Abstract
Background and objective: Lithuania belongs to the group of countries with a high-incidence of tuberculosis (TB). Some scientific studies show that the interferon-gamma release assay is more accurate and correlates more highly with TB exposure as compared to the tuberculin skin test (TST). This study aimed at comparing the efficacy between the T SPOT TB and TST for diagnosing TB among Lithuanian adults. Materials and methods: Individuals with diagnosed TB, healthcare workers with known risk for TB and individuals without any known risk for TB underwent clinical examinations, interviews about their history of TB exposure and chest radiography. Then the TST and the T SPOT TB were performed on patients. Results: A positive T SPOT TB was more common in the group with diagnosed TB compared to healthcare workers and the low risk for TB groups (97.5%, 36.4%, and 0%, respectively, P < 0.01). Positive TST results did not differ between the groups with diagnosed TB and the healthcare workers (92.5% vs. 95.5%, P > 0.05). Agreement between TST and T SPOT TB was poor (kappa 0.14, P > 0.05). T SPOT TB had higher specificity and sensitivity compared to TST (area under the ROC 0.9 ± 0.04, P < 0.01, vs. 0.5 ± 0.06, P > 0.05). Conclusions: The T SPOT TB showed greater accuracy in diagnosing TB than TST did. Positive T SPOT TB result but not the TST was more common in patients with diagnosed TB.
1. Introduction
Despite the fact that the incidence of global tuberculosis (TB) has slowly decreased during the past 13 years, this disease remains widespread worldwide – 9 million incident cases of TB were reported in 2013 [1]. The World Health Organization (WHO) reports the incidence of TB in Lithuania in 2013 at 65/ 100,000 inhabitants [2]. This is more than 50/100,000 inhabitants, which means that Lithuania belongs to the group of high-incidence countries [3]. Moreover, Lithuania has one of the highest rates of TB drug resistance numbering 11% among new TB cases in 2013 [2,4].
Diagnosis of latent TB is very important in high-incidence countries. The definition of latent TB is the presence of immune responses to a previously acquired Mycobacterium tuberculosis (M. tuberculosis) infection without clinical evidence of active TB [5]. Individuals with latent TB are at risk for developing active TB [5]. The WHO guidelines for latent TB management in countries with high or middle-upper incomes and TB incidences of <100/100,000 inhabitants involve systematic testing for and treatment of latent TB. Testing and treatment should be applied for people living with the human immunodeficiency virus (HIV), adults and children in cases of contacts with pulmonary TB, patients for whom antitumor necrosis factor treatments have been initiated, patients receiving dialysis, patients preparing for organ or hematological transplantations and patients with silicosis. Such testing and treatment should also be considered for prisoners, healthcare workers, immigrants from high TB burden countries, homeless persons and illicit drug users [5].
These recommendations suggest using the interferon-gamma (IFN-gamma) release assay or the Mantoux tuberculin skin test (TST) for diagnosing latent TB [5]. The TST has been the most commonly used test in Lithuania for many years. However, some researchers propose that a previous bacille Calmette-Guerin (BCG) vaccination can influence false positive results of the TST [6,7,8]. Moreover, repeated TST can cause a booster effect and show false positive results [6,7,8,9]. The vaccinations among the Lithuanian population are usually with BCG (98.9% of the population) [10,11]. IFN-gamma assays use antigens absent in BCG strains, and therefore are more accurate and have higher correlation with TB exposure compared to TST [12,13,14,15,16,17,18]. One IFN-gamma assay – the T SPOT TB – identifies T cells secreting IFN-gamma by using an enzyme-linked immunospot (ELISPOT) assay technique [16]. The aim of this study was to compare the efficacy between T SPOT TB and TST in diagnosing TB among Lithuanian adults.
2. Material and methods
2.1. Study sample
Individuals from 18 years old with diagnosed TB, healthcare workers with a known risk for TB and individuals with no known risk for TB who attended the Hospital of Lithuanian University of Health Sciences and gave their informed consents were included in the study. The Regional Bioethics Committee of the Lithuanian University of Health Sciences approved this study.
All subjects were divided into the following three groups according to the TB-related anamnesis, clinical symptoms and presence of contacts: (1) TB group of individuals with a bacteriologically confirmed TB diagnosis (smear positive culture) (N = 40), (2) healthcare workers, the subjects who worked in hospitals and had contacts with TB patients but were free of TB clinical symptoms (N = 22) and (3) the low risk for TB group consisting of subjects with no history of contact with TB patients and no clinical symptoms of this disease (N = 21).
