC. Cholecystokinin and leptin act synergistically to reduce body weight. Am J Physiol Regul Integr Comp Physiol 2000, 278, R882–90. [Google Scholar]
- Matson, CA; Ritter, RC. Long-term CCK-leptin synergy suggests a role for CCK in the regulation of body weight. Am J Physiol 1999, 276, R1038–45. [Google Scholar]
- Matson, CA; Wiater, MF; Kuijper, JL; Weigle, DS. Synergy between leptin and cholecystokinin (CCK) to control daily caloric intake. Peptides 1997, 18, 1275–8. [Google Scholar]
- Wang, L; Barachina, MD; Martinez, V; Wei, JY; Tache, Y. Synergistic interaction between CCK and leptin to regulate food intake. Regul Pept 2000, 92, 79–85. [Google Scholar]
- Peters, JH; Karpiel, AB; Ritter, RC; Simasko, SM. Cooperative activation of cultured vagal afferent neurons by leptin and cholecystokinin. Endocrinology 2004, 145, 3652–7. [Google Scholar]
- Heldsinger, A; Grabauskas, G; Song, I; Owyang, C. Synergistic interaction between leptin and cholecystokinin in the rat nodose ganglia is mediated by PI3K and STAT3 signaling pathways: implications for leptin as a regulator of short term satiety. J Biol Chem 2011, 286, 11707–15. [Google Scholar]
- Emond, M; Ladenheim, EE; Schwartz, GJ; Moran, TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav 2001, 72, 123–8. [Google Scholar]
- Wang, L; Martinez, V; Barrachina, MD; Tache, Y. Fos expression in the brain induced by peripheral injection of CCK or leptin plus CCK in fasted lean mice. Brain Res 1998, 79, 157–66. [Google Scholar]
- Maletinska, L; Maixnerova, J; Matyskova, R; Haugvicova, R; Pirnik, Z; Kiss, A; et al. Synergistic effect of CART (cocaine- and amphetamine-regulated transcript) peptide and cholecystokinin on food intake regulation in lean mice. BMC Neurosci 2008, 9, 101. [Google Scholar]
- Heldsinger, A; Lu, Y; Zhou, SY; Wu, X; Grabauskas, G; Song, I; et al. Cocaine- and amphetamine-regulated transcript is the neurotransmitter regulating the action of cholecystokinin and leptin on short-term satiety in rats. Am J Physiol Gastrointest Liver Physiol 2012, 303, G1042–51. [Google Scholar]
- Heldsinger, A; Grabauskas, G; Wu, X; Zhou, S; Lu, Y; Song, I; et al. Ghrelin induces leptin resistance by activation of suppressor of cytokine signaling 3 expression in male rats: implications in satiety regulation. Endocrinology 2014, 155(10), 3956–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, YH; Tache, Y; Sheibel, AB; Go, VL; Wei, JY. Two types of leptin-responsive gastric vagal afferent terminals: an in vitro single-unit study in rats. Am J Physiol 1997, 273, R833–7. [Google Scholar]
- Morton, GJ; Blevins, JE; Williams, DL; Niswender, KD; Gelling, RW; Rhodes, CJ; et al. Leptin action in the forebrain regulates the hindbrain response to satiety signals. J Clin Invest 2005, 115, 703–10. [Google Scholar]
- Loewy, AD. Central regulation of autonomic functions. In Central regulation of autonomic functions; Loewy, AD, Spyer, KM, Eds.; Oxford University Press: New York, 1990; pp. 88–103. [Google Scholar]
- Altschuler, SM; Bao, X; Bieger, D; Hopkins, DA; Miselis, RR. Viscerotopic representation of the upper alimentary tract in the rat: sensory ganglia and nuclei of the solitary and spinal trigeminal tracts. J Comp Neurol 1989, 283, 248–68. [Google Scholar]
