Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study
Abstract
:1. Introduction
2. Materials and Sources
2.1. Data Sources
2.2. Measures of Analgesics Utilization
3. Results
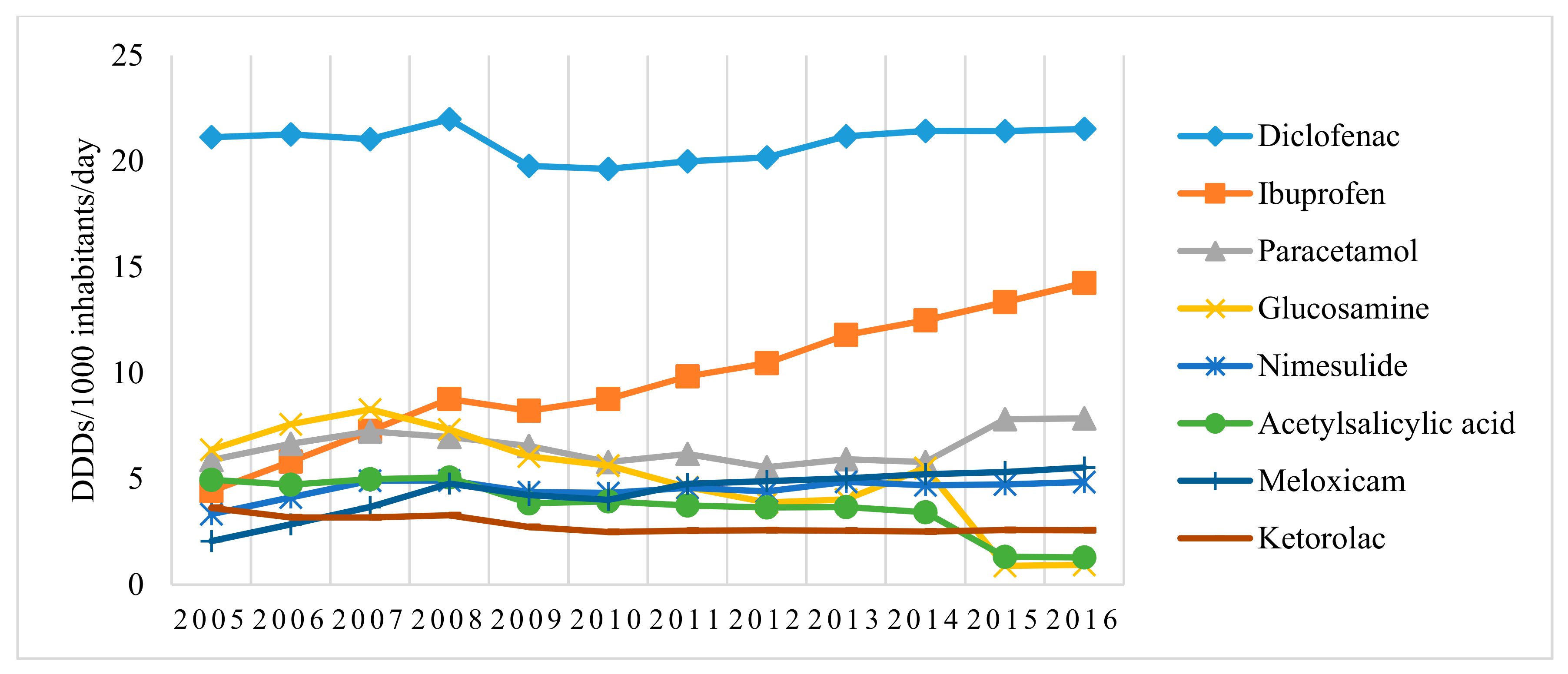
3.1. Utilization Analysis of Pain and Inflammation Relievers (N02B and M01A Pharmacotherapeutic Groups) in Lithuania over the 11-Year Period (2005–2016)
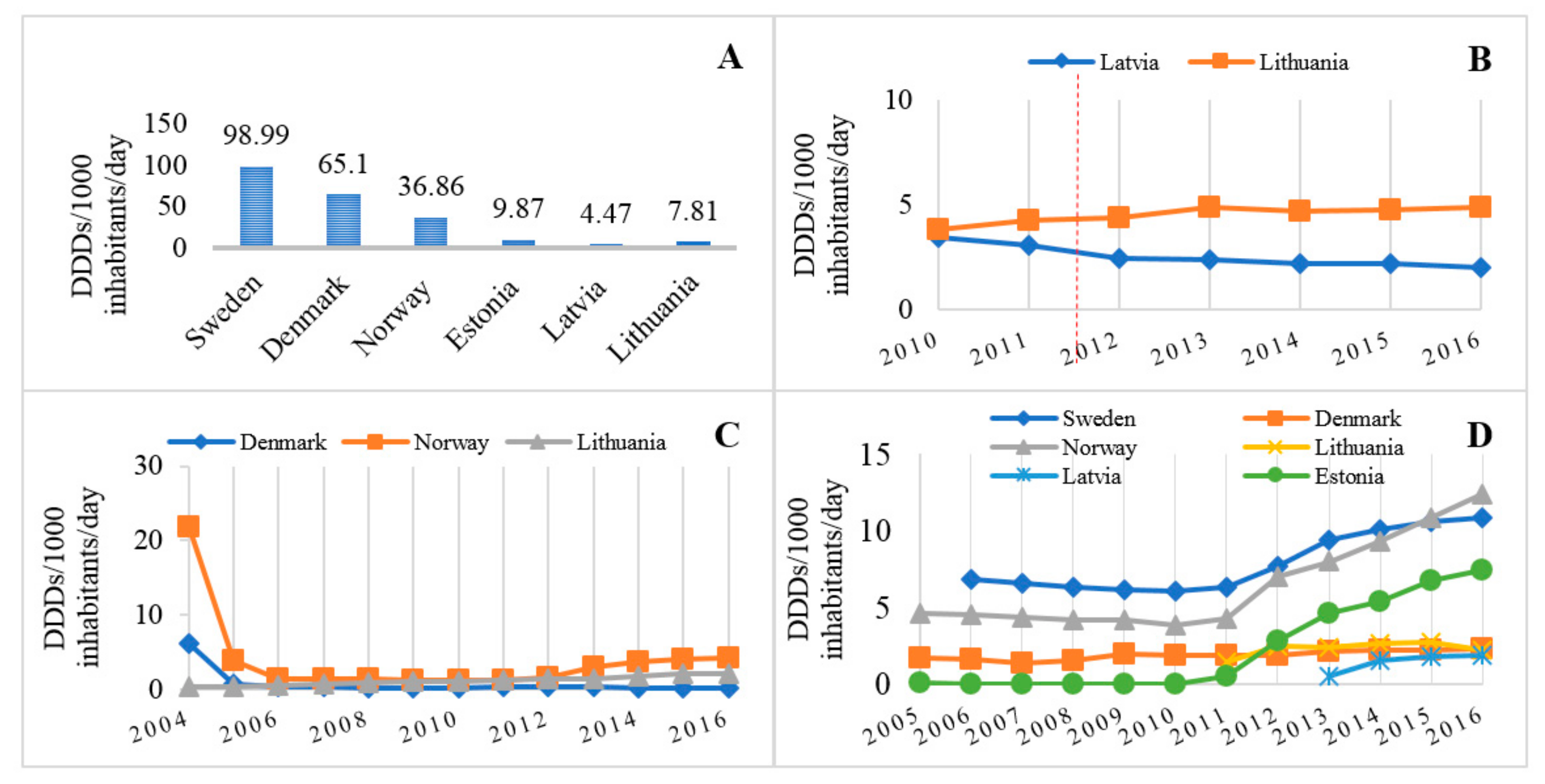
3.2. Comparison of Analgesic Utilization in Lithuania and Other Baltic and Scandinavian Countries
4. Discussion
4.1. General Considerations
4.2. Pain Management Compliance with WHO Pain Treatment Guidelines
4.3. Pain Management Compliance with Recommendations of the European Medicines Agency
4.4. Reasons for Noncompliance with Guidelines
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Hudec, R.; Božeková, L.; Tisoňová, J. Consumption of three most widely used analgesics in six European countries. J. Clin. Pharm. Ther. 2012, 37, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Mijatović, V.; Ćalasan, J.; Horvat, O.; Sabo, A.; Tomić, Z.; Radulović, V. Consumption of non-steroidal anti-inflammatory drugs in Serbia: A comparison with Croatia and Denmark during 2005–2008. Eur. J. Clin. Pharmacol. 2011, 67, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Conaghan, P.G. A turbulent decade for NSAIDs: Update on current concepts of classification, epidemiology, comparative efficacy, and toxicity. Rheumatol. Int. 2012, 32, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Boudreau, D.M.; Freedman, A.N. Trends in the use of aspirin and nonsteroidal anti-inflammatory drugs in the general U.S. population. Pharmacoepidemiol. Drug Saf. 2014, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, D.M.; Mazaud-Guittot, S.; Gaudriault, P.; Lesné, L.; Serrano, T.; Main, K.M.; Jégou, B. Analgesic use—Prevalence, biomonitoring and endocrine and reproductive effects. Nat. Rev. Endocrinol. 2016, 12, 381. [Google Scholar] [CrossRef] [PubMed]
