Abstract
Objective: The aim of this study was to compare the impact of treatment procedures on roots previously treated with resorcinol–formaldehyde resin and analyze the effectiveness of dye and magnification for the detection of dentin cracks. Materials and methods: Distal roots of 80 permanent first mandibular molars with a single canal were sectioned at 3 mm and 9 mm from the anatomical apex. Two groups were formed according to the method used for root canal penetration: group 1 (K-file and Pro Taper instruments) and group 2 (Ultrasound with Pro Ultra and Pro Taper files). Before and after the completion of procedures, photographs of the roots were taken for examination for cracks or/and infraction lines with two levels of magnification and with or without a dye. Results: In groups 1 and 2, either with dye or without it, there were statistically significant differences (P < 0.001) with more fractures observed in the coronal than in the apical part of specimens. Statistically significant proportional differences regarding the location of fractures were observed at both magnifications. When the dye was used, there were no statistically significant differences between the two magnifications in the detection of cracks. In the specimens where the dye was not used, differences between the groups were statistically significant at both magnifications with more complete and intra-dental fractures observed in group 2. Conclusions: Retreatment methods had a damaging effect on the root dentin of teeth previously treated with resorcinol–formaldehyde resin. At magnification ×16, the efficacy of using the dye for the detection of cracks was higher than detection without the dye.
1. Introduction
Dentin cracks can occur due to root canal treatments and tooth restorative procedures. It has been shown that use of a high concentration sodium hypochlorite rinse [1], long-term calcium hydroxide therapy [2], root canal preparation and filling [3], and post-placement [4] might impact the strength of the root dentin and promote formation of dentin cracks that may further develop into complete root fractures. Previous studies mainly focused on primary endodontic cases, while formation of dentin cracks during retreatment procedures was not widely researched.
In Eastern Europe, including other post-Soviet countries, resorcinol–formaldehyde resin has been used since 1960 and retreatment of roots previously treated with this material has been difficult [5]. Endodontists in Western countries face similar challenges when treating teeth of immigrant populations. As teeth treated with resorcinol–formaldehyde resin show staining of the dental hard tissues, such teeth in scientific literature have been described as “red teeth”, “pink teeth” or “Russian red teeth” [6].
The resorcinol–formaldehyde resin method was mainly recommended and used for the treatment of narrow and curved root canals [5,7]. Formaldehyde (liquid) and resorcinol (powder) are two main components and their polymerization occurs after adding 10% sodium hydroxide (catalyst). In the scientific literature, few mixing techniques of this material have been described [5]. In addition, mixing proportions while preparing this material were not accurately followed by dentists, therefore different levels of polymerization of this material in the root canal system can be found. Unsurprisingly, retreatment of roots filled with resorcinol–formaldehyde resin is unpredictable; consequently, depending upon the material consistency different approaches to gain root canal patency need to be used.
There is no previous evidence about how dentin previously treated with resorcinol–formaldehyde resin reacts to machine-driven instruments or ultrasonic devices.
Thus, the aim of the present study was to compare the impact of different endodontic retreatment methods on root dentin previously treated with resorcinol–formaldehyde resin and analyze how a dye or different levels of magnification may aid the detection of dentin cracks in such roots.
2. Materials and methods
Eighty permanent first mandibular molars with mature apices previously filled with resorcinol–formaldehyde resin were chosen. Such categories as patient age, sex and the condition of periradicular tissues were unknown. After extraction, all teeth were gently cleaned with a gauze to avoid any damage to the root surfaces and immediately placed into distilled water to avoid dehydration. Distal roots were sectioned from the tooth’s crown using a water-cooled diamond bur and only roots with a length of 12 mm or more were selected. Only teeth with one canal in a distal root were included. The number of canals was evaluated clinically and radiographically. Radiographs were taken in the buccolingual and mesiodistal directions with a 3 cm distance between the X-ray tube and the root with a 0.08-ms exposition (Planmeca Only, Helsinki, Finland). Roots were coded.