All these subjects underwent a clinical examination, an interview about their history of TB exposure and chest radiography. All these individuals were HIV negative and had previously received a BCG vaccination. The residual scar evaluated the BCG status.
2.2. Tuberculin skin test
Trained nurses performed the TST according to the Mantoux technique using two units of the purified protein derivative (PPD) (Copenhagen Statens Serum Institute, Denmark). A pulmonologist measured the transverse diameter of induration after 72 h. The TST reaction was considered positive when the induration was ≥10 mm [19]. The TST was divided into 4 groups according to the diameter of induration: (1) 0–4 mm, (2) 5–9 mm, (3) 10–14 mm and (4) ≥15 mm.
2.3. T SPOT TB
Before performing the TST, 10 mL of blood was drawn from the peripheral vein for the diagnostic test of M. tuberculosis-specific IFN-γ secreting T-cells (T SPOT TB, Oxford Immunotec, Oxford, UK). The T SPOT TB test was performed using fresh blood (<5 h at room temperature) according to the manufacturer’s instructions. The mononuclear cells from peripheral blood were seeded at 2.5 × 105 cells/well in single-well plates, and two separate pools of overlapping peptides spanning the full length of ESAT-6 and CFP-10 proteins were used together for the negative and for the positive controls. The count of individual spots for ESAT-6 (T SPOT A) and CFP-10 (T SPOT B) was taken by using manual counting with Trypan Blue and a hemocyometer. The results were evaluated according to the manufacturer’s instructions.
2.4. Statistical analysis
The statistical analysis was performed by using the Statistical Package for Social Sciences (SPSS) 13. Values are expressed as mean ± SEM. Methods of statistical analysis were selected after performing the Kolmogorov–Smirnov test. Consequently the Kruskal–Wallis H test was applied for comparing the T SPOT A and T SPOT B with the groups of TB patients, healthcare workers and low risk for TB. Application of the Mann–Whitney U test was for comparing data between two groups. The One-way ANOVA method was used to compare age and the TST diameter of induration between TB patients, high risk for TB and low risk for TB groups. The χ2 test was used for data comparisons of binary variables. The Kendall tau-b coefficient was used for evaluating the correlation between T SPOT A, T SPOT B and TST. Calculations of sensitivity, Specificity, positive predictive value (PPV), negative predictive value (NPV) and the area under the ROC curve evaluated the accuracy of the TST and the T SPOT TB for diagnosing TB. All these measurements were taken for the entire study sample. Agreement between the T SPOT TB and the TST was assessed by using the kappa measure. A P value of <0.05 was considered statistically significant.
3. Results
Eighty-three persons with a mean age of 43.5 ± 1.6 years participated in the study. Positive T SPOT TB results were significantly more common in the group with diagnosed TB than it was in the other groups under study. Positive TST was higher in the diagnosed TB group as compared to the low risk of the TB group; however, it did not differ between the diagnosed with TB and the healthcare worker groups. One patient with diagnosed TB had a negative T SPOT TB result, and three patients from the TB group had negative TST results. Table 1 presents the characteristics of the studied groups.

Table 1.
Group characteristics.
T SPOT A and T SPOT B were statistically significantly higher in the diagnosed TB group compared to the healthcare workers and low risk for TB groups (Table 1). The mean size of TST did not differ significantly between the groups (Table 1).