- Altschuler, SM; Ferenci, DA; Lynn, RB; Miselis, RR. Representation of the cecum in the lateral dorsal motor nucleus of the vagus nerve and commissural subnucleus of the nucleus tractus solitarii in rat. J Comp Neurol 1991, 304, 261–74. [Google Scholar]
- Altschuler, SM; Escardo, J; Lynn, RB; Miselis, RR. The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 1993, 104, 502–9. [Google Scholar]
- Smith, BN; Dou, P; Barber, WD; Dudek, FE. Vagally evoked synaptic currents in the immature rat nucleus tractus solitarii in an intact in vitro preparation. J Physiol 1998, 512, 149–62. [Google Scholar]
- Glatzer, NR; Smith, BN. Modulation of synaptic transmission in the rat nucleus of the solitary tract by endomorphin-1. J Neurophysiol 2005, 93, 2530–40. [Google Scholar]
- de Lartigue, G; Barbier de la Serre, C; Espero, E; Lee, J; Raybould, HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 2012, 7, e32967. [Google Scholar]
- Gillespie, BR; Burns, GA; Ritter, RC. NMDA channels control meal size via central vagal afferent terminals. Am J Physiol Regul Integr Comp Physiol 2005, 289, R1504–11. [Google Scholar]
- Zheng, H; Corkern, MM; Crousillac, SM; Patterson, LM; Phifer, CB; Berthoud, HR. Neurochemical phenotype of hypothalamic neurons showing Fos expression 23 h after intracranial AgRP. Am J Physiol Regul Integr Comp Physiol 2002, 282, R1773–81. [Google Scholar]
- Saadé, NE; Abdallah, LE; Barada, KA; Atweh, SF; Nassar, CF. Effects of intracerebral injections of VIP on jejunal alanine absorption and gastric acid secretion in rats. Regul Pept 1995, 55, 269–76. [Google Scholar]
- Helke, CJ; Niederer, AJ. Studies on the coexistence of substance P with other putative transmitters in the nodose and petrosal ganglia. Synapse 1990, 5, 144–51. [Google Scholar]
- Helke, CJ; Hill, KM. Immunohistochemical study of neuropeptides in vagal and glossopharyngeal afferent neurons in the rat. Neuroscience 1988, 26, 539–51. [Google Scholar]
- Sekizawa, S; Joad, JP; Bonham, AC. Substance P presynaptically depresses the transmission of sensory input to bronchopulmonary neurons in the guinea pig nucleus tractus solitarii. J Physiol 2003, 552, 547–59. [Google Scholar]
- Holzer, P; Lippe, IT. Stimulation of afferent nerve endings by intragastric capsaicin protects against ethanol-induced damage of gastric mucosa. Neuroscience 1988, 27, 981–7. [Google Scholar]
- Holzer, P; Guth, PH. Neuropeptide control of rat gastric mucosal blood flow: increase by calcitonin gene-related peptide and vasoactive intestinal polypeptide, but not substance P and neurokinin A. Circ Res 1991, 68, 100–5. [Google Scholar]
- Holzer, P; Sametz, W. Gastric mucosal protection against ulcerogenic factors in the rat mediated by capsaicin-sensitive afferent neurons. Gastroenterology 1986, 91, 975–81. [Google Scholar]
- Lin, LH; Cassell, MD; Sandra, A; Talman, WT. Direct evidence for nitric oxide synthase in vagal afferents to the nucleus tractus solitarii. Neuroscience 1998, 84, 549–58. [Google Scholar]
- Page, AJ; O'Donnell, TA; Cooper, NJ; Young, RL; Blackshaw, LA. Nitric oxide as an endogenous peripheral modulator of visceral sensory neuronal function. J Neurosci 2009, 29, 7246–55. [Google Scholar]