- Craftman, Å.G.; Johnell, K.; Fastbom, J.; Westerbotn, M.; von Strauss, E. Time trends in 20 years of medication use in older adults: Findings from three elderly cohorts in Stockholm, Sweden. Arch. Gerontol. Geriatr. 2016, 63, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Motov, S.M.; Nelson, L.S. Advanced Concepts and Controversies in Emergency Department Pain Management. Anesthesiol. Clin. 2016, 34, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Desai, R.J.; Agarwal, S.J.; Aparasu, R.R. Drug use trends for arthritis and other rheumatic conditions and effect of patient’s age on treatment choice. N. C. Med. J. 2011, 72, 432–438. [Google Scholar] [PubMed]
- Saedder, E.A.; Brock, B.; Nielsen, L.P.; Bonnerup, D.K.; Lisby, M. Identifying high-risk medication: A systematic literature review. Eur. J. Clin. Pharmacol. 2014, 70, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Singh, G. Gastrointestinal complications of prescription and over-the-counter nonsteroidal anti-inflammatory drugs: A view from the ARAMIS database. Arthritis, Rheumatism, and Aging Medical Information System. Am. J. Ther. 2000, 7, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Sandler, R.S.; Bresalier, R.S.; Lanas, A.; Morton, D.G.; Riddell, R.; Iverson, E.R.; DeMets, D.L. Cardiovascular events associated with rofecoxib: Final analysis of the APPROVe trial. Lancet 2008, 372, 1756–1764. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Sandler, R.S.; Quan, H.