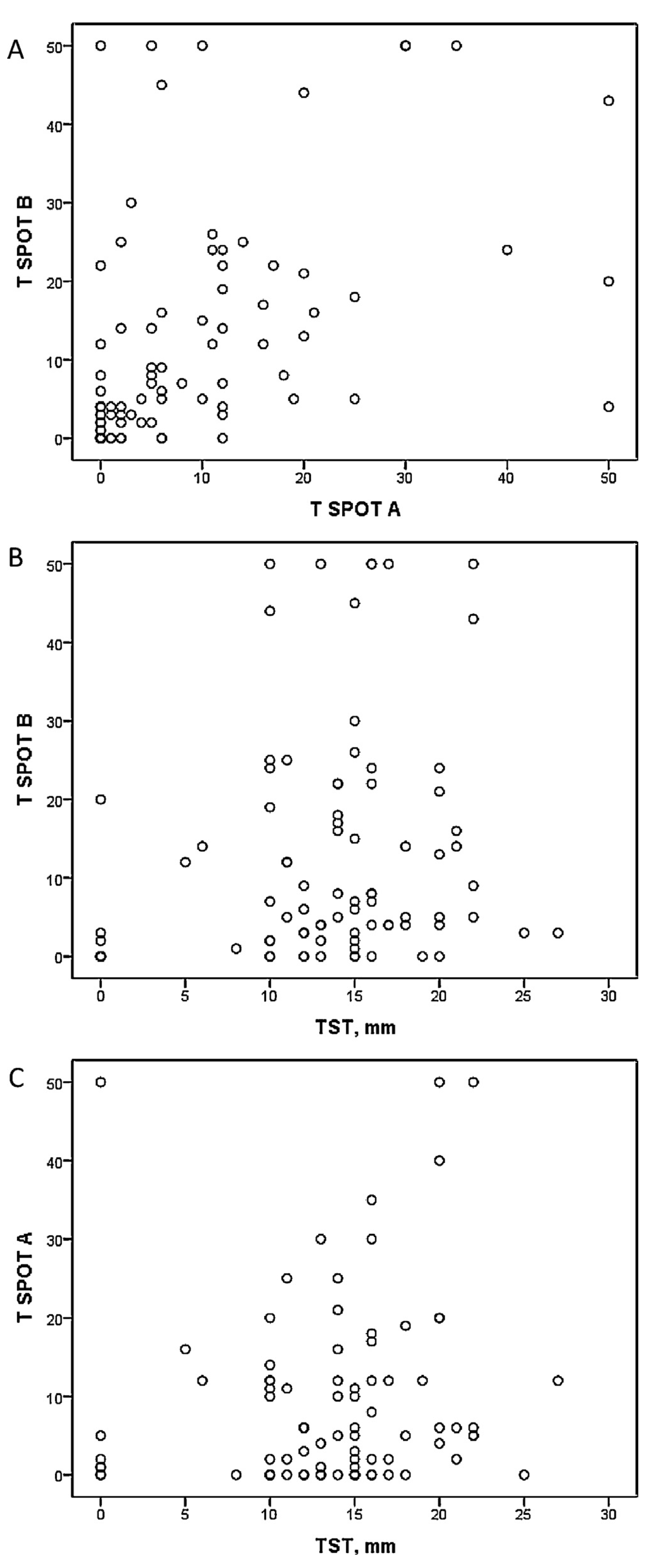
There was a significant correlation found between T SPOT A and T SPOT B, but there was no relation observed between T SPOT A or T SPOT B and mean TST size after analyzing all subjects (Fig. 1). The correlation between the mentioned variables in different groups separately showed only one significant correlation between T SPOT A and mean TST size in the healthcare worker group (Table 2). The agreement between TST and T SPOT TB was poor (kappa 0.14, P > 0.05).
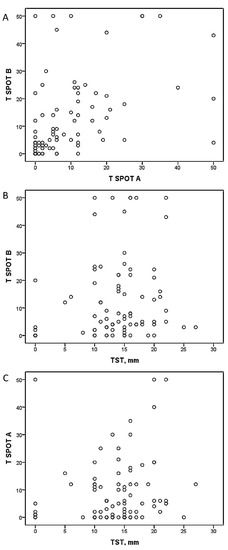
Fig. 1.
Correlation (Kendall tau-b coefficient) between spots number of T SPOT A, T SPOT B and mean TST diameter in all studied subjects. A, Kendall tau-b coefficient 0.42 (P < 0.01); B, Kendall tau-b coefficient 0.14 (P > 0.05); C, Kendall tau-b coefficient 0.13 (P > 0.05).

Table 2.
Correlation between spots number of T SPOT A and T SPOT B and mean TST diameter in different groups (Kendall tau-b correlation coefficient).
No significant differences were found in the T SPOT TB results between different TST cut-offs overall and in separate groups (Table 3).

Table 3.
Comparison of T SPOT TB results between TST cut-offs.