2.1. Preparation of specimens
For the stability, 2 mm of a root’s coronal part was fixed in a resin and then sectioned horizontally at 3 mm and 9 mm distances from anatomical apices using a water-cooled lowspeed saw (Leica SP 1600, Wetzlar, Germany). Flat surfaces of the specimens were observed and photographs were taken under ×10 and ×16 magnifications (Zeiss Stemi SV6, Carl Zeiss, Jena, Germany). Two observers independently inspected all specimens in order to exclude the ones with cracks prior to retreatment procedures. Images of the specimens prior to the procedures (no cracks) served as the controls. Buccolingual and mesiodistal diameters of the apical and coronal sections were measured three times and an average estimate was calculated. The degree of polymerization and the consistency of a filling material were also evaluated. If a root canal patency could be gained with a #15 K file (Dentsplay Maillefer, Bellaigues, Switzerland), the filling material was considered soft and if no patency was gained, the filling material was considered hard.
2.2. Preparation of specimens (Groups 1 and 2)
Specimens were divided into two groups according to the instruments used for the penetration of root canals. Group 1 contained those specimens where a filling material was soft, i.e. a canal patency was easy to gain with a #15K file; the Pro Taper rotary system with a full sequence of rotary files was used following the manufacturer’s recommendations (Densplay Maillefer, Ballaigues, Switzerland). For this root preparation, an electric motor (Densplay Maillefer, Ballaigues, Switzerland) with a torque control at a constant speed of 300 rpm and gentle in-and-out motions were employed.
Group 2 contained those specimens where the filling material was hard and patency could not be gained with a #15 K file; therefore the Pro Ultra tip #4 (Densplay Maillefer, Ballaigues, Switzerland) and Master Piezon Scaler (EMS) were used to remove the filling material. Subsequently, the Pro Taper instruments were used to enlarge all canals as it was done for specimens in Group 1.
All treatment procedures were completed by the same operator (EN). In order to simulate periodontal ligament space and to mimic the mechanisms of stress distribution, a silicon impression material (Panasil, Kettenbach, Gmbh and Co, Germany) was used as matrix around roots during their canal preparation procedures. A 2 mL 2% sodium hypochlorite rinse prior to each subsequent instrument was used and after the completion of procedures, canals were rinsed with 2 mL of distilled water.
