Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Research Ethics
2.3. Anthropometric Measurements
2.4. Psychological Condition: Pain, Anxiety, and Symptom
2.5. Physiological Conditions
2.5.1. Blood Pressure and Heart Rate Measurement
2.5.2. Autonomic Nervous System Measurement
2.5.3. Range of Motion Measurement
2.5.4. Isokinetic Strength Measurement
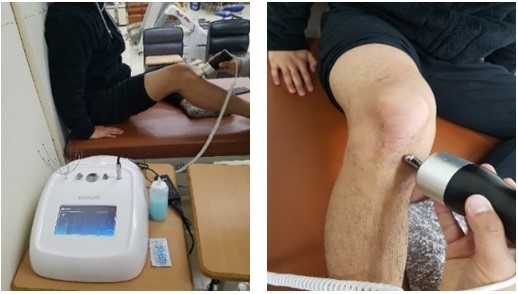
2.6. Rehabilitation Program and Local Body Vibration Administration
2.7. Data Analysis
3. Results
3.1. Effect of Local Body Vibration on Psychological Condition
3.2. Effect of Local Body Vibration on Physiological Condition
3.2.1. Effect of Local Body Vibration on Cardiorespiratory Variables
3.2.2. Effect of Local Body Vibration on Autonomic Nervous System
3.2.3. Effect of Local Body Vibration on Strength and Range of Motion
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ellis, P. Vibroacoustic sound therapy: Case studies with children with profound and multiple learning difficulties and the elderly in long-term residential care. Stud. Health Technol. Inf. 2004, 103, 36–42. [Google Scholar]
- Patrick, G. The effects of vibroacoustic music on symptom reduction. IEEE Eng. Med. Biol. Mag. 1999, 18, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Iwamoto, S.; Kimata, Y.; Nohno, T.; Hiragami, F.; Kawamura, K.; Numata, K.; Murai, H.; Okisima, K.; Iwata, M.; et al. Low frequency vibratory sound induces neurite outgrowth in PC12m3 cells in which nerve growth factor-induced neurite outgrowth is impaired. Tiss Cult. Res. Commun. 2004, 2, 81–90. [Google Scholar]
- Łukasiak, A.; Krystosiak, M.; Widłak, P.; Woldańska-Okońska, M. Evaluation of the effectiveness of vibroacoustic therapy treatment of patients with so-called “heel spur”. A preliminary report. Ortop. Traumatol. Rehabil. 2013, 15, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bautmans, I.; Van Hees, E.; Lemper, J.C.; Mets, T. The feasibility of whole body vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: A randomised controlled trial. BMC Geriatr. 2005, 22, 17. [Google Scholar] [CrossRef]
- Jackson, K.J.; Merriman, H.L.; Vanderburgh, P.M.; Brahler, C.J. Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2008, 32, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.; Sakari, R.; Cheng, S.M.; Hietikko, A.; Moilanen, P.; Timonen, J.; Fagerlund, K.M.; Kärkkäinen, M.; Alèn, M.; Cheng, S. Effects of a low-frequency sound wave therapy programme on functional capacity, blood circulation and bone metabolism in frail old men and women. Clin. Rehabil. 2009, 23, 897–908. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Grampp, S.; Henk, C.; Resch, H.; Preisinger, E.; Fialka-Moser, V.; Imhof, H. Whole-body vibration exercise leads to alterations in muscle blood volume. Clin. Physiol. 2001, 21, 377–382. [Google Scholar] [CrossRef]
- Altman, R.; Asch, E.; Bloch, D.; Bole, G.; Borenstein, D.; Brandt, K.; Christy, W.; Cooke, T.D.; Greenwald, R.; Hochberg, M.; et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Arthritis Rheum. 1986, 29, 1039–1049. [Google Scholar] [CrossRef]
