Pediatric Celiac Disease in Central and East Asia: Current Knowledge and Prevalence
Abstract
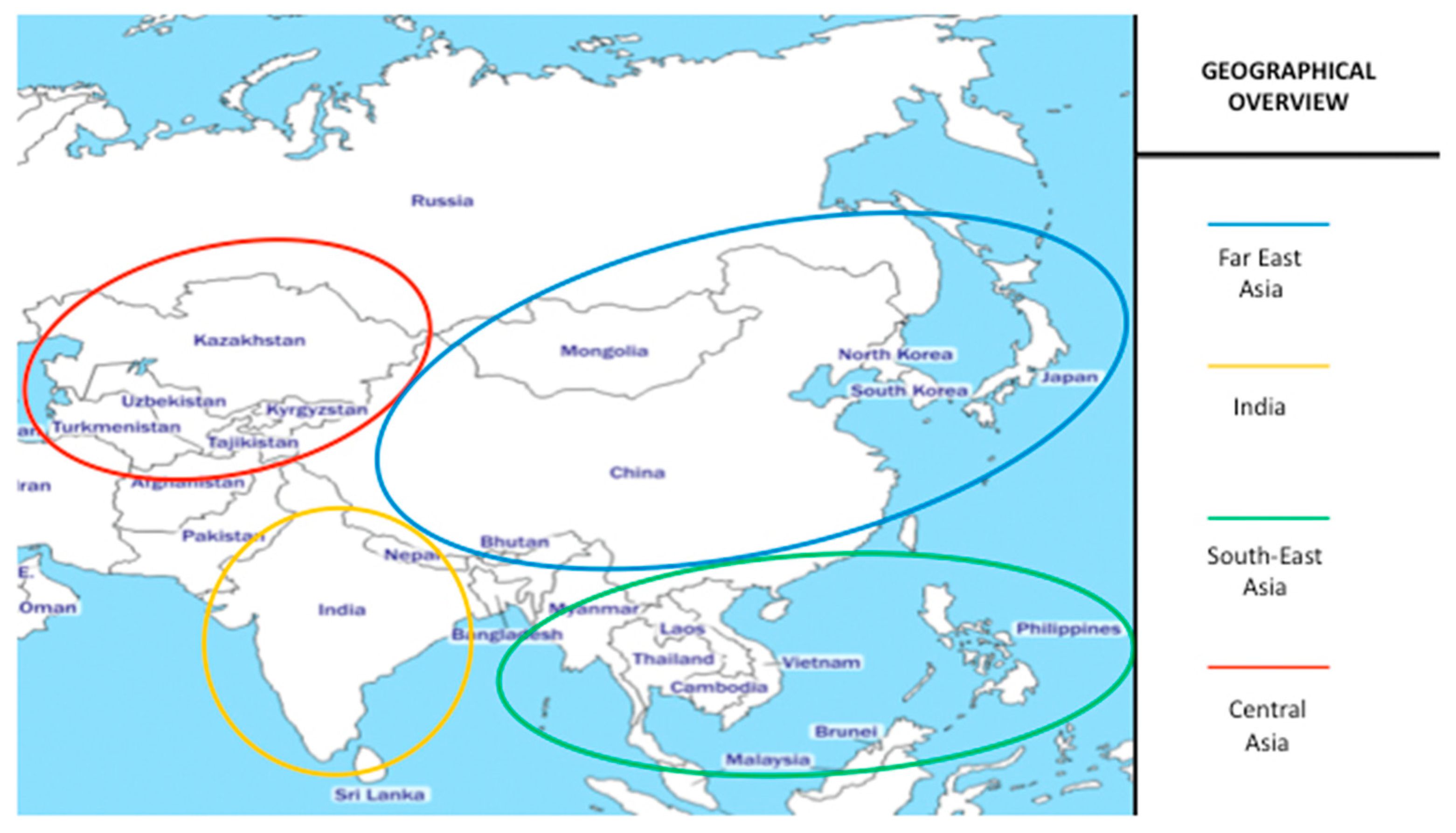
:1. Introduction
2. Far East Asia
3. India
4. South-East Asia
5. Central Asia
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Sabatino, A.; Corazza, G.R. Coeliac disease. Lancet 2009, 373, 1480–1493. [Google Scholar] [CrossRef]
- Lionetti, E.; Castellaneta, S.; Francavilla, R.; Pulvirenti, A.; Tonutti, E.; Amarri, S.; Barbato, M.; Barbera, C.; Barera, G.; Bellantoni, A.; et al. Introduction of gluten, HLA status, and the risk of celiac disease in children. N. Engl. J. Med. 2014, 371, 1295–1303. [Google Scholar] [CrossRef] [PubMed]
- De Silvestri, A.; Capittini, C.; Poddighe, D.; Valsecchi, C.; Marseglia, G.L.; Tagliacarne, S.C.; Scotti, V.; Rebuffi, C.; Pasi, A.; Martinetti, M.; et al. HLA-DQ genetics in children with celiac disease: A meta-analysis suggesting a two-step genetic screening procedure starting with HLA-DQ β chains. Pediatr Res. 2018, 83, 564–572. [Google Scholar] [CrossRef] [PubMed]
- Husby, S.; Koletzko, S.; Korponay-Szabó, I.R.; Mearin, M.L.; Phillips, A.; Shamir, R.; Troncone, R.; ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition; et al. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J. Pediatr. Gastroenterol. Nutr. 2012, 54, 136–160. [Google Scholar] [CrossRef] [PubMed]
- Admou, B.; Essaadouni, L.; Krati, K.; Zaher, K.; Sbihi, M.; Chabaa, L.; Belaabidia, B.; Alaoui-Yazidi, A. Atypical celiac disease: From recognizing to managing. Gastroenterol. Res. Pract. 2012, 2012, 637187. [Google Scholar] [CrossRef] [PubMed]
- Wessels, M.M.S.; de Rooij, N.; Roovers, L.; Verhage, J.; de Vries, W.; Mearin, M.L. Towards an individual screening strategy for first-degree relatives of celiac patients. Eur. J. Pediatr. 2018, 177, 1585–1592. [Google Scholar] [CrossRef] [PubMed]
- Poddighe, D. Individual screening strategy for pediatric celiac disease. Eur. J. Pediatr. 2018, 177, 1871. [Google Scholar] [CrossRef]
- Singh, P.; Arora, A.; Strand, T.A.; Leffler, D.A.; Catassi, C.; Green, P.H.; Kelly, C.P.; Ahuja, V.; Makharia, G.K. Global Prevalence of Celiac Disease: Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2018, 16, 823–836. [Google Scholar] [CrossRef]
- Catassi, C.; Gatti, S.; Lionetti, E. World perspective and celiac disease epidemiology. Dig. Dis. 2015, 33, 141–146. [Google Scholar] [CrossRef]
- Singh, P.; Arora, S.; Singh, A.; Strand, T.A.; Makharia, G.K. Prevalence of celiac disease in Asia: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 1095–1101. [Google Scholar] [CrossRef]