2.3. Evaluation of specimens
After canal preparation, images of apical and coronal flat surfaces were examined under both magnifications ×10 and ×16 (Zeiss Stemi SV6, Carl Zeiss, Jena, Germany). In order to evaluate the use of dye as an aid for the detection of dentin cracks, the coronal and apical flat surfaces of slices of root dentin were stained for two minutes with a 2% methylene blue dye and subsequently rinsed with water. Similarly to undyed specimens, the stained images were also examined under magnification ×10 and ×16 (Zeiss Stemi SV6, Carl Zeiss, Jena, Germany).
Three observers blinded to a retreatment method evaluated microphotographs twice with a two week interval in-between these evaluations. The number and type of dentin defects observed after the procedures (after intervention) were compared with the same specimen prior to the procedures (control condition). In cases of discrepancy among the three examiners, the images were re-inspected and consensus was reached.
2.4. Classification of dentin defects
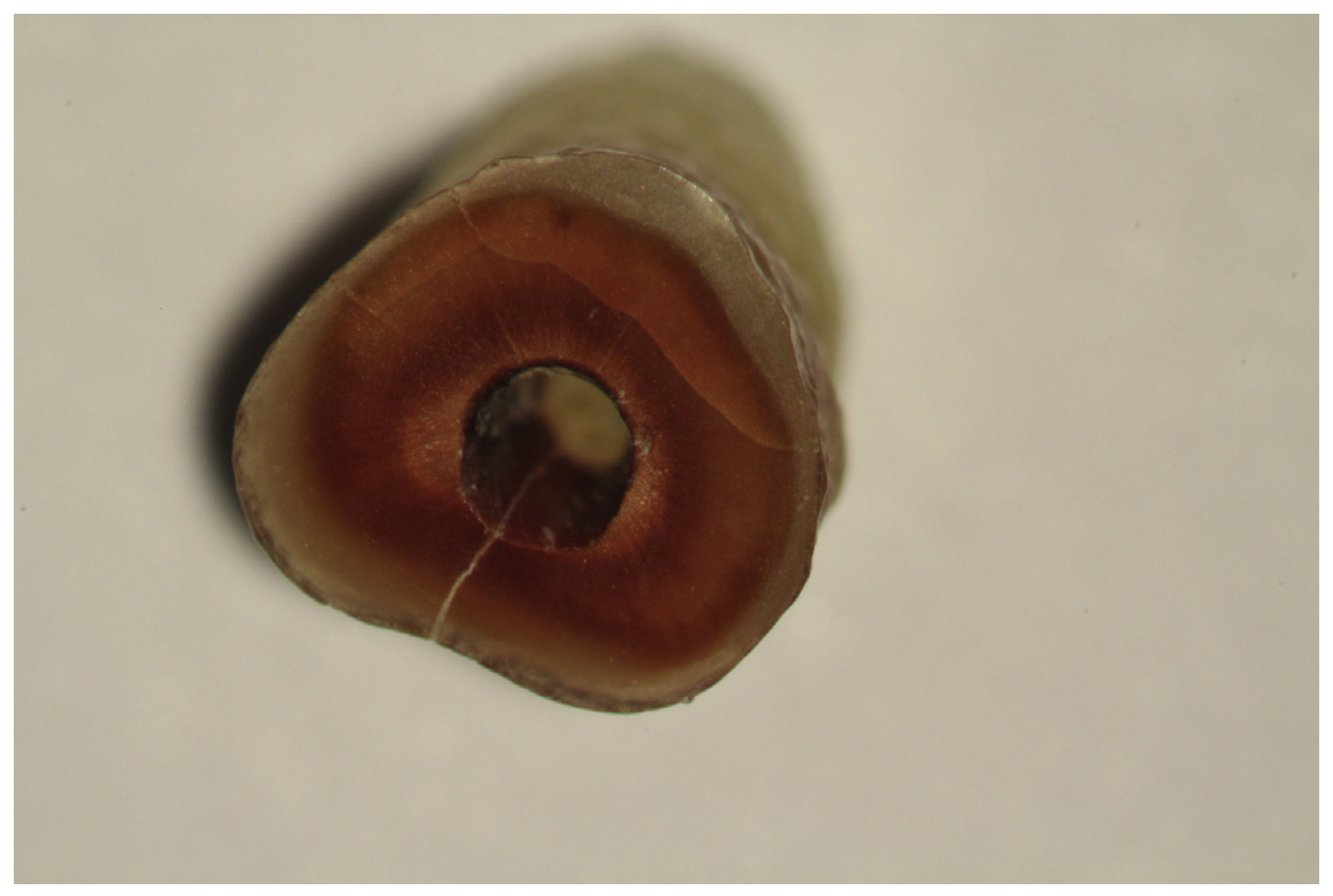
The following scheme for recording root defects was used: no defect – root dentin devoid of any cracks or lines (Fig. 1). In complete fractures, the line extended from an inner root canal wall to an outer root surface (Fig. 2). In incomplete (partial) fractures, the line started from an outer or inner root surface but it did not extend throughout the whole dentin surface (Fig. 3). In intradental fractures, the line was localized inside the dentin without reaching the outer or inner surface of a root (Fig. 4).

Fig. 1.
Root dentin devoid of any cracks or lines.
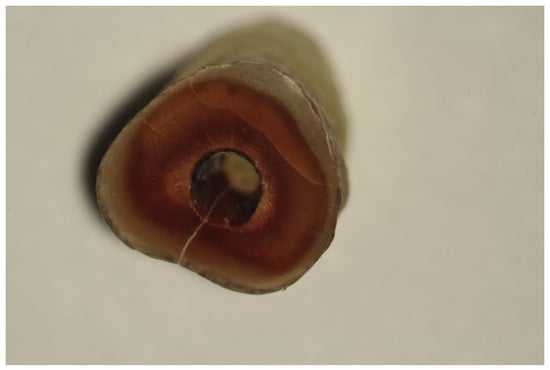
Fig. 2.
Complete root dentin fracture.

Fig. 3.
Incomplete (partial) root dentin fracture.

Fig. 4.
Intradental fractures.
2.5. Statistical analysis
For the comparison of defects identified after employing two different methods (ProTaper versus ProTaper + Ultrasound) and for the evaluation of the effectiveness of magnification to aid the crack identification, chi-square or Fisher exact tests were used.
Data analysis was performed employing the IBM SPSS, Version 21.0 statistical software and the threshold for statistical significance for all tests was set at P < 0.05. Risk Ratios (RR) and their 95% CI were used to compare findings between ProTaper and ProTaper + Ultrasound preparation systems.