- Jee, Y.S. The efficacy and safety of whole-body electromyostimulation in applying to human body: Based from graded exercise test. J. Exerc. Rehabil. 2018, 14, 49–57. [Google Scholar] [CrossRef]
- Lane, R.D.; McRae, K.; Reiman, E.M.; Chen, K.; Ahern, G.L.; Thayer, J.F. Neural correlates of heart rate variability during emotion. Neuroimage 2009, 44, 213–222. [Google Scholar] [CrossRef]
- White, D.W.; Raven, P.B. Autonomic neural control of heart rate during dynamic exercise: Revisited. J. Physiol. 2014, 592, 2491–2500. [Google Scholar] [CrossRef]
- Kilinc, B.E.; Kara, A.; Celik, H.; Oc, Y.; Camur, S. Is anterior cruciate ligament surgery technique important in rehabilitation and activity scores? J. Exerc. Rehabil. 2016, 12, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Oh, H.W.; Park, E.K.; Ko, I.G.; Kim, S.E.; Kim, J.D.; Jin, J.J.; Jee, Y.S. Effects of rehabilitation program on functional scores and isokinetic torques of knee medial plica-operated patients. Isokinet. Exerc. Sci. 2013, 21, 19–28. [Google Scholar] [CrossRef]
- Lundeberg, T.; Nordemar, R.; Ottoson, D. Pain alleviation by vibratory stimulation. Pain 1984, 20, 25–44. [Google Scholar] [CrossRef]
- Petrofsky, J.S.; Lee, S. The effects of type 2 Diabetes and aging on vascular endothelial and autonomic function. Med. Sci. Monit. 2005, 11, CR247–CR254. [Google Scholar]
- Henry, L.L. Music therapy: A nursing intervention for the control of pain and anxiety in the ICU: A review of the research literature. Dimens. Crit. Care Nurs. 1995, 14, 295–304. [Google Scholar] [CrossRef]
- Boyd-Brewer, C.; McCaffrey, R. Vibroacoustic sound therapy improves pain management and more. Holist. Nurs. Pract. 2004, 18, 111–118. [Google Scholar] [CrossRef]
- Hooper, J. An introduction to vibroacoustic therapy and an examination of its place in music therapy practice. Br. J. Music Ther. 2001, 5, 69–77. [Google Scholar] [CrossRef]
- Thayer, J.; Brosschot, J. Psychosomatics and psychopathology: Looking up and down from the brain. Psychoneuroendocrinology 2005, 30, 1050–1058. [Google Scholar] [CrossRef]
- Saul, J. Beat-to-beat variations of heart rate reflect modulation of cardiac autonomic outflow. Am. Physiol. Soc. 1990, 5, 32–37. [Google Scholar] [CrossRef]
- Grossman, P. Respiratory and cardiac rhythms as windows to central and autonomic biobehavioral regulation: Selection of window frames, keeping the panes clean and viewing the neural topography. Biol. Psychol. 1992, 34, 131–161. [Google Scholar] [CrossRef]
- Zahn, D.; Wenzel, M.; Kubiak, T. Response: Commentary: Heart rate variability and self-control—A meta-analysis. Front. Psychol. 2016, 7, 1070. [Google Scholar] [CrossRef] [PubMed]
- Skille, O.; Wigram, T.; Weeks, L. Vibroacoustic Therapy: The therapeutic effect of low frequency sound on specific physical disorders and disabilities. Br. J. Music Ther. 1989, 3, 6–10. [Google Scholar] [CrossRef]