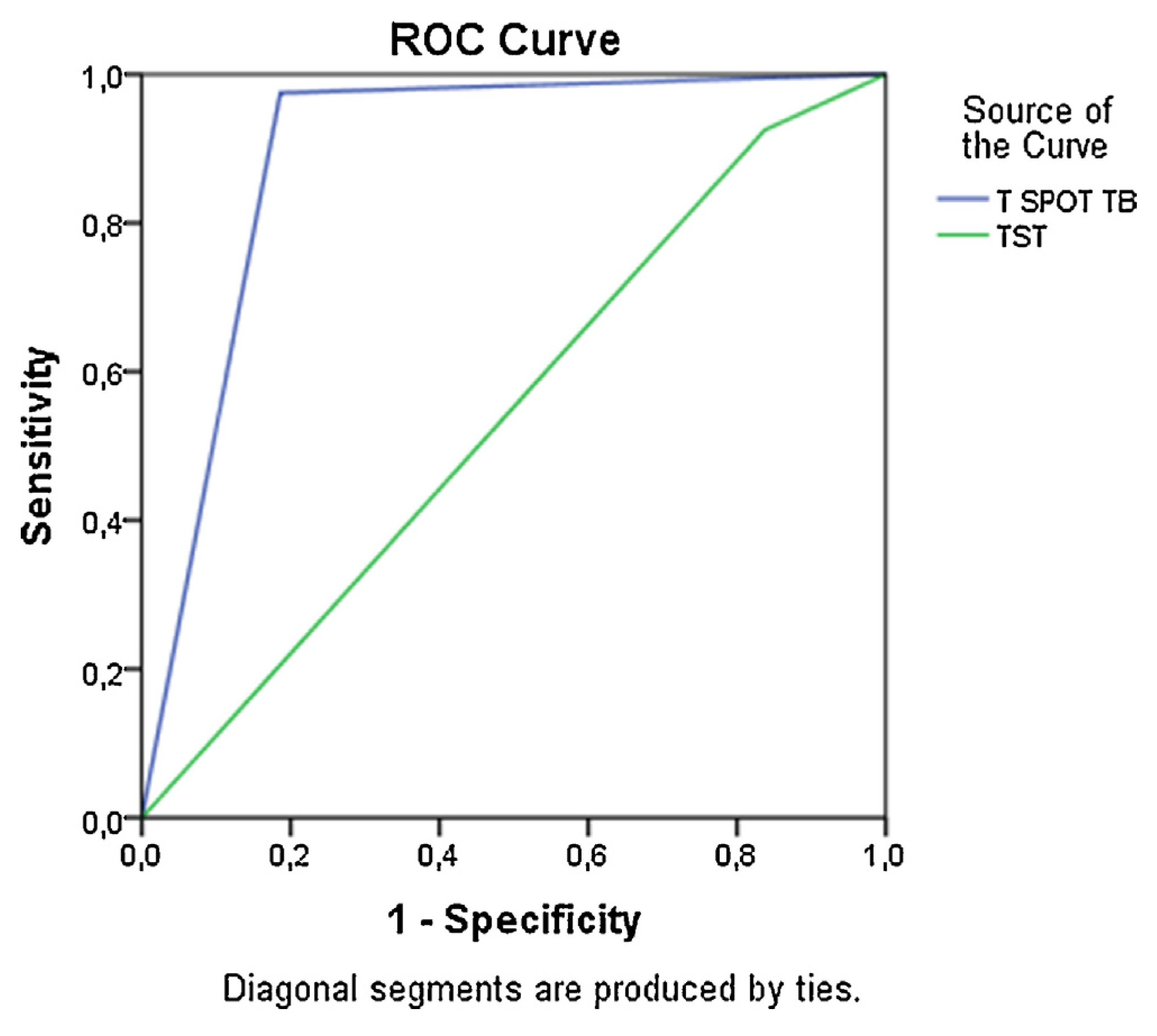
Calculation of sensitivity and Specificity of the TST and T SPOT TB methods showed that the T SPOT TB had statistically significant accuracy for diagnosing TB (Fig. 2). Specificity for T SPOT TB was 100%, whereas Specificity for the TST was only 28.6% (Table 4). However, sensitivity was high for both tests (Table 4).