3. Results
After the root canal preparations, cracks were observed in both groups. In the Pro Taper group, no complete and intradental cracks were observed and only a single case of fracture was observed at the apical root surface under magnification ×16 that was aided by a methylene blue dye.
3.1. Bivariate comparisons
Table 1 compares total numbers of root fractures after two different root preparation techniques. The crack identification was compared under two levels of magnification (×10 vs. ×16), in two different locations (coronal vs. apical) and crack identification was compared between the groups with or without the use of dye.

Table 1.
Distribution by root fracture and magnification between the ProTaper and ProTaper + Ultrasound methods.
3.2. Crack identification – aid of magnification
Overall, the use of higher magnification (×16) did not aid significantly the identification of cracks as compared to lower magnification (×10), e.g., at the coronal location in the ProTaper group when methylene was used 37.5% of the cracks were found at magnification ×10 as compared to 40.0% of the cracks at magnification ×16 (P = 0.818). Similar nonsignificant differences between the two magnifications were observed in the ProTaper + Ultrasound group.
3.3. Dentin cracks in different root locations
An overall statistically significant trend was that more cracks were observed in the coronal as compared to the apical location of the roots. This finding was consistent in both the ProTaper + Ultrasound groups.
3.4. The influence in methylene dye in aiding crack identification
The use of a dye to aid crack identification presented differently for the two preparation techniques (Protaper vs. ProTaper + Ultrasound) as well as for different root locations (coronal vs. apical). In the ProTaper group at the coronal location, there were statistically significantly more cracks identified at the coronal location when methylene was used as compared to similar samples but without the use of dye. This finding was consistent for both magnifications. Concomitantly, the use of dye did not significantly aid crack identification at the apical location of roots.
In the Protaper + Ultrasound samples, use of the dye did not help to identify more cracks at any of root locations or at any magnification levels.
Table 2 compares more specifically different types of root fractures after two different preparation techniques.

Table 2.
Distribution by the type of root fracture and magnification between the ProTaper and ProTaper + Ultrasound methods.
In both samples with or without methylene blue under both magnifications, there were statistically significantly more complete fractures after ProTaper + Ultrasound preparation as compared to the numbers of complete fractures after ProTaper preparation.
When comparing the occurrence of intradental fractures, in both samples with or without dye and under both magnifications, there were statistically significantly more intradental fractures in the ProTaper + Ultrasound group (Table 2).
Similar, although non-significant findings were reported when incomplete fractures from the outer root surfaces or incomplete fractures from the root canal were compared between the ProTaper and the ProTaper + Ultrasound groups (Table 2).
Table 3 presents risk ratios among different types of fractures between the two preparation methods (ProTaper vs. ProTaper + Ultrasound). Under magnification ×10, two statistically significant differences were found regarding complete fractures with (RR = 0.2) or without (RR = 0.1) using methylene blue. An additional significant difference for the incomplete fractures from the outer root surface (RR = 0.2) was found under magnification ×16.

Table 3.
Location of root fractures – a comparison between the ProTaper and ProTaper + Ultrasound methods.
4. Discussion
Retreatment procedures typically make up a large portion of a dentist’s daily work load. Despite the prohibition by both the European Union Directive [8] and the European Society of Endodontists [9], on the use of resorcinol–formaldehyde resin as root canal filling material, roots filled with this material still constitute a substantial part of retreatment cases in clinical dental practice. Consequently, it is important to know the outcomes of treating roots previously treated with resorcinol–formaldehyde resin. Therefore, the present study compared the impact of different treatment procedures on root dentin previously treated with resorcinol–formaldehyde resin and analyzed the effectiveness of dye and different levels of magnification for detection of dentin cracks in such roots.
The important findings of the present in vitro study were that both ProTaper and ProTaper combined with ultrasound preparation techniques had detrimental effects on root dentin, as both techniques led to crack development in roots previously treated with resorcinol–formaldehyde resin. Most of the cracks were observed in the coronal rather than in the apical location of roots. Seemingly the ProTaper combined with ultrasound preparation technique is more damaging to roots as compared to the ProTaper alone. To identify cracks in such roots, two types of aids were considered; the use of higher magnification and the use of dye. Seemingly, there was no benefit in using higher levels of magnification to identify the cracks. On the other hand, the use of dye assisting in better crack identification at least in identifying cracks in the coronal locations after root preparation of teeth previously treated with resorcinol–formaldehyde resin. It is likely that resorcinol–formaldehyde resin weakens the root dentin that is at a further risk of needing further root preparation.