- Ekhtiari, S.; Horner, N.S.; de Sa, D.; Simunovic, N.; Hirschmann, M.T.; Ogilvie, R.; Berardelli, R.L.; Whelan, D.B.; Ayeni, O.R. Arthrofibrosis after ACL reconstruction is best treated in a step-wise approach with early recognition and intervention: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 3929–3937. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Hu, M.; Lou, Z.; Liao, B. Effects of strength and neuromuscular training on functional performance in athletes after partial medial meniscectomy. J. Exerc. Rehabil. 2017, 28, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Warth, M.; Kessler, J.; Kotz, S.; Hillecke, T.K.; Bardenheuer, H.J. Effects of vibroacoustic stimulation in music therapy for palliative care patients: A feasibility study. BMC Complement. Altern. Med. 2015, 15, 436. [Google Scholar] [CrossRef] [PubMed]
- Dottie, R. Alternative therapies for arthritis treatment: Part 2. Holist. Nurs. Pract. 2004, 3, 167–174. [Google Scholar]
- Lundqvist, L.O.; Andersson, J.; Viding, J. Effects of vibroacoustic music on challenging behaviors in individuals with autism and developmental disabilities. Res. Autism Spectr. Disord. 2009, 3, 390–400. [Google Scholar] [CrossRef]
- Koike, Y.; Hoshitani, M.; Tabata, Y.; Seki, K.; Nishimura, R.; Kano, Y. Effects of vibroacoustic therapy on elderly nursing home residents with depression. J. Phys. Ther. Sci. 2012, 24, 291–294. [Google Scholar] [CrossRef]
- Imai, R.; Osumi, M.; Ishigaki, T.; Kodama, T.; Shimada, S.; Morioka, S. Effects of illusory kinesthesia by tendon vibratory stimulation on the postoperative neural activities of distal radius fracture patients. Neuroreport 2017, 28, 1144–1149. [Google Scholar] [CrossRef] [PubMed]
- Skille, O.; Wigram, T. The effect of music, vocalization and vibration on brain and muscle tissue: Studies in vibroacoustic therapy. In The Art and Science of Music Therapy: A Handbook; Wigram, T., Saperston, B., West, R., Eds.; Harwood Academic Press: London, UK, 1995. [Google Scholar]


| Items | Groups | Z | p * | |
|---|---|---|---|---|
| LBVG (n = 11) | nLBVG (n = 13) | |||
| Age, year | 26.82 ± 12.08 | 31.31 ± 16.49 | −0.150 | 0.910 |
| Height, cm | 173.00 ± 4.02 | 174.23 ± 2.35 | −0.703 | 0.494 |
| Weight, kg | 74.00 ± 5.37 | 75.50 ± 6.54 | −0.321 | 0.776 |
| Muscle mass, kg | 36.04 ± 5.99 | 36.94 ± 4.26 | −0.933 | 0.361 |
| Fat mass, kg | 14.09 ± 5.38 | 15.38 ± 3.60 | −1.341 | 0.186 |
| Body mass index, kg/m2 | 24.72 ± 1.57 | 24.85 ± 1.72 | −0.349 | 0.733 |
| Type (Length) | Program Type | Intensity/Time |
|---|---|---|
| Warm-up (1 day–8 weeks) | Cycling | RPE 13/15 min |
| Upper and lower leg stretching | RPE 13/20 min | |
| 1st workout phase (Week 0–2) | Vibroacoustic intervention (331.6 Hz) | 20% × 1658 Hz × 30 min |
| Q-set | 5 s × 10 reps × 3 sets | |
| Straight leg raise | 12 reps × 3 sets | |
| Ball adduction | 6 s × 10 reps × 3 sets | |
| Half squat | 10 reps × 3 sets | |
| Weight shift | 15 reps × 3 sets | |
| Weight bearing | 15 reps × 3 sets | |
| Calf raise | 10 reps × 3 sets | |
| Balance on floor | 2 min × 3 sets | |