- Lawrence, AJ. Neurotransmitter mechanisms of rat vagal afferent neurons. Clin Exp Pharmacol Physiol 1995, 22, 869–73. [Google Scholar] [CrossRef] [PubMed]
- Schaffar, N; Rao, H; Kessler, JP; Jean, A. Immunohistochemical detection of glutamate in rat vagal sensory neurons. Brain Res 1997, 778, 302–8. [Google Scholar]
- Saha, S; Batten, TF; McWilliam, PN. Glutamate-immunoreactivity in identified vagal afferent terminals of the cat: a study combining horseradish peroxidase tracing and postembedding electron microscopic immunogold staining. Exp Physiol 1995, 80, 193–202. [Google Scholar]
- Ambalavanar, R; Ludlow, CL; Wenthold, RJ; Tanaka, Y; Damirjian, M; Petralia, RS. Glutamate receptor subunits in the nucleus of the tractus solitarius and other regions of the medulla oblongata in the cat. J Comp Neurol 1998, 402, 75–92. [Google Scholar]
- Andresen, MC; Yang, MY. Non-NMDA receptors mediate sensory afferent synaptic transmission in medial nucleus tractus solitarius. Am J Physiol 1990, 259, H1307–11. [Google Scholar]
- Kessler, JP; Jean, A. Evidence that activation of N-methyl-d-aspartate (NMDA) and non-NMDA receptors within the nucleus tractus solitarii triggers swallowing. Eur J Pharmacol 1991, 201, 59–67. [Google Scholar]
- Treece, BR; Covasa, M; Ritter, RC; Burns, GA. Delay in meal termination follows blockade of N-methyl-d-aspartate receptors in the dorsal hindbrain. Brain Res 1998, 810, 34–40. [Google Scholar]
- Burns, GA; Ritter, RC. Visceral afferent participation in delayed satiation following NMDA receptor blockade. Physiol Behav 1998, 65, 361–6. [Google Scholar]
- Wright, J; Campos, C; Herzog, T; Covasa, M; Czaja, K; Ritter, RC. Reduction of food intake by cholecystokinin requires activation of hindbrain NMDA-type glutamate receptors. Am J Physiol Regul Integr Comp Physiol 2011, 301, R448–55. [Google Scholar]
- Zheng, H; Kelly, L; Patterson, LM; Berthoud, HR. Effect of brain stem NMDA-receptor blockade by MK-801 on behavioral and fos responses to vagal satiety signals. Am J Physiol 1999, 277, R1104–11. [Google Scholar]
- Zheng, H; Patterson, LM; Berthoud, HR. CART in the dorsal vagal complex: sources of immunoreactivity and effects on Fos expression and food intake. Brain Res 2002, 957, 298–310. [Google Scholar]
- Glaum, SR; Miller, RJ. Metabotropic glutamate receptors mediate excitatory transmission in the nucleus of the solitary tract. J Neurosci 1992, 12, 2251–8. [Google Scholar]
- Glaum, SR; Miller, RJ. Activation of metabotropic glutamate receptors produces reciprocal regulation of ionotropic glutamate and GABA responses in the nucleus of the tractus solitarius of the rat. J Neurosci 1993, 13, 1636–41. [Google Scholar]
- Page, AJ; Young, RL; Martin, CM; Umaerus, M; O'Donnell, TA; Cooper, NJ; et al. Metabotropic glutamate receptors inhibit mechanosensitivity in vagal sensory neurons. Gastroenterology 2005, 128, 402–10. [Google Scholar]
- Young, RL; Page, AJ; O'Donnell, TA; Cooper, NJ; Blackshaw, LA. Peripheral versus central modulation of gastric vagal pathways by metabotropic glutamate receptor 5. Am J Physiol Gastrointest Liver Physiol 2007, 292, G501–11. [Google Scholar]