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Lines, C.; Riddell, R.; Morton, D.; Lanas, A.; et al. Cardiovascular events associated with rofecoxib in a colorectal adenoma chemoprevention trial. N. Engl. J. Med. 2005, 352, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Baron, J.A.; Sandler, R.S.; Bresalier, R.S.; Quan, H.; Riddell, R.; Lanas, A.; Bolognese, J.A.; Oxenius, B.; Horgan, K.; Loftus, S.; et al. A randomized trial of rofecoxib for the chemoprevention of colorectal adenomas. Gastroenterology 2006, 131, 1674–1682. [Google Scholar] [CrossRef] [PubMed]
- Nissen, S.E. Adverse Cardiovascular Effects of Rofecoxib. N. Engl. J. Med. 2006, 355, 203–205. [Google Scholar] [PubMed]
- Lagakos, S.W. Time-to-Event Analyses for Long-Term Treatments—The APPROVe Trial. N. Engl. J. Med. 2006, 355, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.J.; Campen, D.; Hui, R.; Spence, M.; Cheetham, C.; Levy, G.; Shoor, S.; Ray, W.A. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: Nested case-control study. Lancet 2005, 365, 475–481. [Google Scholar] [CrossRef]
- Nussmeier, N.A.; Whelton, A.A.; Brown, M.T.; Langford, R.M.; Hoeft, A.; Parlow, J.L.; Boyce, S.W.; Verburg, K.M. Complications of the COX-2 inhibitors parecoxib and valdecoxib after cardiac surgery. N. Engl. J. Med. 2005, 352, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; McMurray, J.J.; Pfeffer, M.A.; Wittes, J.; Fowler, R.; Finn, P.; Anderson, W.F.; Zauber, A.; Hawk, E.; Bertagnolli, M. Cardiovascular Risk Associated with Celecoxib in a Clinical Trial for Colorectal Adenoma Prevention. N. Engl. J. Med. 2005, 352, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Trelle, S.; Reichenbach, S.; Wandel, S.; Hildebrand, P.; Tschannen, B.; Villiger, P.M.; Egger, M.; Jüni, P. Cardiovascular safety of non-steroidal anti-inflammatory drugs: Network meta-analysis. BMJ 2011, 