Nitric Oxide Donor NOC-18-Induced Changes of Mitochondrial Phosphoproteome in Rat Cardiac Ischemia Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Animals and Induction of Heart Ischemia
2.3. Mitochondrial Preparation
2.4. Mitochondrial Incubation with PKG
2.5. Phosphoprotein Enrichment
2.6. In-Gel Protein Digestion for Mass Spectrometry Analysis
2.7. Protein Digestion and Sample Preparation for LC–MS/MS Analysis
2.8. Liquid Chromatography and Mass Spectrometry
2.9. Data Processing, Searching, and Analysis
2.10. Statistical Data Analysis
3. Results
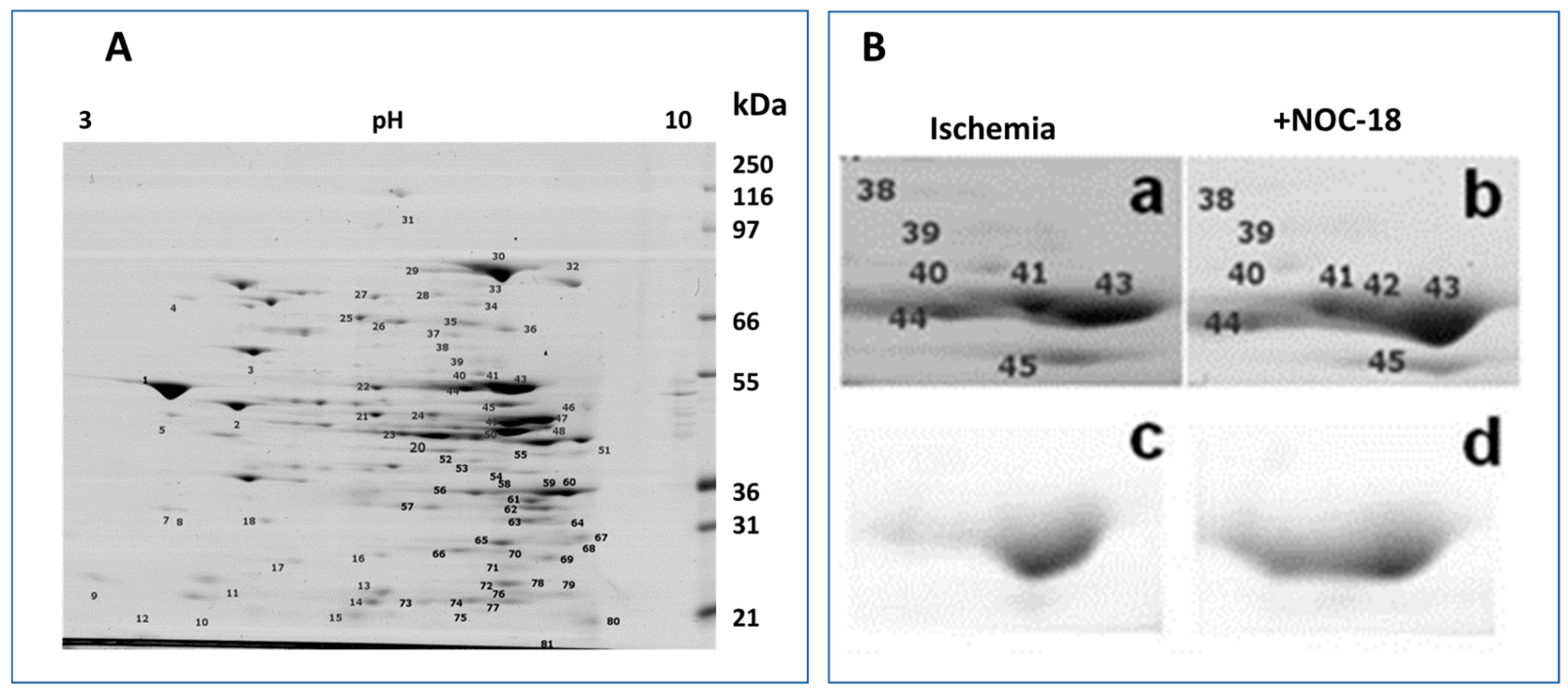
3.1. Mitochondrial Phosphoproteins in Response to NOC-18 versus Ischemia
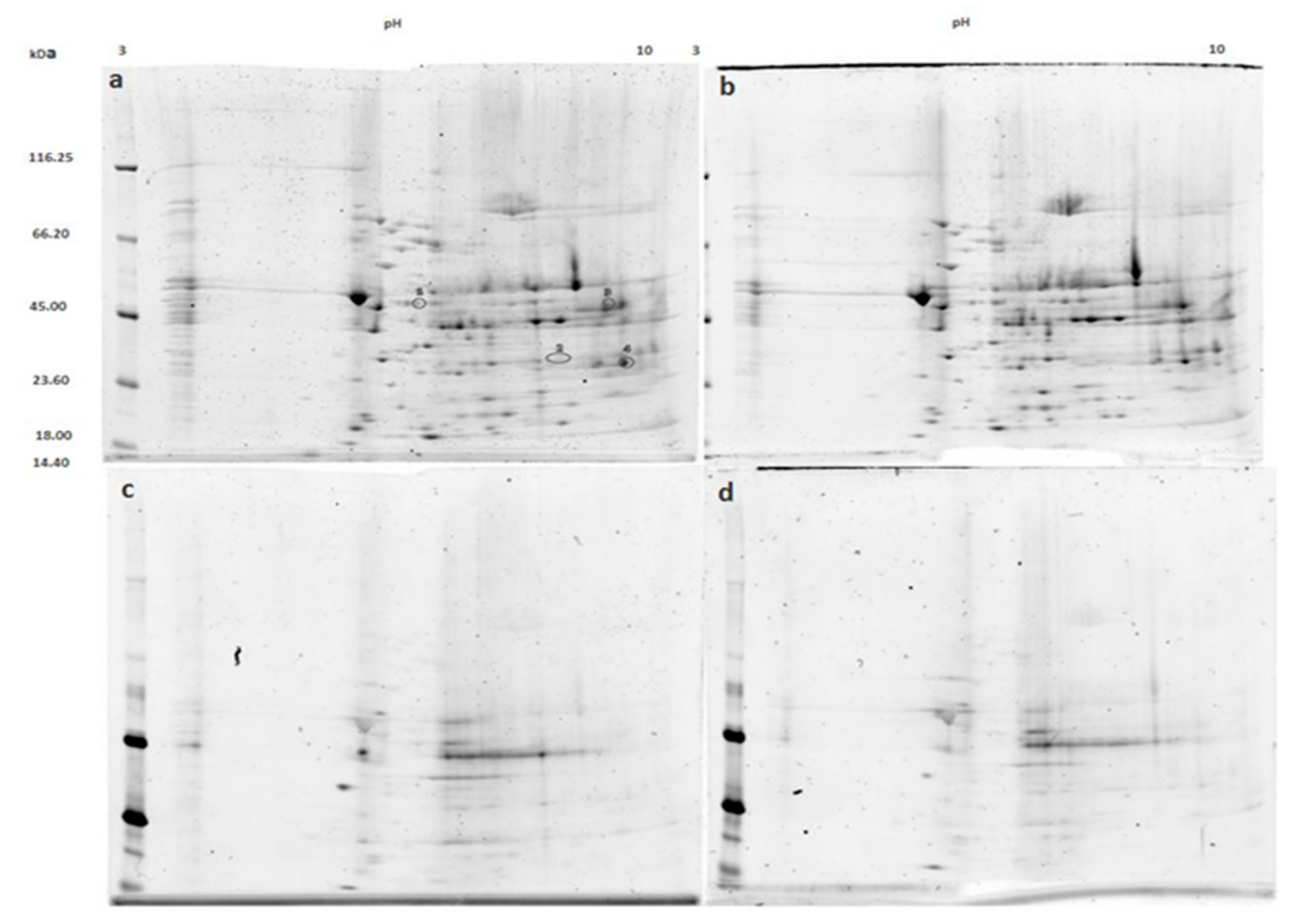
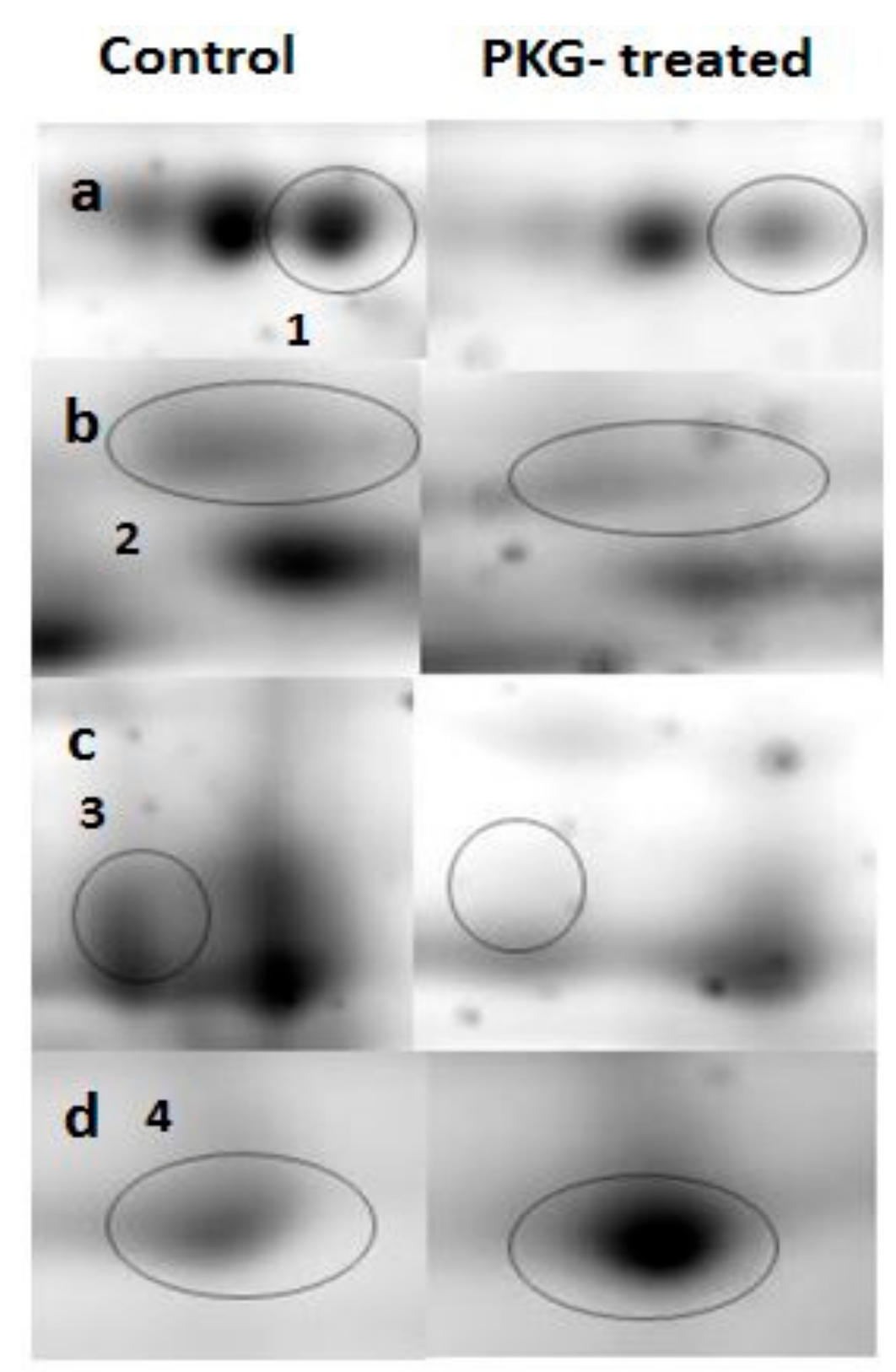
3.2. Mitochondrial Proteins after Incubation with PKG
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Crompton, M.; Ellinger, H.; Costi, A. Inhibition by cyclosporin A of a Ca2+ dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem. J. 1988, 255, 357–360. [Google Scholar] [PubMed]
- Crow, M.T.; Mani, K.; Nam, Y.-J.; Kitsis, R.N. The Mitochondrial Death Pathway and Cardiac Myocyte Apoptosis. Circ. Res. 2004, 95, 957–970. [Google Scholar] [CrossRef] [PubMed]
- Boengler, K.; Lochnit, G.; Schulz, R. “THE” target of myocardial conditioning. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H1215–H1231. [Google Scholar] [CrossRef] [PubMed]