- Ramakrishna, B.S.; Makharia, G.K.; Chetri, K.; Dutta, S.; Mathur, P.; Ahuja, V.; Amarchand, R.; Balamurugan, R.; Chowdhury, S.D.; Daniel, D.; et al. Prevalence of Adult Celiac Disease in India: Regional Variations and Associations. Am. J. Gastroenterol. 2016, 111, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Rostami Nejad, M.; Rostami, K.; Emami, M.; Zali, M.; Malekzadeh, R. Epidemiology of celiac disease in iran: A review. Middle East J. Dig. Dis. 2011, 3, 5–12. [Google Scholar] [PubMed]
- Saito, S.; Ota, S.; Yamada, E.; Inoko, H.; Ota, M. Allele frequencies and haplotypic associations defined by allelic DNA typing at HLA class I and class II loci in the Japanese population. Tissue Antigens 2000, 56, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Cummins, A.G.; Roberts-Thomson, I.C. Prevalence of celiac disease in the Asia-Pacific region. J. Gastroenterol. Hepatol. 2009, 24, 1347–1351. [Google Scholar] [CrossRef] [PubMed]
- Fukunaga, M.; Ishimura, N.; Fukuyama, C.; Izumi, D.; Ishikawa, N.; Araki, A.; Oka, A.; Mishiro, T.; Ishihara, S.; Maruyama, R.; et al. Celiac disease in non-clinical populations of Japan. J. Gastroenterol. 2018, 53, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Kou, G.J.; Guo, J.; Zuo, X.L.; Li, C.Q.; Liu, C.; Ji, R.; Liu, H.; Wang, X.; Li, Y.Q. Prevalence of celiac disease in adult Chinese patients with diarrhea-predominant irritable bowel syndrome: A prospective, controlled, cohort study. J. Dig. Dis. 2018, 19, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhou, G.; Luo, L.; Crusius, J.B.; Yuan, A.; Kou, J.; Yang, G.; Wang, M.; Wu, J.; von Blomberg, B.M.; et al. Serological Screening for Celiac Disease in Adult Chinese Patients with Diarrhea Predominant Irritable Bowel Syndrome. Medicine 2015, 94, e1779. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Gao, J.; Li, X.; Liu, F.; Wijmenga, C.; Chen, H.; Gilissen, L.J. The tip of the “celiac iceberg” in China: A systematic review and meta-analysis. PLoS ONE 2013, 8, e81151. [Google Scholar] [CrossRef]
- Yuan, J.; Zhou, C.; Gao, J.; Li, J.; Yu, F.; Lu, J.; Li, X.; Wang, X.; Tong, P.; Wu, Z.; et al. Prevalence of Celiac Disease Autoimmunity Among Adolescents and Young Adults in China. Clin. Gastroenterol. Hepatol. 2017, 15, 1572–1579. [Google Scholar] [CrossRef]
- Zhao, Z.; Zou, J.; Zhao, L.; Cheng, Y.; Cai, H.; Li, M.; Liu, E.; Yu, L.; Liu, Y. Celiac Disease Autoimmunity in Patients with Autoimmune Diabetes and Thyroid Disease among Chinese Population. PLoS ONE 2016, 11, e0157510. [Google Scholar] [CrossRef]
- Wang, X.Q.; Liu, W.; Xu, C.D.; Mei, H.; Gao, Y.; Peng, H.M.; Yuan, L.; Xu, J.J. Celiac disease in children with diarrhea in 4 cities in China. J. Pediatr. Gastroenterol. Nutr. 2011, 53, 368–370. [Google Scholar] [PubMed]
- Kaur, G.; Sarkar, N.; Bhatnagar, S.; Kumar, S.; Rapthap, C.C.; Bhan, M.K.; Mehra, N.K. Pediatric celiac disease in India is associated with multiple DR3-DQ2 haplotypes. Hum. Immunol. 2002, 63, 677–682. [Google Scholar] [CrossRef]