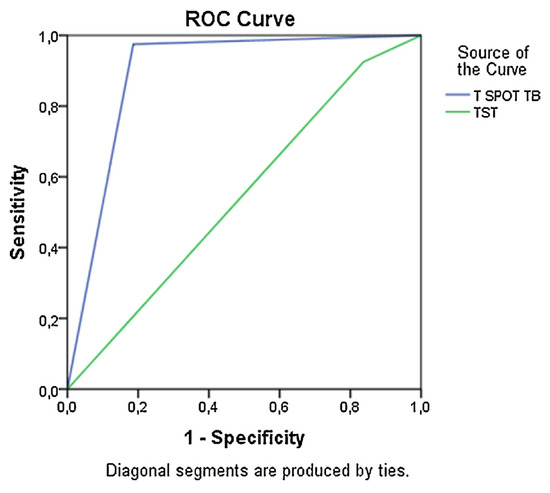
Fig. 2.
Sensitivity and specificity of T SPOT TB and TST. Area under the ROC for T SPOT TB = 0.9 W 0.04 (P < 0.01); area under the ROC for TST = 0.5 W 0.06 (P > 0.05).

Table 4.
Specificity, sensitivity, negative predictive value (NPV) and positive predictive value (PPV) of TST and T SPOT TB.
4. Discussion
This study analyzed efficacy and the differences between the two diagnostic tests of TB – TST and one of the IFN-gamma assays – the T SPOT TB.
Currently use of the TST is still widespread, even though its development by Koch was as long ago as 1890 [7]. Charles Mantoux, a French physician, described the intradermal technique currently under use in 1912 [7]. The most widely used tuberculin is PPD, which is derived from cultures of M. tuberculosis. The reaction to intracutaneously injected tuberculin is an example of a delayed hypersensitivity reaction: Tcells sensitized by prior infection are recruited to the skin site where they release cytokines, which induce induration through local vasodilatation, edema, fibrin deposition and recruitment of other inflammatory cells to the area [7]. Studies show that previous BCG vaccination and non-tuberculous mycobacteria exposures increase risk of false positive TST [6,18].
Development of the first IFN-gamma release assay took place in 2001 [20]. Later, in 2008, the fourth IFN-gamma release assay T SPOT TB test was approved [20]. This test is based on the ex vivo ELISPOT technique to measure T-cell responses to the region of deletion 1 (RD1)-encoded antigens ESAT-6 and CFP-10, which are present in M. tuberculosis but not in BCG and not in most non-tuberculous mycobacteria [18]. The risk of false positive results is lower. The recommendation is to test samples during the first few hours after drawing blood, because this assay relies on functional living T cells [18].
This study revealed that the T SPOT TB resulted in higher accuracy for diagnosing TB than the TST did. Positive T SPOT TB was significantly more common in patients with bacteriologically confirmed TB, compared to the other groups, whereas the TST did not differ between the groups of diagnosed TB and of healthcare workers. However, positive TST was more common in the diagnosed TB group than it was in the low risk for TB group, but there were no significant differences found in the mean TST diameter between the studied groups.
Results of this study are similar to the previous study conducted by these same authors comparing the T SPOT TB and the TST in diagnosing TB among Lithuanian children: the T SPOT TB was more accurate than the TST was for identifying latent TB infection in children who had been BCG vaccinated previously [21]. However, one patient from the TB group had a negative T SPOT TB result, but the TST was positive. Pan et al. [22] suggested that older age, being overweight, and prolonged hospitalization of a patient could increase the risk of a false negative T SPOT TB result. Three of the TB patients with positive T SPOT TB showed negative TST results. The absence of positive TST in patients with diagnosed TB could be associated with a congenital or acquired defective T cell response to the PPD [23].
Our results agree with the findings of the study carried out by Zelweger et al. That study indicated that positive T SPOT TBs were more common in the close TB contact group than they were in the no close TB contact group, whereas the number of positive TST did not differ significantly between the groups [12]. Moreover, Storla et al. found that positive TST was more common than positive T SPOT TB in healthcare workers with TB exposure and in individuals from the control group [24]. A possible interpretation of these results can be false positive TST due to previous BCG vaccination.
This study finds no relation between T SPOT A or T SPOT B and mean TST size in separate groups and in all subjects together. Moreover, the agreement between these tests was poor. These results are similar to the study performed by Leung et al. where agreement between T SPOT TB and TST cutoffs were relatively poor among household contacts [25]. Other authors also indicated poor agreement between these two tests [12,26].
Sensitivity of the TST and T SPOT TB was similar, but Specificity, NPV, PPV and area under ROC of the T SPOT TB were much higher than they were for TST in our study. Other studies also showed high Specificity, sensitivity, NPV and PPV of the T SPOT TB [13,27], which differed from the TST significantly [14,27].