- Javadov, S.A.; Clarke, S.; Das, M.; Griffiths, E.J.; Lim, K.H.H.; Halestrap, A.P. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. J. Physiol. 2003, 549, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Argaud, L.; Gateau-Roesch, O.; Raisky, O.; Loufouat, J.; Robert, D.; Ovize, M. Postconditioning Inhibits Mitochondrial Permeability Transition. Circulation 2005, 111, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Münzel, T.; Daiber, A.; Gori, T. Nitrate therapy: New aspects concerning molecular action and tolerance. Circulation 2011, 123, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Nathan, C.; Xie, Q.-W. Nitric oxide synthases: Roles, tolls, and controls. Cell 1994, 78, 915–918. [Google Scholar] [CrossRef]
- Andreadou, I.; Iliodromitis, E.K.; Rassaf, T.; Schulz, R.; Papapetropoulos, A.; Ferdinandy, P. The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning. Br. J. Pharmacol. 2015, 172, 1587–1606. [Google Scholar] [CrossRef] [PubMed]
- Borutaite, V.; Morkuniene, R.; Arandarcikaite, O.; Jekabsone, A.; Barauskaite, J.; Brown, G.C. Nitric oxide protects the heart from ischemia-induced apoptosis and mitochondrial damage via protein kinase G mediated blockage of permeability transition and cytochrome c release. J. Biomed. Sci. 2009, 16, 70. [Google Scholar] [CrossRef]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Boil. Chem. 1949, 177, 751–766. [Google Scholar]
- Hellman, U.; Wernstedt, C.; Gonez, J.; Heldin, C. Improvement of an “In-Gel” Digestion Procedure for the Micropreparation of Internal Protein Fragments for Amino Acid Sequencing. Anal. Biochem. 1995, 224, 451–455. [Google Scholar] [CrossRef] [PubMed]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359. [Google Scholar] [CrossRef] [PubMed]