- Krigel, A.; Turner, K.O.; Makharia, G.K.; Green, P.H.; Genta, R.M.; Lebwohl, B. Ethnic Variations in Duodenal Villous Atrophy Consistent with Celiac Disease in the United States. Clin. Gastroenterol. Hepatol. 2016, 14, 1105–1111. [Google Scholar] [CrossRef]
- Makharia, G.K.; Verma, A.K.; Amarchand, R.; Bhatnagar, S.; Das, P.; Goswami, A.; Bhatia, V.; Ahuja, V.; Datta Gupta, S.; Anand, K. Prevalence of celiac disease in the northern part of India: A community based study. J. Gastroenterol. Hepatol. 2011, 26, 894–900. [Google Scholar] [CrossRef]
- Agrawal, S.; Srivastava, S.K.; Borkar, M.; Chaudhuri, T.K. Genetic affinities of north and northeastern populations of India: Inference from HLA-based study. Tissue Antigens 2008, 72, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Sood, A.; Midha, V.; Sood, N.; Avasthi, G.; Sehgal, A. Prevalence of celiac disease among school children in Punjab, North India. J. Gastroenterol. Hepatol. 2006, 21, 1622–1625. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M.; Dubey, A.P.; Mathur, N.B. Prevalence of celiac disease in north Indian children. Indian Pediatr. 2009, 46, 415–417. [Google Scholar]
- Srivastava, A.; Yachha, S.K.; Mathias, A.; Parveen, F.; Poddar, U.; Agrawal, S. Prevalence, human leukocyte antigen typing and strategy for screening among Asian first-degree relatives of children with celiac disease. J. Gastroenterol. Hepatol. 2010, 25, 319–324. [Google Scholar] [CrossRef] [Green Version]
- Singla, S.; Kumar, P.; Singh, P.; Kaur, G.; Rohtagi, A.; Choudhury, M. HLA Profile of Celiac Disease among First-Degree Relatives from a Tertiary Care Center in North India. Indian J. Pediatr. 2016, 83, 1248–1252. [Google Scholar] [CrossRef]
- Gautam, A.; Jain, B.K.; Midha, V.; Sood, A.; Sood, N. Prevalence of celiac disease among siblings of celiac disease patients. Indian J. Gastroenterol. 2006, 25, 233–235. [Google Scholar]
- Yachha, S.K.; Poddar, U. Celiac disease in India. Indian J. Gastroenterol. 2007, 26, 230–237. [Google Scholar] [PubMed]
- Zanella, S.; De Leo, L.; Nguyen-Ngoc-Quynh, L.; Nguyen-Duy, B.; Not, T.; Tran-Thi-Chi, M.; Phung-Duc, S.; Le-Thanh, H.; Malaventura, C.; Vatta, S.; et al. Cross-sectional study of coeliac autoimmunity in a population of Vietnamese children. BMJ Open 2016, 6, e011173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thammarakcharoen, T.; Hirankarn, N.; Sahakitrungruang, T.; Thongmee, T.; Kuptawintu, P.; Kanoonthong, S.; Chongsrisawat, V. Frequency of HLA-DQB1*0201/02 and DQB1*0302 alleles and tissue transglutaminase antibody seropositivity in children with type 1 diabetes mellitus. Asian Pac. J. Allergy Immunol. 2017, 35, 82–85. [Google Scholar] [PubMed]