342, c7086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odom, D.M.; Mladsi, D.M.; Saag, K.G.; Sherif, B.N.; Miles, L.; Ronquest, N.; Wang, J. Relationship between diclofenac dose and risk of gastrointestinal and cardiovascular events: Meta-regression based on two systematic literature reviews. Clin. Ther. 2014, 36, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Salvo, F.; Antoniazzi, S.; Duong, M.; Molimard, M.; Bazin, F.; Fourrier-Réglat, A.; Moore, N. Cardiovascular events associated with the long-term use of NSAIDs: A review of randomized controlled trials and observational studies. Expert Opin. Drug Saf. 2014, 13, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Bhala, N.; Emberson, J.; Merhi, A.; Abramson, S.; Arber, N.; Baron, J.A.; Bombardier, C.; Cannon, C.; Farkouh, M.E.; FitzGerald, G.A.; et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: Meta-analyses of individual participant data from randomised trials. Lancet 2013, 382, 769–779. [Google Scholar] [PubMed]
- European Medicines Agency. News and Events—PRAC Recommends Updating Advice on Use of High-Dose Ibuprofen. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2015/04/news_detail_002306.jsp&mid=WC0b01ac058004d5c1 (accessed on 24 May 2016).
- European Medicines Agency. News and Events—European Medicines Agency Concludes Action on COX-2 Inhibitors. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/news/2010/01/news_detail_000969.jsp&mid=WC0b01ac058004d5c1 (accessed on 9 July 2016).
- European Medicines Agency. Nimesulide. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Nimesulide/human_referral_000275.jsp&mid=WC0b01ac0580024e9a (accessed on 9 July 2016).
- European Medicines Agency. Piroxicam. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/referrals/Piroxicam/human_referral_000109.jsp (accessed on 9 July 2016).