- Šimoliūnas, E.; Kaliniene, L.; Stasilo, M.; Truncaite, L.; Zajančkauskaite, A.; Staniulis, J.; Nainys, J.; Kaupinis, A.; Valius, M.; Meškys, R. Isolation and Characterization of vB_ArS-ArV2—First Arthrobacter sp. Infecting Bacteriophage with Completely Sequenced Genome. PLoS ONE 2014, 9, e111230. [Google Scholar] [CrossRef] [PubMed]
- Bonora, M.; Pinton, P. A New Current for the Mitochondrial Permeability Transition. Trends Biochem. Sci. 2019, 44, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef]
- Fontaine, E.; Detaille, D.; Vial, G. Preventing cell death with a ‘check valve’ in mitochondrial complex I? Cell Death Dis. 2016, 7, e2165. [Google Scholar] [CrossRef][Green Version]
- Arandarcikaite, O.; Jokubka, R.; Borutaite, V. Neuroprotective effects of nitric oxide donor NOC-18 against brain ischemia-induced mitochondrial damages: Role of PKG and PKC. Neurosci. Lett. 2015, 586, 65–70. [Google Scholar] [CrossRef]
- Costa, A.D.T.; Garlid, K.D. Intramitochondrial signaling: Interactions among mitoK ATP, PKCε, ROS, and MPT. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H874–H882. [Google Scholar] [CrossRef]
- Oldenburg, O.; Qin, Q.; Krieg, T.; Yang, X.M.; Philipp, S.; Critz, S.D.; Cohen, M.V.; Downey, J.M. Bradykinin induces mitochondrial ROS generation via NO, cGMP, PKG, and mitoK ATP channel opening and leads to cardioprotection. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H468–H476. [Google Scholar] [CrossRef]
- Costa, A.D.; Garlid, K.D.; West, I.C.; Lincoln, T.M.; Downey, J.M.; Cohen, M.V.; Critz, S.D. Protein Kinase G Transmits the Cardioprotective Signal from Cytosol to Mitochondria. Circ. Res. 2005, 97, 329–336. [Google Scholar] [CrossRef]
