Infantile hemangioma: Predicting proliferation by infrared thermography
Abstract
1. Introduction
2. Materials and methods
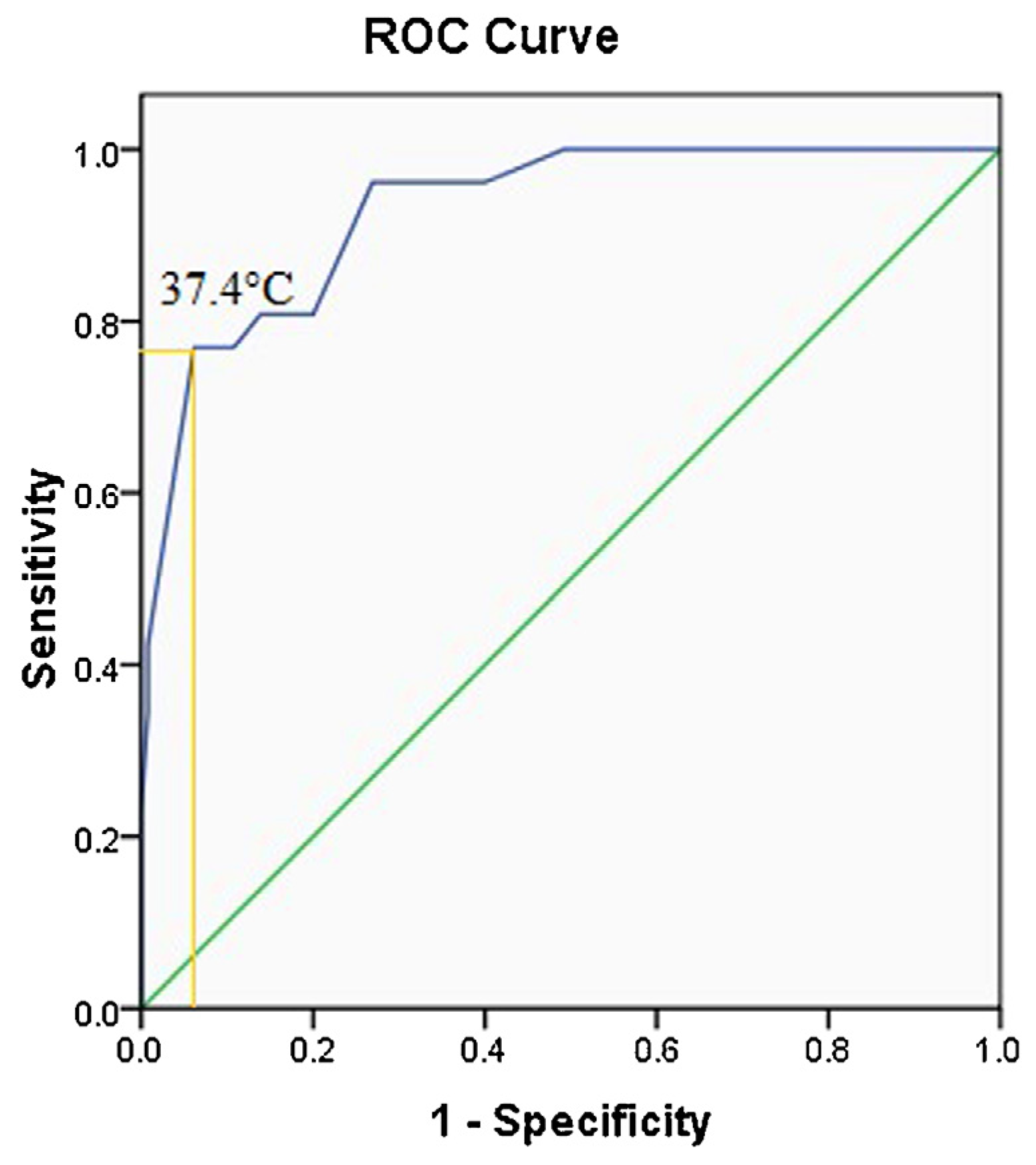
3. Results
4. Discussion
5. Conclusions
Conflict of interest
Acknowledgments
R E F E R E N C E S
- Jinnin, M; Ishihara, T; Boye, E; Olsen, BR. Recent progress in studies of infantile hemangioma. J Dermatol 2010, 37, 283–98. [Google Scholar] [CrossRef] [PubMed]
- Chiller, KG; Passaro, D; Frieden, IJ. Hemangiomas of infancy: clinical characteristics, morphologic subtypes, and their relationship to race, ethnicity, and sex. Arch Dermatol 2002, 138, 1567–76. [Google Scholar] [CrossRef] [PubMed]
- Frieden, IJ; Haggstrom, AN; Drolet, BA; Mancini, AJ; Friedlander, SF; Boon, L; et al. Infantile hemangiomas: current knowledge, future directions. Proceedings of a research workshop on infantile hemangiomas. Pediatr Dermatol 2005, 22, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Soltani, AM; Reinisch, JF. Algorithmic approach to the management of hemangiomas. J Craniofac Surg 2011, 22, 585–8. [Google Scholar] [CrossRef] [PubMed]
- Greenberger, S; Bischoff, J. Pathogenesis of infantile haemangioma. Br J Dermatol 2013, 169(1), 12–9. [Google Scholar] [CrossRef] [PubMed]
- Boscolo, E; Bischoff, J. Vasculogenesis in Infantile Hemangioma. Angiogenesis 2008, 12(2), 197–207. [Google Scholar] [CrossRef] [PubMed]
- Chang, LC; Haggstrom, AN; Drolet, BA; Baselga, E; Chamlin, SL; Garzon, MC; et al. Growth characteristics of infantile hemangiomas: implications for management. Pediatrics 2008, 122, 360–7. [Google Scholar] [CrossRef] [PubMed]
- Bang, GM; Setabutr, P. Periocular capillary hemangiomas: indications and options for treatment. Middle East Afr J Ophthalmol 2010, 17, 121–8. [Google Scholar] [PubMed]
- Léauté-Labrèze, C. Infantile hemangioma: update and treatment. Arch Pediatr 2013, 20, 517–22. [Google Scholar]
- Kwon, EK; Seefeldt, M; Drolet, BA. Infantile hemangiomas: an update. Am J Clin Dermatol 2013, 14, 111–23. [Google Scholar] [CrossRef] [PubMed]
- Alcantara-Gonzalez, J; Boixeda, P; Truchuelo-Díez, MT; Pérez-García, B; Alonso-Castro, L; Jaén Olasolo, P. Infantile hemangiomas treated by sequential application of pulsed dye laser and Nd:YAG laser radiation: a retrospective study. Actas Dermosifiliogr 2013, 104, 504. [Google Scholar] [CrossRef] [PubMed]
- Kessels, JP; Hamers, ET; Ostertag, JU. Superficial hemangioma: pulsed dye laser versus wait-and-see. Dermatol Surg 2013, 39, 414–21. [Google Scholar] [CrossRef] [PubMed]