It is known that components of resorcinol–formaldehyde resin may lead to allergic reactions after endodontic treatments [10] and they even could provoke the sequestration of the bone [11]. However the scientific data about the retreatment of teeth previously treated with resorcinol–formaldehyde resin is scarce. A few studies evaluated the quality and success of retreatment of the teeth previously treated with this material [12,13,14].
It has been proposed that treatment procedures alter the surface of root canal in ways that depend on the root canal anatomy and the chemicals used and this effect ranges from displacement and/or deformation of soft and/or hard tissue components, in the biological, mechanical, and chemical properties of the root canal dentin surface. These changes may have a profound effect on survival of the tooth [15].
A recent study attempted to mimic the technical procedure of the root canal mechanical preparation as it is done in a clinical setting, i.e. in order to simulate stress absorption during the present experiment a silicon layer surrounding the specimen was used [16]. Although we followed this recommendation in the present study, no artificial material could reproduce accurately the viscoelastic properties of periodontal ligament [17]. We also considered that the storage of specimens throughout all phases of an experiment may affect results, particularly when mechanical properties of specimens are investigated [18]. Therefore, in the present study teeth were kept in distilled water as a storage medium in order to evade relative dehydration during setting of resin, polymerization of silicone, preparation and image recording.
It is also important to emphasize that irrigation with NaOCl can significantly decrease the elastic modulus and flexural strength of dentin [1]. In order to mimic the clinical situation where 2% NaOCl is commonly used the solution of the same concentration was used in this study. Although performing the root canal preparation after removing the crown and the apical portion of the root leaving 6-mm samples does not wholly reflect clinical conditions accurately, but this sectioning method allows us to evaluate the impact on root dentin by a direct inspection of a quality of root dentin before and after the root canal preparation. The advantage of our study was that we examined both the inner and outer surfaces of roots in contrast to other studies where only the outer surfaces of roots were inspected [3,19].
In addition to the experimental in vitro nature of the present study, another limitation was that the age of the teeth in our sample was unknown. It is known that aging can provoke microstructural changes in dentin that may influence the number of micro-cracks that can be detected, therefore results acquired from experiments involving older teeth should not be used to generalize how similar operations and conditions would influence young dentin [20]. It is also important to acknowledge that forces during tooth extraction, pre-existing occlusal dysfunctions, trauma, or other factors might potentially influence the results [21].
In the present study, a machine-driven system for mechanical preparation of the root canal was chosen because this type of preparation is frequently used in many countries. It has been reported in in vitro studies that the instrumentation of root canals alone substantially reduces the resistance of teeth and consequently contributes to their fracture [22]. The machine-driven system has progressively varying taper and removes relatively more of the dentin coronally as compared to other systems [23]. Thus, the taper preparation could be a contributing factor in the generation of dentin cracks [24]. It was concluded that the remaining volume of the dentin after a root canal preparation was most relevant to the tooth strength [25], while other studies showed that presence of the thicker dentin did not necessarily lead to higher resistance to fractures [26].
Recent studies have shown that using various rotary instruments contributes to a higher potential of dentinal cracks. During canal preparation, the canal is shaped via contact between the instrument and the dentinal walls, creating a momentary stress concentration in the dentin which may lead to dentinal defects [23,27,28]. On the other hand, it has been demonstrated that the use of hand files leads to less damage to the root dentin due to their less aggressive movements [3] and less taper [29]. Evidence shows that not only instruments, but also a number of procedures can influence the development of dentinal cracks. Shemesh et al. found that the retreatment groups developed more defects than the primary treated groups [30]. Retreatment procedures require more mechanical manipulations in the root canal, consequently in retreatment cases more dentin tissue is removed from root canal walls.
Ultrasonic action can also result in roughening of the canal walls [31]. When the ultrasonic retrograde preparation was introduced to endodontics, it was associated with increased crack formation following endodontic root preparation [32].
Methylene blue was recommended as the dye to use when looking for cracks due to the small dye molecules that allow deeper penetration than other dyes [33]. The present study confirmed that this may aid to identify cracks in laboratory studies.