According to Ballmeli et al. and Moreno et al., positive TST was more common in healthcare workers who were vaccinated with BCG compared to those who were not vaccinated [6,28]. Moreover, Zelweger et al. performed the TST and the T SPOT TB in patients with smear-positive TB and found that subjects with BCG were more likely to have positive TST [12]. On the contrary, previous BCG did not influence the T SPOT TB results [12]. The study performed by Katsenos revealed that a BCG vaccination after infancy increased the risk of a false positive TST [29]. The subjects in this study had all been vaccinated with BCG previously; therefore the TST and the T SPOT TB could not be compared between BCG vaccinated and non-vaccinated groups. Positive TST in this study tended to be more common than positive T SPOT TB was. The aforementioned studies maintaining the hypothesis that BCG impacts TST results could explain this [6,7]. However, other scientists suggest that a BCG vaccination has no or minimal impact on TST results, especially when TST is performed ≥10 years after vaccination [30,31,32].
There is lack of clear data about the impact of a previous BCG vaccination on TST results, but scientific literature shows that this factor has no influence on the T SPOT TB. Thus, this test is recommended for BCG-vaccinated populations [12,18,27,33,34,35]. This is very relevant for Lithuania, where very many people are vaccinated with BCG and latent TB is commonly diagnosed using the TST. This can lead to a hyperdiagnosis of TB, especially of latent TB. Research comparing the TST and the T SPOT TB in intermediate and high TB burden countries with high BCG coverage show that the T SPOT TB is more sensitive and specific than the TST is for diagnosing TB [26,34]. However, more studies need to be performed.
The cost-effectiveness of diagnostic tools is also a very important factor. Use of IFN-gamma release assays resulted in cost decreases, as shown by all 13 available studies according to the systematic review performed by Nienhaus et al. [36]. Six studies compared the use of an IFN-gamma release assay as a test to confirm a positive TST. Four of these studies indicated that the two-step strategy was more cost-effective than the TST, and two studies indicated that only the IFN-gamma release assay based strategy was more cost effective than the TST. That is why IFN-gamma release assays are recommended for screening TB risk groups, such as health care workers, immigrants from high-incidence countries and close contacts with TB [36].
5. Conclusions
To conclude, this study shows that the TST has low accuracy for diagnosing TB and poor agreement with the T SPOT TB, whereas the T SPOT TB has high Specificity and sensitivity. The IFN-gamma release assay would be useful for diagnosing latent TB in Lithuania, where TB is highly prevalent and where most people have BCG vaccinations.
Conflict of Interest
The authors report no conflicts of interest. All authors have approved the final article. The authors alone are responsible for the content and writing of the paper.
Contributions
All the authors of this study participated in the collection of data, performance of laboratory tests, statistical analysis and preparation of this manuscript.t
Acknowledgments
Authors would like to express their gratitude to Vijole Arbas for editing this manuscript and to Oxford Immunotec for kindly providing free T SPOT TB kits.ext for this section.
R E F E R E N C E S
- Dheda, K; Barry, CE, 3rd; Maartens, G. Tuberculosis. Lancet 2015. [Google Scholar] [CrossRef]
- World Health Organization. Tuberculosis. Tuberculosis country profiles. https://extranet.who.int/sree/Reports?op=Replet&name=%2FWHO_HQ_Reports%2FG2%2FPROD%2FEXT%2FTBCountryProfile&ISO2=LT&LAN=EN&outtype=html [accessed 29.09.15].