- Baptista, V; Zheng, Z; Coleman, FH; Rogers, RC; Travagli, RA. Cholecystokinin octapeptide increases spontaneous glutamatergic synaptic transmission to neurons of the nucleus tractus solitarius centralis. J Neurophysiol 2005, 94, 2763–71. [Google Scholar]
- Appleyard, SM; Bailey, TW; Doyle, MW; Jin, YH; Smart, JL; Low, MJ; et al. Proopiomelanocortin neurons in nucleus tractus solitarius are activated by visceral afferents: regulation by cholecystokinin and opioids. J Neurosci 2005, 25, 3578–85. [Google Scholar]
- Emch, GS; Hermann, GE; Rogers, RC. TNF-a activates solitary nucleus neurons responsive to gastric distention. Am J Physiol Gastrointest Liver Physiol 2000, 279, G582–6. [Google Scholar]
- Wan, S; Browning, KN; Coleman, FH; Sutton, G; Zheng, H; Butler, A; et al. Presynaptic melanocortin-4 receptors on vagal afferent fibers modulate the excitability of rat nucleus tractus solitarius neurons. J Neurosci 2008, 28, 4957–66. [Google Scholar]
- Wan, S; Browning, KN. Glucose increases synaptic transmission from vagal afferent central nerve terminals via modulation of 5-HT3 receptors. Am J Physiol Gastrointest Liver Physiol 2008, 295, G1050–7. [Google Scholar]
- Jin, YH; Bailey, TW; Li, BY; Schild, JH; Andresen, MC. Purinergic and vanilloid receptor activation releases glutamate from separate cranial afferent terminals in nucleus tractus solitarius. J Neurosci 2004, 24, 4709–17. [Google Scholar]
- Zhang, S; Grabauskas, G; Wu, X; Joo, MK; Heldsinger, A; Song, I; et al. Role of prostaglandin D2 in mast cell activation-induced sensitization of esophageal vagal afferents. Am J Physiol Gastrointest Liver Physiol 2013, 304, G908–16. [Google Scholar]
- Ter Horst, GJ; de Boer, P; Luiten, PG; Van Willigen, JD. Ascending projections from the solitary tract nucleus to the hypothalamus. A Phaseolus vulgaris lectin tracing study in the rat. Neuroscience 1989, 31, 785–97. [Google Scholar]
- Sawchenko, PE; Swanson, LW. The organization of noradrenergic pathways from the brainstem to the paraventricular and supraoptic nuclei in the rat. Brain Res 1982, 257, 275–325. [Google Scholar]
- Rogers, RC; McCann, MJ. Intramedullary connections of the gastric region in the solitary nucleus: a biocytin histochemical tracing study in the rat. J Auton Nerv Syst 1993, 42, 119–30. [Google Scholar]
- Grabauskas, G; Moises, HC. Gastrointestinal-projecting neurones in the dorsal motor nucleus of the vagus exhibit direct and viscerotopically organized sensitivity to orexin. J Physiol 2003, 549, 37–56. [Google Scholar]
- Grabauskas, G; Zhou, SY; Das, S; Lu, Y; Owyang, C; Moises, HC. Prolactin-releasing peptide affects gastric motor function in rat by modulating synaptic transmission in the dorsal vagal complex. J Physiol 2004, 56, 821–39. [Google Scholar]
- Glatzer, NR; Hasney, CP; Bhaskaran, MD; Smith, BN. Synaptic and morphologic properties in vitro of premotor rat nucleus tractus solitarius neurons labeled transneuronally from the stomach. J Comp Neurol 2003, 464(4), 525–39. [Google Scholar]
- Holmes, GM; Browning, KN; Babic, T; Fortna, SR; Coleman, FH; Travagli, RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol 2013, 591, 3081–100. [Google Scholar]
- Grabauskas, G; Wu, X; Song, I; Zhou, SY; Lanigan, T; Owyang, C. Increased Activation of the TRESK K+ Mediates Vago-Vagal Reflex Malfunction in Diabetic Rats. Gastroenterology 2016, 151, 910–22. [Google Scholar]