- Erickson Foster, J.; Velasco, J.M.; Hieken, T.J. Adverse Outcomes Associated with Noncompliance with Melanoma Treatment Guidelines. Ann. Surg. Oncol. 2008, 15, 2395–2402. [Google Scholar] [CrossRef] [PubMed]
- Perrier, L.; Buja, A.; Mastrangelo, G.; Vecchiato, A.; Sandonà, P.; Ducimetière, F.; Blay, J.Y.; Gilly, F.N.; Siani, C.; Biron, P.; et al. Clinicians’ adherence versus non adherence to practice guidelines in the management of patients with sarcoma: A cost-effectiveness assessment in two European regions. BMC Health Serv. Res. 2012, 12, 82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Visser, B.C.; Ma, Y.; Zak, Y.; Poultsides, G.A.; Norton, J.A.; Rhoads, K.F. Failure to comply with NCCN guidelines for the management of pancreatic cancer compromises outcomes. HPB 2012, 14, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Chandrananth, J.; Rabinovich, A.; Karahalios, A.; Guy, S.; Tran, P. Impact of adherence to local antibiotic prophylaxis guidelines on infection outcome after total hip or knee arthroplasty. J. Hosp. Infect. 2016, 93, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Quaglini, S.; Ciccarese, P.; Micieli, G.; Cavallini, A. Guideline Application for Decision Making in Ischemic Stroke (GLADIS) Study Group. Non-compliance with guidelines: Motivations and consequences in a case study. Stud. Health Technol. Inform. 2004, 101, 75–87. [Google Scholar] [PubMed]
- Puymirat, E.; Caudron, J.; Steg, P.G.; Lemesle, G.; Cottin, Y.; Coste, P.; Schiele, F.; de Labriolle, A.; Bataille, V.; Ferrières, J.; et al. Prognostic impact of non-compliance with guidelines-recommended times to reperfusion therapy in ST-elevation myocardial infarction. The FAST-MI 2010 Registry. Eur. Heart J. 2015, 6, 26–33. [Google Scholar]
- Statens Serum Institut. Statistikker. Available online: http://medstat.dk/en (accessed on 2 June 2016).
- Statistikdatabas för Läkemedel. Available online: http://www.socialstyrelsen.se/statistik/statistikdatabas/lakemedel (accessed on 2 June 2016).
- a6cce59ca6.pdf. Available online: http://www.fhi.no/dokumenter/a6cce59ca6.pdf (accessed on 2 June 2016).
- baltic_statistics_on_medicines_2010_2012.pdf. Available online: http://www.ravimiamet.ee/sites/default/files/documents/publications/baltic_statistics_on_medicines_2010_2012/baltic_statistics_on_medicines_2010_2012.pdf (accessed on 2 June 2016).
- Lazzaroni, M.; Bianchi Porro, G. Gastrointestinal side-effects of traditional non-steroidal anti-inflammatory drugs and new formulations. Aliment. Pharmacol. Ther. 2004, 20 (Suppl. 2), 48–58. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, F.E.; Faich, G.; Goldstein, J.L.; Simon, L.S.; Pincus, T.; Whelton, A.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: The CLASS study: A randomized controlled trial. Celecoxib Long-term Arthritis Safety Study. JAMA 2000, 284, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Bajador, E.; Serrano, P.; Fuentes, J.; Carreño, S.; Guardia, J.; Makuch, R.; Eisen, G.; Agrawal, N.M.; Stenson, W.F.; et al. Nitrovasodilators, low-dose aspirin, other nonsteroidal antiinflammatory drugs, and the risk of upper gastrointestinal bleeding. N. Engl. J. Med. 2000, 343, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; Baron, J.A.; Sandler, R.S.; Horgan, K.; Bolognese, J.; Oxenius, B.; Quan, H.; Watson, D.; Cook, T.J.; Schoen, R.; et al. Peptic ulcer and bleeding events associated with rofecoxib in a 3-year colorectal adenoma chemoprevention trial. Gastroenterology 2007, 132, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, S.E.; Jaakkimainen, L.; Bombardier, C. Risk for serious gastrointestinal complications related to use of nonsteroidal anti-inflammatory drugs. A meta-analysis. Ann. Intern. Med. 1991, 115, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Lanas, A.; García-Rodríguez, L.A.; Arroyo, M.T.; Gomollón, F.; Feu, F.; Pérez, A.G.; Zapata, E.; Bástida, G.; Rodrigo, L.; Santolaria, S.; et al. Risk of upper gastrointestinal ulcer bleeding associated with selective cyclo-oxygenase-2 inhibitors, traditional non-aspirin non-steroidal anti-inflammatory drugs, aspirin and combinations. BMJ 2006, 55, 1731–1738. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.A. COX-2 inhibition: What we learned—A controversial update on safety data. Pain Med. 2013, 14 (Suppl. 1), S29–S34. [Google Scholar] [CrossRef] [PubMed]
- Harirforoosh, S.; Asghar, W.; Jamali, F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J. Pharm. Pharm. 2013, 16, 821–847. [Google Scholar] [CrossRef]
- Møllersen, M.V.; Norgård, H.; Spigset, O.; Slørdal, L. Cardiovascular safety of non-steroidal anti-inflammatory drugs. Tidsskr. Den Nor. Lægeforen. 2015, 135, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.M.; Gislason, G.H.; Fosbøl, E.L. Time-perspective in cardiovascular risk of NSAID use after first-time myocardial infarction. Curr. Opin. Cardiol. 2013, 28, 683–688. [Google Scholar] [CrossRef] [PubMed]
- NSAIDs and Coxibs: The Stomach, The Heart and The Brain; Arthritis Research UK. Available online: http://www.arthritisresearchuk.org/health-professionals-and-students/reports/topical-reviews/topical-reviews-spring-2010.aspx (accessed on 11 June 2016).