- Talaat, AA; Elbasiouny, MS; Elgendy, DS; Elwakil, TF. Propranolol treatment of infantile hemangioma: clinical and radiologic evaluations. J Pediatr Surg 2012, 47, 707–14. [Google Scholar] [CrossRef] [PubMed]
- Akcay, A; Karakas, Z; Saribeyoglu, ET; Unuvar, A; Baykal, C; Garipardic, M; et al. Infantile hemangiomas, complications and follow-up. Indian Pediatr 2012, 49, 805–9. [Google Scholar] [CrossRef] [PubMed]
- Tambe, K; Munshi, V; Dewsbery, C; Ainsworth, JR; Willshaw, H; Parulekar, MV. Relationship of infantile periocular hemangioma depth to growth and regression pattern. J AAPOS 2009, 13, 567–70. [Google Scholar] [CrossRef] [PubMed]
- Bruckner, AL; Frieden, IJ. Hemangiomas of infancy. J Am Acad Dermatol 2003, 48, 671–82. [Google Scholar] [CrossRef] [PubMed]
- Serra, AM; Soares, FM; Cunha Júnior, AG; Costa, IM. Therapeutic management of skin hemangiomas in children. An Bras Dermatol 2010, 85, 307–17. [Google Scholar] [CrossRef] [PubMed]
- Saxena, AK; Willital, GH. Infrared thermography: experience from a decade of pediatric imaging. Eur J Pediatr 2008, 167, 757–64. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, JA; Balma-Mena, A; Chakkittakandiyil, A; Matea, F; Pope, E. Infrared thermography to assess proliferation and involution of infantile hemangiomas: a prospective cohort study. JAMA Dermatol 2014, 150(9), 964–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Garcia-Romero, MT; Chakkittakandiyil, A; Pope, E. The role of infrared thermography in evaluation of proliferative infantile hemangiomas. Results of a pilot study. Int J Dermatol 2014, 53(3), e216–7. [Google Scholar] [CrossRef] [PubMed]
- Haggstrom, AN; Drolet, BA; Baselga, E; Chamlin, SL; Garzon, MC; Horii, KA; et al. Prospective study of infantile hemangiomas: clinical characteristics predicting complications and treatment. Pediatrics 2006, 118, 882–7. [Google Scholar] [CrossRef] [PubMed]
- Berlien, HP. Principles of therapy of infantile hemangiomas and other congenital vascular tumors of the newborns and infants. In Hemangiomas and Vascular Malformations. An Atlas of Diagnosis and Treatment part II; Mattassi, R, Belov, S, Loose, DA, Vaghi, M, Eds.; Springer Verlag Italia: Milan, 2003; [chapter 4]. [Google Scholar]
- Frieden, IJ; Eichenfield, LF; Esterly, NB; Geronemus, R; Mallory, SB. Guidelines of care for hemangiomas of infancy. American Academy of Dermatology Guidelines/Outcomes Committee. J Am Acad Dermatol 1997, 37, 631–7. [Google Scholar] [CrossRef]