- Applegate, M.A.B.; Humphries, K.M.; Szweda, L.I. Reversible Inhibition of α-Ketoglutarate Dehydrogenase by Hydrogen Peroxide: Glutathionylation and Protection of Lipoic Acid. Biochemistry 2008, 47, 473–478. [Google Scholar] [CrossRef]
- Jo, S.H.; Son, M.K.; Koh, H.J.; Lee, S.M.; Song, I.H.; Kim, Y.O.; Lee, Y.S.; Jeong, K.S.; Kim, W.B.; Park, J.W.; et al. Control of Mitochondrial Redox Balance and Cellular Defense against Oxidative Damage by Mitochondrial NADP+-dependent Isocitrate Dehydrogenase. J. Biol. Chem. 2001, 276, 16168–16176. [Google Scholar] [CrossRef]
- Yu, W.; Dittenhafer-Reed, K.E.; Denu, J.M. SIRT3 Protein Deacetylates Isocitrate Dehydrogenase 2 (IDH2) and Regulates Mitochondrial Redox Status. J. Boil. Chem. 2012, 287, 14078–14086. [Google Scholar] [CrossRef]
- Kim, E.Y.; Kim, W.K.; Kang, H.J.; Kim, J.-H.; Chung, S.J.; Seo, Y.S.; Park, S.G.; Lee, S.C.; Bae, K.-H. Acetylation of malate dehydrogenase 1 promotes adipogenic differentiation via activating its enzymatic activity. J. Lipid Res. 2012, 53, 1864–1876. [Google Scholar] [CrossRef]
- Shi, Q.; Gibson, G.E. Up-regulation of the mitochondrial malate dehydrogenase by oxidative stress is mediated by miR-743a. J. Neurochem. 2011, 118, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Fu, Y.; Wang, X.; Shi, H.; Huang, Y.; Song, X.; Li, L.; Song, N.; Luo, Y. Voltage-dependent anion channel 1 is involved in endostatin-induced endothelial cell apoptosis. FASEB J. 2008, 22, 2809–2820. [Google Scholar] [CrossRef]