- World Health Organization. WHO’s Cancer Pain Ladder for Adults. Available online: http://www.who.int/cancer/palliative/painladder/en/ (accessed on 11 June 2016).
- Vargas-Schaffer, G. Is the WHO analgesic ladder still valid? Twenty-four years of experience. Can. Fam. Phys. 2010, 56, 514–517. [Google Scholar]
- The College of Emergency Medicine. Best Practice guidelines. CEM4681-Pain in Adults BPG—Revised 5-12-14 (1).pdf. Available online: https://www.rcem.ac.uk/docs/College%20Guidelines/5w.%20Management%20of%20Pain%20in%20Adults%20 (accessed on 6 October 2017).
- Gain Pain Final.pdf. Available online: http://www.gain-ni.org/images/Uploads/Guidelines/Gain%20pain%20final.pdf (accessed on 11 June 2016).
- Management of Chronic Pain. A National Clinical Guideline.pdf. Available online: https://www.guidelinecentral.com/summaries/management-of-chronic-pain-a-national-clinical-guideline/ (accessed on 11 June 2016).
- MTUS_ChronicPainMedicalTreatmentGuidelines.pdf. Available online: http://www.dir.ca.gov/dwc/DWCPropRegs/MTUS_Regulations/MTUS_ChronicPainMedicalTreatmentGuidelines.pdf (accessed on 11 June 2016).
- Pharma-Mgmt-Cancer-Pain.pdf. Available online: http://health.gov.ie/wp-content/uploads/2016/01/Pharma-Mgmt-Cancer-Pain.pdf (accessed on 11 June 2016).
- Scottish Palliative Care Guidelines—Pain Management. Scottish Palliative Care Guidelines. Available online: http://www.palliativecareguidelines.scot.nhs.uk/guidelines/pain/pain-management.aspx (accessed on 11 June 2016).
- Pham, P.C.T.; Toscano, E.; Pham, P.M.T.; Pham, P.A.T.; Pham, S.V.; Pham, P.T.T. Pain management in patients with chronic kidney disease. NDT Plus 2009, 2, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Assessment Report for Nimesulide Containing Medicinal Products for Systemic Use. 20 January 2012 EMA/73856/2012 Patient Health Protection. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/Nimesulide_31/WC500125574.pdf (accessed on 6 October 2017).
- Roberto, G.; Simonetti, M.; Piccinni, C.; Lora Aprile, P.; Cricelli, I.; Fanelli, A.; Cricelli, C.; Lapi, F. Risk of Acute Cerebrovascular and Cardiovascular Events Among Users of Acetaminophen or an Acetaminophen-Codeine Combination in a Cohort of Patients with Osteoarthritis: A Nested Case-Control Study. Pharmacotherapy 2015, 35, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Fulton, R.L.; Walters, M.R.; Morton, R.; Touyz, R.M.; Dominiczak, A.F.; Morrison, D.S.; Padmanabhan, S.; Meredith, P.A.; McInnes, G.T.; Dawson, J. Acetaminophen use and risk of myocardial infarction and stroke in a hypertensive cohort. Hypertension 2015, 65, 1008–1014. [Google Scholar] [CrossRef] [PubMed]
- Barozzi, N.; Tett, S.E. What happened to the prescribing of other COX-2 inhibitors, paracetamol and non-steroidal anti-inflammatory drugs when rofecoxib was withdrawn in Australia? Pharmacoepidemiol. Drug Saf. 2007, 16, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- CVD Statistics 2017. Available online: http://www.ehnheart.org/cvd-statistics/cvd-statistics-2017.html (accessed on 27 December 2017).