However, staining alone should only be seen as aid as it may not enhance the detection of dentin defects due to the fact that dye cannot flow into crazy lines unless there is a break on the surface [34]. The age of tooth and permeability of dentin which is dependent on the configuration of the intertubular dentin also could influence the dye penetration [35].
5. Conclusions
The use of machine-driven or ultrasonic instruments during retreatment procedures showed a damaging effect on the root dentin of teeth previously treated with resorcinol–formaldehyde resin. The use of methylene dye aided the crack detection, but the benefits of using higher ×16 magnification for the crack identification were minimal at best.
Conflicts of Interest
The authors declare no conflict of interest as they did not receive any personal benefits in connection with this study.
Acknowledgments
The authors had full control of this study at all stages of its implementation, analyses and presentation.
R E F E R E N C E S
- Sim, TP; Knowles, JC; Ng, YL; Shelton, J; Gulabivala, K. Effect of sodium hypochlorite on mechanical properties of dentine and tooth surface strain. Int Endod J 2001, 34, 120–32. [Google Scholar] [CrossRef] [PubMed]
- Doyon, GE; Dumsha, T; Fraunhofer, JA. Fracture resistance of human root dentin exposed to intracanal calcium hydroxide. J Endod 2005, 31, 895–7. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, H; Bier, CA; Wu, MK; Tanomaru-Filho, M; Wesselink, PR. The effects of canal preparation and filling on the incidence of dentinal defects. Int Endod J 2009, 42, 208–13. [Google Scholar] [CrossRef] [PubMed]
- Kishen, A. Mechanism and risk factors for fracture predilection in endodontically treated teeth. Endod Top 2006, 13, 57–83. [Google Scholar] [CrossRef]
- Schwandt, NW; Gound, G. Resorcinol–formaldehyde resin “Russian red” endodontic therapy. J Endod 2003, 29, 435–7. [Google Scholar] [CrossRef] [PubMed]
- Matthews, JD. Pink teeth resulting from Russian endodontic therapy. J Am Dent Assoc 2000, 131, 1598–9. [Google Scholar] [CrossRef] [PubMed]
- Gluskin, AH. Mishapes and serious complications in endodontic obturation. Endod Top 2005, 12, 52–70. [Google Scholar] [CrossRef]
- Medical Device Directive 93/42 EEC.
- Quality guidelines for endodontic treatment: consensus report of the European Society of Endodontology. Int Endod 2006, 39, 921–30.
- Hensten, A; Jacobsen, N. Allergic reactions in endodontic practice. Endod Top 2005, 12, 44–51. [Google Scholar] [CrossRef]
- Ongenae, K; Matthieu, L; Constandt, L; Van Hecke, E. Contact allergy to resorcinol monobenzonate. Dermatology 1998, 196, 470–3. [Google Scholar] [CrossRef] [PubMed]
- Gound, TG; Marx, D; Schwandt, NA. Incidence of flare-ups and evaluation of quality after retreatment of resorcinol–formaldehyde resin (“Russian Red Cement”) endodontic therapy. J Endod 2003, 29, 624–6. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, MK; Wang, M. Clinical and experimental observation on resinifying therapy. Oral Surg Oral Med Oral Pathol 1986, 62, 441–8. [Google Scholar] [CrossRef]
- Gambrel, MG; Hartwell, GR; Moon, PC; Cardon, JW. The effect of endodontic solutions on resorcinol–formalin paste in teeth. J Endod 2005, 31, 25–9. [Google Scholar] [CrossRef] [PubMed]
- Gulabivala, K; Patel, B; Evans, G; Yuan-Ling, NG. Effect of mechanical and chemical procedures on root canal surfaces. Endod Top 2005, 10, 103–22. [Google Scholar] [CrossRef]
- Okitsu, M; Takahashi, H; Yoshioka, T; Ivasaki, N; Suda, H. Effective factor including periodontal ligament on vertical root fractures. J Endod 2005, 20, 32–7. [Google Scholar] [CrossRef]
- Soros, C; Zinelis, S; Lambrianidis, T; Palaghias, G. Spreader load required for vertical root fracture during lateral compaction ex vivo: evaluation of periodontal simulation and fracture load information. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2008, 106, e64–70. [Google Scholar] [CrossRef] [PubMed]