- Ottani, F.; Latini, R.; Staszewsky, L.; La Vecchia, L.; Locuratolo, N.; Sicuro, M.; Masson, S.; Barlera, S.; Milani, V.; Lombardi, M.; et al. CYCLE Investigators. Cyclosporine A in Reperfused Myocardial Infarction: The Multicenter, Controlled, Open-Label CYCLE Trial. J. Am. Coll. Cardiol. 2016, 67, 365–374. [Google Scholar] [CrossRef]
| Mitochondrial Membrane-Bound Proteins | UniProt/SwissProt Number | Fold Change NOC-18/Ischemia | P-Value |
|---|---|---|---|
| Mitochondrial pyruvate carrier 1 (MPC1) | P63031 | 1.64 | 0.003 |
| NADH dehydrogenase ubiquinone flavoprotein 2 (NDUFV2) | P19234 | −1.53 | 0.009 |
| Very long chain specific acyl CoA dehydrogenase mitochondrial (ACADVL) | P45953 | 1.58 | 0.013 |
| Mitochondrial 2 oxoglutarate malatecarrier protein (SLC25A11) | P97700 | 1.66 | 0.013 |
| ADP/ATP translocase 1 (SLC25A4) | Q05962 | 1.57 | 0.022 |
| ATP synthase subunit alpha mitochondrial (ATP5A1) | P15999 | 1.55 | 0.028 |
| Trifunctional enzyme subunit beta mitochondrial (HADHB) | Q60587 | 1.61 | 0.032 |
| Electron transfer flavoprotein ubiquinone oxidoreductase mitochondrial (ETFD) | Q6UPE1 | 1.63 | 0.041 |
| Cytochrome c oxidase subunit 4isoform 1 mitochondrial (COX4I1) | P10888 | 1.54 | 0.042 |
| Cytochrome b c1 complex subunit Rieske (UQCRFS1) | P20788 | 1.54 | 0.043 |
| Mitochondrial Matrix Proteins | |||
| Pyruvate dehydrogenase E1 component subunit alpha somatic form mitochondrial (PDHA1) | P26284 | 1.55 | 0.024 |
| Short chain specific acyl CoAdehydrogenase mitochondrial (ACADS) | P15651 | 1.58 | 0.027 |
| Long chain specific acyl CoAdehydrogenase mitochondrial (ACADVL) | P15650 | 1.59 | 0.027 |
| Isovaleryl CoA dehydrogenase mitochondrial (IVD) | P12007 | 1.59 | 0.030 |