- Boelsterli, U.A. Mechanisms of NSAID-induced hepatotoxicity: Focus on nimesulide. Drug Saf. 2002, 25, 633–648. [Google Scholar] [CrossRef] [PubMed]
- McNaughton, R.; Huet, G.; Shakir, S. An investigation into drug products withdrawn from the EU market between 2002 and 2011 for safety reasons and the evidence used to support the decision-making. BMJ Open 2014, 4, e004221. [Google Scholar] [CrossRef] [PubMed]
- WC500144451.pdf. Available online: http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2013/06/WC500144451.pdf (accessed on 24 May 2016).
- NICE. Non-Steroidal Anti-Inflammatory Drugs; Guidance and Guidelines. Available online: https://www.nice.org.uk/advice/ktt13/chapter/evidence-context (accessed on 11 December 2017).
- Statistinių Rodiklių Analizė—OSP. Available online: http://osp.stat.gov.lt/web/guest/statistiniu-rodikliu-analize?portletFormName=visualization&hash=f201640d-ef5e-421d-bed9-845eb20e505e (accessed on 9 July 2016).
- Vartotojui—Tik Saugūs ir Efektyvūs Vaistai!—Metinės NRV Ataskaitos. Available online: http://www.vvkt.lt/lit/Metines-NRV-ataskaitos/897 (accessed on 9 July 2016).
- Denmark Population 2016. Current Population of Denmark. Available online: http://countrymeters.info/en/Denmark (accessed on 9 July 2016).
- Annual Pharmacovigilance Report 2015. Available online: https://laegemiddelstyrelsen.dk/en/publications/2016/annual-pharmacovigilance-report-2015 (accessed on 9 July 2016).
- Berreni, A.; Montastruc, F.; Bondon-Guitton, E.; Rousseau, V.; Abadie, D.; Durrieu, G.; Chebane, L.; Giroud, J.P.; Bagheri, H.; Montastruc, J.L. Adverse drug reactions to self-medication: A study in a pharmacovigilance database. Fundam. Clin. Pharmacol. 2015, 29, 517–520. [Google Scholar] [CrossRef] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasciuškevičiūtė, S.; Gumbrevičius, G.; Vendzelytė, A.; Ščiupokas, A.; Petrikonis, K.; Kaduševičius, E. Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study. Medicina 2018, 54, 30. https://doi.org/10.3390/medicina54020030
Kasciuškevičiūtė S, Gumbrevičius G, Vendzelytė A, Ščiupokas A, Petrikonis K, Kaduševičius E. Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study. Medicina. 2018; 54(2):30. https://doi.org/10.3390/medicina54020030
Chicago/Turabian StyleKasciuškevičiūtė, Skaistė, Gintautas Gumbrevičius, Aušra Vendzelytė, Arūnas Ščiupokas, Kęstutis Petrikonis, and Edmundas Kaduševičius. 2018. "Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study" Medicina 54, no. 2: 30. https://doi.org/10.3390/medicina54020030
APA StyleKasciuškevičiūtė, S., Gumbrevičius, G., Vendzelytė, A., Ščiupokas, A., Petrikonis, K., & Kaduševičius, E. (2018). Impact of the World Health Organization Pain Treatment Guidelines and the European Medicines Agency Safety Recommendations on Nonsteroidal Anti-Inflammatory Drug Use in Lithuania: An Observational Study. Medicina, 54(2), 30. https://doi.org/10.3390/medicina54020030