- Burklein, S; Tsotsis, P; Schafer, E. Incidence of dentinal defects after root canal preparation: reciprocating versus rotary instrumentation. J Endod 2013, 39, 501–4. [Google Scholar] [CrossRef] [PubMed]
- Adorno, CG; Yoshioka, T; Suda, H. The effect of root preparation technique and instrumentation length on the development of apical root cracks. J Endod 2009, 35(3), 389–92. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A; Bajaj, D; Zhang, D; Romberg, E; Arola, D. Aging and the reduction in fracture toughness of human dentin. J Mech Behav Biomed Mater 2009, 2, 550–9. [Google Scholar] [CrossRef] [PubMed]
- Barreto, MS; Moraes Rdo, A; Rosa, RA; Moreira, CH; Só, MV; Bier, CA. Vertical root fracture and dentin defects: effects of root canal preparation, filling and mechanical cycling. J Endod 2012, 38, 1135–9. [Google Scholar] [CrossRef] [PubMed]
- Zandbiglari, T; Davids, H; Schafer, E. Influence of instrument taper on the resistance to fracture of endodontically treated roots. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2006, 101, 126–31. [Google Scholar] [CrossRef] [PubMed]
- Bergmans, L; Van Cleynenbreugel, J; Beullens, M; Wevers, M; Van Meerbeek, B; Lambrechts, P. Smooth flexible versus active tapered shaft design using NiTi rotary instruments. Int Endod J 2002, 35, 820–8. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, LR; Roskelley, C; Sutton, T. The relationship of root canal enlargement to finger-spreader induced vertical root fracture. J Endod 1997, 23, 533–4. [Google Scholar] [CrossRef]
- Sedgley, CM; Messer, HH. Are endodontically treated teeth more brittle? J Endod 1992, 18, 332–5. [Google Scholar] [CrossRef]
- Kivan, BH; Alacam, T; Ulusoy, OI; Genc, O; Gorgul, G. Fracture resistance of thin-walled roots restored with different post systems. Int Endod J 2009, 42, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Bier, CAS; Shemesh, H; Tanomaru-Filho, M; Wesselink, PR; Wu, M-K. The ability of different nickel-titanium rotary instrument to induce dentinal damage during canal preparation. J Endod 2009, 35, 236–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, R; Kaiwar, A; Shemesh, H; Wesselink, PR; Hou, B; Wu, MK. Incidence of apical root cracks and apical dentinal detachments after canal preparation with hand and rotary files at different instrumentation length. J Endod 2013, 39, 129–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, HC; Lee, MH; Yum, J; Versluis, A; Lee, CJ; Kim, BM. Potential relationship between design of nickel-titanium rotary instruments and vertical root fracture. J Endod 2010, 36, 1195–9. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, H; Alida, C; Roeleveld; Paul, R; Wesselink; Min-Kai, Wu. Damage to root dentin during retreatment procedures. JOE 2011, 37, 63–6. [Google Scholar] [CrossRef] [PubMed]
- Stock, CJ. Current status of the use of ultrasound in endodontics. Int Dent J 1991, 41, 175–82. [Google Scholar] [PubMed]
- Navarre, SW; Steiman, HR. Root-end fracture during retropreparation: a comparison between zirconium nitride-coated and stainless steel microsurgical ultrasonic instruments. J Endod 2002, 28, 330–2. [Google Scholar] [CrossRef] [PubMed]
- Wright, HM, Jr.; Loushine, RJ; Weller, RN; Kimbrough, WF; Waller, J; Pashley, DH. Identification of resected root-end dentinal cracks: a comparative study of transillumination and dyes. J Endod 2004, 30, 712–5. [Google Scholar] [CrossRef] [PubMed]
- Von Arx, T; Kunz, R; Schneider, AC; Burgin, W; Lussi, A. Detection of dentinal cracks after root-end resection: an ex vivo study comparing microscopy and endoscopy with scanning electron microscopy. J Endod 2010, 36, 1563–8. [Google Scholar] [CrossRef] [PubMed]
- Thaler, A; Ebert, J; Petschelt, A; Pelka, M. Influence of tooth age and root section on root dentin dye penetration. Int Endod J 2008, 41, 1115–22. [Google Scholar] [CrossRef] [PubMed]
© 2017 The Lithuanian University of Health Sciences. Production and hosting by Elsevier Sp. z o.o. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/). (http://creativecommons.org/licenses/by-nc-nd/4.0/).