- de Vries, G; Aldridge, RW; Cayla, JA; Haas, WH; Sandgren, A; van Hest, NA; et al. Epidemiology of tuberculosis in big cities of the European Union and European Economic Area countries. Euro Surveill 2014, 19(9). [Google Scholar] [CrossRef]
- Ignatyeva, O; Balabanova, Y; Nikolayevskyy, V; Koshkarova, E; Radiulyte, B; Davidaviciene, E; et al. Resistance profile and risk factors of drug resistant tuberculosis in the Baltic countries. Tuberculosis (Edinb) 2015, 95(5), 581–8. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H; Matteelli, A; Abubakar, I; Aziz, MA; Baddeley, A; Barreira, D; et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur Respir J 2015. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S; Blázquez, R; Novoa, A; Carpena, I; Menasalvas, A; Ramírez, C; et al. The effect of BCG vaccination on tuberculin reactivity and the booster effect among hospital employees. Arch Intern Med 2001, 161(14), 1760–5. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S; Acharjya, B. Mantoux test and its interpretation. Indian Dermatol Online J 2012, 3(1), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Singh, D; Sutton, C; Woodcock, A. Repeat tuberculin testing in BCG-vaccinated subjects in the United Kingdom. The booster effect varies with the time of reading. Am J Respir Crit Care Med 2001, 164(6), 962–4. [Google Scholar] [CrossRef] [PubMed]
- Tissot, F; Zanetti, G; Francioli, P; Zellweger, JP; Zysset, F. Influence of bacille Calmette-Guérin vaccination on size of tuberculin skin test reaction: to what size? Clin Infect Dis 2005, 40(2), 211–7. [Google Scholar] [CrossRef] [PubMed]
- The BCG world atlas. A database of global BCG vaccination policies and practices. http://www.bcgatlas.org/ [accessed 21.11.15].
- Infuso, A; Falzon, D. EuroTB network. European survey of BCG vaccination policies and surveillance in children, 2005. Euro Surveill 2006, 11(3), 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, JP; Zellweger, A; Ansermet, S; de Senarclens, B; Wrighton-Smith, P. Contact tracing using a new T-cell-based test: better correlation with tuberculosis exposure than the tuberculin skin test. Int J Tuberc Lung Dis 2005, 9(11), 1242–7. [Google Scholar] [PubMed]
- King, TC; Upfal, M; Gottlieb, A; Adamo, P; Bernacki, E; Kadlecek, CP; et al. T-SPOT.TB interferon-γ release assay performance in healthcare worker screening at nineteen U.S. Hospitals. Am J Respir Crit Care Med 2015, 192(3), 367–73. [Google Scholar] [CrossRef] [PubMed]
- De Keyser, E; De Keyser, F; De Baets, F. Tuberculin skin test versus interferon-gamma release assays for the diagnosis of tuberculosis infection. Acta Clin Belg 2014, 69(5), 358–66. [Google Scholar] [CrossRef] [PubMed]
- Borkowska, D; Zwolska, Z; Michałowska-Mitczuk, D; Korzeniewska-Koseła, M; Zabost, A; Napiórkowska, A; et al. Interferon-gamma assays T-SPOT.TB for the diagnosis of latent tuberculosis infection. Pneumonol Alergol Pol 2011, 79(4), 264–71. [Google Scholar] [PubMed]
- Yan, L; Xiao, H; Han, M; Zhang, Q. Diagnostic value of T-SPOT.TB interferon-γ release assays for active tuberculosis. Exp Ther Med 2015, 10(1), 345–51. [Google Scholar] [CrossRef] [PubMed]
- Arenas Miras Mdel, M; Hidalgo-Tenorio, C; Jimenez-Gamiz, P; Jiménez-Alonso, J. Diagnosis of latent tuberculosis in patients with systemic lupus erythematosus: T.SPOT.TB versus tuberculin skin test. Biomed Res Int 2014, 291031. [Google Scholar]
- Meier, T; Eulenbruch, HP; Wrighton-Smith, P; Enders, G; Regnath, T. Sensitivity of a new commercial enzyme-linked immunospot assay (T SPOT-TB) for diagnosis of tuberculosis in clinical practice. Eur J Clin Microbiol Infect Dis 2005, 24(8), 529–36. [Google Scholar] [CrossRef] [PubMed]
- Landizabal, AA; Reichman, LB. Diagnosis of latent tuberculosis infection. Tuberculosis and nontuberculous mycobacterial infections, 5th ed.; McGraw-Hill Medical Publishing Division: New York, USA, 2006; pp. 61–70. [Google Scholar]
- Mazurek, GH; Jereb, J; Vernon, A; LoBue, P; Goldberg, S; Castro, K; et al. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection United States, 2010. MMWR Recomm Rep 2010, 59(RR-5), 1–25. [Google Scholar] [PubMed]