- Admani, S; Krakovski, AC; Nelson, JS; Eichenfield, LF; Friedlander, SF. Beneficial effects of early pulsed dye laser therapy in individuals with infantile hemangiomas. Dermatol Surg 2012, 38, 1732–8. [Google Scholar] [CrossRef] [PubMed]
- Couto, RA; Maclellan, RA; Zurakowski, D; Greene, AK. Infantile hemangioma: clinical assessment of the involuting phase and implications for management. Plast Reconstr Surg 2012, 130(3), 619–24. [Google Scholar] [CrossRef] [PubMed]
- Bauland, CG; Lüning, TH; Smit, JM; Zeebregts, CJ; Spauwen, PH. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg 2011, 127(4), 1643–8. [Google Scholar] [CrossRef] [PubMed]
- Hoeger, PH. An update on infantile haemangiomas. Br J Dermatol 2013, 169, 11. [Google Scholar] [CrossRef] [PubMed]
- Jian, D; Chen, X; Babajee, K; Su, J; Li, J; Hu, X; et al. Adverse effects of propranolol treatment for infantile hemangiomas in China. J Dermatol Treat 2014, 25, 388–90. [Google Scholar] [CrossRef] [PubMed]

| Variable | Growing profile | ||
|---|---|---|---|
| Stable IHs (n = 100) | Slightly growing His (n = 30) | Growing His (n = 26) | |
| Temperature (°C) Age, months | 36.7 (36.4; 36.9)*,† 3 (2; 4)‡ | 37 (36.7; 37.3)* 3 (2; 4)½ | 37.4 (37.2; 37.6) 2 (1; 3) |
| Values are median (25th; 75th quartiles). *P < 0.01 versus growing. †P < 0.01 versus slightly growing. ‡P < 0.01 versus growing. ½P = 0.02 versus growing (Mann–Whitney test to compare the groups). | |||
© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Share and Cite
Strumila, A.; Kazlauskas, V.; Pošiūnas, G.; Verkauskas, G.; Beiša, V. Infantile hemangioma: Predicting proliferation by infrared thermography. Medicina 2017, 53, 85-89. https://doi.org/10.1016/j.medici.2017.04.002
Strumila A, Kazlauskas V, Pošiūnas G, Verkauskas G, Beiša V. Infantile hemangioma: Predicting proliferation by infrared thermography. Medicina. 2017; 53(2):85-89. https://doi.org/10.1016/j.medici.2017.04.002
Chicago/Turabian StyleStrumila, Arūnas, Vytis Kazlauskas, Gintas Pošiūnas, Gilvydas Verkauskas, and Virgilijus Beiša. 2017. "Infantile hemangioma: Predicting proliferation by infrared thermography" Medicina 53, no. 2: 85-89. https://doi.org/10.1016/j.medici.2017.04.002
APA StyleStrumila, A., Kazlauskas, V., Pošiūnas, G., Verkauskas, G., & Beiša, V. (2017). Infantile hemangioma: Predicting proliferation by infrared thermography. Medicina, 53(2), 85-89. https://doi.org/10.1016/j.medici.2017.04.002