- Yap, T.W.; Chan, W.K.; Leow, A.H.; Azmi, A.N.; Loke, M.F.; Vadivelu, J.; Goh, K.L. Prevalence of serum celiac antibodies in a multiracial Asian population-a first study in the young Asian adult population of Malaysia. PLoS ONE 2015, 10, e0121908. [Google Scholar] [CrossRef] [PubMed]
- Savvateeva, L.V.; Erdes, S.I.; Antishin, A.S.; Zamyatnin, A.A., Jr. Overview of Celiac Disease in Russia: Regional Data and Estimated Prevalence. J. Immunol. Res. 2017, 2017, 2314813. [Google Scholar] [CrossRef] [PubMed]
- Kurtanov, H.A.; Danilova, A.L.; Yakovleva, A.E.; Savvina, A.D.; Maximova, H.P. Genetic research of HLA genes I and II class—DRB1, DQA1, DQB1 in patients with celiac disease. Bull. Hematol. 2015, 11, 44–47. (In Russian) [Google Scholar]
- Tlif, A.I.; Kondratyeva, E.I.; Chernyak, I.Y.; Dolbneva, O.V.; Shtoda, I.I.; Golovenko, I.M. The prevalence of polymorphisms of genes HLA DQA1 and DQB1 in patients with type 1 diabetes and celiac disease in the Krasnodar Region. Kuban Sci. Med. Bull. 2012, 5, 65–69. (In Russian) [Google Scholar]
- Sharipova, M.N. Clinical, epidemiological and genetic characteristics of celiac disease in children in Kazakhstan. Pediatr. Named G.N. Speransky 2009, 87, 106–108. (In Russian) [Google Scholar]
- Verma, A.K.; Gatti, S.; Lionetti, E.; Galeazzi, T.; Monachesi, C.; Franceschini, E.; Balanzoni, L.; Scattolo, N.; Cinquetti, M.; Catassi, C. Comparison of Diagnostic Performance of the IgA Anti-tTG Test vs IgA Anti-Native Gliadin Antibodies Test in Detection of Celiac Disease in the General Population. Clin. Gastroenterol. Hepatol. 2018, 16, 1997–1998. [Google Scholar] [CrossRef]
- Abduzhabarova, Z.M. Immunogenetic Profile in children with celiac disease from Uzbek population. Eur. Sci. Rev. 2016, 3–4, 34–36. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poddighe, D.; Rakhimzhanova, M.; Marchenko, Y.; Catassi, C. Pediatric Celiac Disease in Central and East Asia: Current Knowledge and Prevalence. Medicina 2019, 55, 11. https://doi.org/10.3390/medicina55010011
Poddighe D, Rakhimzhanova M, Marchenko Y, Catassi C. Pediatric Celiac Disease in Central and East Asia: Current Knowledge and Prevalence. Medicina. 2019; 55(1):11. https://doi.org/10.3390/medicina55010011
Chicago/Turabian StylePoddighe, Dimitri, Marzhan Rakhimzhanova, Yelena Marchenko, and Carlo Catassi. 2019. "Pediatric Celiac Disease in Central and East Asia: Current Knowledge and Prevalence" Medicina 55, no. 1: 11. https://doi.org/10.3390/medicina55010011
APA StylePoddighe, D., Rakhimzhanova, M., Marchenko, Y., & Catassi, C. (2019). Pediatric Celiac Disease in Central and East Asia: Current Knowledge and Prevalence. Medicina, 55(1), 11. https://doi.org/10.3390/medicina55010011