- Covasa, M; Ritter, RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides 1998, 19, 1407–15. [Google Scholar]
- Covasa, M; Grahn, J; Ritter, RC. High fat maintenance diet attenuates hindbrain neuronal response to CCK. Regul Pept 2000, 86, 83–8. [Google Scholar]
- Kentish, SJ; Vincent, AD; Kennaway, DJ; Wittert, GA; Page, AJ. High-fat diet-induced obesity ablates gastric vagal afferent circadian rhythms. J Neurosci 2016, 36, 3199–207. [Google Scholar]
- Waise, TM; Toshinai, K; Naznin, F; NamKoong, C; Md Moin, AS; Sakoda, H; et al. One-day high-fat diet induces inflammation in the nodose ganglion and hypothalamus of mice. Biochem Biophys Res Commun 2015, 464, 1157–62. [Google Scholar] [CrossRef] [PubMed]
- Naznin, F; Toshinai, K; Waise, TM; NamKoong, C; Md Moin, AS; Sakoda, H; et al. Diet-induced obesity causes peripheral and central ghrelin resistance by promoting inflammation. J Endocrinol 2015, 226, 81–92. [Google Scholar]
- Lundberg, JM; Hokfelt, T; Kewenter, J; Pettersson, G; Ahlman, H; Edin, R; et al. Substance P, VIP and enkephalin-like immunoreactivity in the human vagus nerve. Gastroenterology 1979, 77, 468–71. [Google Scholar]
- Rytel, L; Palus, K; Całka, J. Co-expression of PACAP with VIP, SP and CGRP in the porcine nodose ganglion sensory neurons. Anat Histol Embryol 2015, 44(2), 86–91. [Google Scholar]
- Suzuki, T; Kagoshima, M; Shibata, M; Inaba, H; Onodera, S; Yamamura, T; et al. Effects of several denervation procedures on distribution of calcitonin gene-related peptide and substance P immunreactive in rat stomach. Dig Dis Sci 1997, 42/6, 1242–54. [Google Scholar] [CrossRef]
- Konturek, SJ; Brzozowski, T; Pytko-Polonczyk, J; Drozdowic, D. Exogenous and endogenous cholecystokinin protects gastric mucosa against the damage caused by ethanol in rats. Eur J Pharmacol 1995, 273, 57–62. [Google Scholar] [PubMed]
- Heinemann, A; Jocic, M; Peskar, BM; Holzer, P. CCK-evoked hyperemia in rat gastric mucosa involves neural mechanisms and nitric oxide. Am J Physiol 1996, 270, 253–8. [Google Scholar] [CrossRef] [PubMed]
- Yonei, Y; Holzer, P; Guth, PH. Laparotomy-induced gastric protection against ethanol injury is mediated by capsaicin-sensitive sensory neurons. Gastroenterology 1990, 99, 3–9. [Google Scholar] [CrossRef]
- Stead, RH; Dixon, RF; Bramwell, NH. Mast cells are closely opposed to nerves in the human gastrointestinal mucosa. Gastroenterology 1989, 97, 575–85. [Google Scholar] [CrossRef]
© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Grabauskas, G.; Owyang, C. Plasticity of vagal afferent signaling in the gut. Medicina 2017, 53, 73-84. https://doi.org/10.1016/j.medici.2017.03.002
Grabauskas G, Owyang C. Plasticity of vagal afferent signaling in the gut. Medicina. 2017; 53(2):73-84. https://doi.org/10.1016/j.medici.2017.03.002
Chicago/Turabian StyleGrabauskas, Gintautas, and Chung Owyang. 2017. "Plasticity of vagal afferent signaling in the gut" Medicina 53, no. 2: 73-84. https://doi.org/10.1016/j.medici.2017.03.002
APA StyleGrabauskas, G., & Owyang, C. (2017). Plasticity of vagal afferent signaling in the gut. Medicina, 53(2), 73-84. https://doi.org/10.1016/j.medici.2017.03.002