| Succinyl CoA ligase ADP GDP forming subunit alpha mitochondrial (SUCLG1) | P13086 | 1.62 | 0.030 |
| Lon protease homolog mitochondrial (LONP1) | Q924S5 | 1.75 | 0.032 |
| Hexaprenyldihydroxybenzoatemethyltransferase mitochondrial (COQ3) | Q63159 | 1.67 | 0.032 |
| Citrate synthase mitochondrial (CS) | Q8VHF5 | 1.57 | 0.034 |
| 3-ketoacyl CoA thiolase mitochondrial (ACAA2) | P13437 | 1.57 | 0.044 |
| Malate dehydrogenase mitochondrial (MDH2) | P04636 | 1.54 | 0.049 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Umbrasas, D.; Jokubka, R.; Kaupinis, A.; Valius, M.; Arandarčikaitė, O.; Borutaitė, V. Nitric Oxide Donor NOC-18-Induced Changes of Mitochondrial Phosphoproteome in Rat Cardiac Ischemia Model. Medicina 2019, 55, 631. https://doi.org/10.3390/medicina55100631
Umbrasas D, Jokubka R, Kaupinis A, Valius M, Arandarčikaitė O, Borutaitė V. Nitric Oxide Donor NOC-18-Induced Changes of Mitochondrial Phosphoproteome in Rat Cardiac Ischemia Model. Medicina. 2019; 55(10):631. https://doi.org/10.3390/medicina55100631
Chicago/Turabian StyleUmbrasas, Danielius, Ramūnas Jokubka, Algirdas Kaupinis, Mindaugas Valius, Odeta Arandarčikaitė, and Vilmantė Borutaitė. 2019. "Nitric Oxide Donor NOC-18-Induced Changes of Mitochondrial Phosphoproteome in Rat Cardiac Ischemia Model" Medicina 55, no. 10: 631. https://doi.org/10.3390/medicina55100631
APA StyleUmbrasas, D., Jokubka, R., Kaupinis, A., Valius, M., Arandarčikaitė, O., & Borutaitė, V. (2019). Nitric Oxide Donor NOC-18-Induced Changes of Mitochondrial Phosphoproteome in Rat Cardiac Ischemia Model. Medicina, 55(10), 631. https://doi.org/10.3390/medicina55100631