- Hansted, E; Andriuskeviciene, A; Sakalauskas, R; Kevalas, R; Sitkauskiene, B. T-cell-based diagnosis of tuberculosis infection in children in Lithuania: a country of high incidence despite a high coverage with bacille Calmette-Guerin vaccination. BMC Pulm Med 2009, 9, 41. [Google Scholar] [CrossRef] [PubMed]
- Pan, L; Jia, H; Liu, F; Sun, H; Gao, M; Du, F; et al. Risk factors for false-negative T-SPOT.TB assay results in patients with pulmonary and extra-pulmonary TB. J Infect 2015, 70(4), 367–80. [Google Scholar] [CrossRef] [PubMed]
- Encinales, L; Zuñiga, J; Granados-Montiel, J; Yunis, M; Granados, J; Almeciga, I; et al. Humoral immunity in tuberculin skin test anergy and its role in high-risk persons exposed to active tuberculosis. Mol Immunol 2010, 47(5), 1066–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Storla, DG; Kristiansen, I; Oftung, F; Korsvold, GE; Gaupset, M; Gran, G; et al. Use of interferon gamma-based assay to diagnose tuberculosis infection in health care workers after short term exposure. BMC Infect Dis 2009, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Leung, CC; Yam, WC; Ho, PL; Yew, WW; Chan, CK; Law, WS; et al. T-Spot.TB outperforms tuberculin skin test in predicting development of active tuberculosis among household contacts. Respirology 2015, 20(3), 496–503. [Google Scholar] [CrossRef] [PubMed]
- Dilektasli, AG; Erdem, E; Durukan, E; Eyüboğlu, FÖ. Is the T-cell-based interferon-gamma releasing assay feasible for diagnosis of latent tuberculosis infection in an intermediate tuberculosis-burden country? Jpn J Infect Dis 2010, 63(6), 433–6. [Google Scholar] [PubMed]
- Feng, Y; Diao, N; Shao, L; Wu, J; Zhang, S; Jin, J; et al. Interferon gamma release assay performance in pulmonary and extrapulmonary tuberculosis. PLoS ONE 2012, 7(3), e32652. [Google Scholar] [CrossRef] [PubMed]
- Balmelli, C; Zysset, F; Pagnamenta, A; Francioli, P; Lazor-Blanchet, C; Zanetti, G; et al. Contact tracing investigation after professional exposure to tuberculosis in a Swiss hospital using both tuberculin skin test and IGRA. Swiss Med Wkly 2014, 144, w13988. [Google Scholar] [CrossRef] [PubMed]
- Katsenos, S; Nikolopoulou, M; Konstantinidis, AK; Gartzonika, C; Gogali, A; Margelis, I; et al. Interferon-gamma release assay clarifies the effect of bacille Calmette-Guérin vaccination in Greek army recruits. Int J Tuberc Lung Dis 2010, 14(5), 545–50. [Google Scholar] [PubMed]
- Hizel, K; Maral, I; Karakus, R; Aktas, F. The influence of BCG immunisation on tuberculin reactivity and booster effect in adults in a country with a high prevalence of tuberculosis. Clin Microbiol Infect 2004, 10(11), 980–3. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M; Greenaway, C; Pai, M; Menzies, D. False-positive tuberculin skin tests: what is the absolute effect of BCG and non-tuberculous mycobacteria? Int J Tuberc Lung Dis 2006, 10(11), 1192–204. [Google Scholar] [PubMed]
- Araujo, Z; de Waard, JH; de Larrea, CF; Borges, R; Convit, J. The effect of Bacille Calmette-Guérin vaccine on tuberculin reactivity in indigenous children from communities with high prevalence of tuberculosis. Vaccine 2008, 26(44), 5575–81. [Google Scholar] [CrossRef] [PubMed]
- Belknap, R; Daley, CL. Interferon-gamma release assays. Clin Lab Med 2014, 34(2), 337–49. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S; Shao, L; Mo, L; Chen, J; Wang, F; Meng, C; et al. Evaluation of gamma interferon release assays using Mycobacterium tuberculosis antigens for diagnosis of latent and active tuberculosis in Mycobacterium bovis BCG-vaccinated populations. Clin Vaccine Immunol 2010, 17(12), 1985–90. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pavić, I; Hojsak, I; Žmak, L; Tješić-Drinković, D; Bogović, JČ; Katalinić-Janković, V. Choosing a tuberculin skin test for the child with hyperreactive tuberculin skin test results. Lijec Vjesn 2015, 137(7–8), 241–5. [Google Scholar]
- Nienhaus, A; Schablon, A; Costa, JT; Diel, R. Systematic review of cost and cost-effectiveness of different TB-screening strategies. BMC Health Serv Res 2011, 11, 247. [Google Scholar] [CrossRef] [PubMed]
© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).