| 2nd workout phase (Week 3–5) | Vibroacoustic intervention (663.2 Hz) | 40% × 1658 Hz × 30 min |
| Q-set | 10 s × 10 reps × 3 sets | |
| Straight leg raise | 15 reps × 3 sets | |
| Ball adduction | 8 s × 10 reps × 4 sets | |
| Leg press | 10 reps × 3 sets | |
| Leg extension | 15 reps × 3 sets | |
| Leg curl | 15 reps × 3 sets | |
| Half squat | 12 reps × 3 sets | |
| Calf raise | 12 reps × 3 sets | |
| Balance on pad | 4 min × 3 sets | |
| 3rd workout phase (Week 6–8) | Vibroacoustic intervention (994.8 Hz) | 60% × 1658 Hz × 30 min |
| Ball adduction | 10 s × 10 reps × 4 sets | |
| Leg press | 12 reps × 3 sets | |
| Leg extension | 15 reps × 3 sets | |
| Leg curl | 15 reps × 3 sets | |
| Half squat | 15 reps × 3 sets | |
| Calf raise | 15 reps × 3 sets | |
| Balance on air-pad | 6 min × 3 sets | |
| Cool-down (1 day–8 weeks) | Upper and lower leg stretching | RPE 13–15/20 min |
| Icing | 10 min |
| Item (Points) | Groups | |||
|---|---|---|---|---|
| Week | LBVG (n = 11) | nLBVG (n = 13) | Z (p) * | |
| Pain | 0 | 7.56 ± 1.21 | 7.70 ± 1.58 | −0.511 (0.649) |
| 4 | 4.10 ± 1.44 | 4.53 ± 1.20 | −0.503 (0.649) | |
| 8 | 2.13 ± 0.90 | 4.54 ± 1.33 | −3.661 (0.001) | |
| X2 (p) ** | 20.150 (0.001) | 17.280 (0.001) | ||
| Anxiety | 0 | 6.39 ± 1.09 | 6.10 ± 1.24 | −1.000 (0.361) |
| 4 | 3.72 ± 0.64 | 4.08 ± 0.95 | −1.193 (0.277) | |
| 8 | 3.01 ± 0.91 | 3.58 ± 0.95 | −1.230 (0.252) | |
| X2 (p) ** | 17.714 (0.001) | 17.522 (0.001) | ||
| Symptom | 0 | 7.04 ± 1.46 | 7.12 ± 1.89 | −0.353 (0.733) |
| 4 | 3.50 ± 1.16 | 6.71 ± 1.10 | −4.119 (0.001) | |
| 8 | 2.31 ± 0.90 | 3.95 ± 1.21 | −3.090 (0.002) | |
| X2 (p) ** | 18.558 (0.001) | 12.875 (0.002) | ||
| Item (Units) | Groups | |||
|---|---|---|---|---|
| Week | LBVG (n = 11) | nLBVG (n = 13) | Z (p) * | |
| Systolic blood pressure (mmHg) | 0 | 135.09 ± 5.17 | 134.69 ± 12.59 | −0.351 (0.733) |
| 4 | 127.09 ± 12.99 | 126.23 ± 7.17 | −0.350 (0.733) | |
| 8 | 123.45 ± 5.63 | 124.31 ± 4.85 | −0.235 (0.820) | |
| X2 (p) ** | 13.905 (0.001) | 7.714 (0.021) | ||
| Diastolic blood pressure (mmHg) | 0 | 80.55 ± 7.15 | 81.38 ± 12.49 | −0.991 (0.331) |
| 4 | 79.73 ± 7.73 | 79.08 ± 11.11 | −0.872 (0.392) | |
| 8 | 79.09 ± 7.11 | 81.15 ± 11.01 | −1.546 (0.134) | |
| X2 (p) ** | 0.800 (0.670) | 0.792 (0.673) | ||
| Heart rate (beats/min) | 0 | 77.09 ± 6.47 | 71.08 ± 8.30 | −1.887 (0.063) |
| 4 | 73.45 ± 6.22 | 78.62 ± 9.99 | −1.457 (0.150) | |
| 8 | 69.09 ± 7.71 | 73.69 ± 7.22 | −1.484 (0.150) | |
| X2 (p) ** | 11.538 (0.003) | 3.720 (0.156) | ||
| Breathing rate (reps.) | 0 | 29.61 ± 7.16 | 27.65 ± 5.54 | −1.251 (0.228) |
| 4 | 19.85 ± 4.19 | 19.14 ± 3.06 | −0.611 (0.569) | |
| 8 | 19.60 ± 4.34 | 18.94 ± 3.48 | −0.669 (0.531) | |
| X2 (p) ** | 13.818 (0.001) | 16.769 (0.001) | ||
| Item | Groups | |||
|---|---|---|---|---|
| Week | LBVG (n = 11) | nLBVG (n = 13) | Z (p) * | |
| Sympathetic activation | 0 | 7.61 ± 0.72 | 7.23 ± 0.51 | −1.352 (0.186) |
| 4 | 6.32 ± 0.63 | 7.69 ± 1.15 | −3.023 (0.002) | |
| 8 | 5.77 ± 0.57 | 6.46 ± 1.33 | −1.257 (0.228) | |
| X2 (p) ** | 14.727 (0.001) | 7.882 (0.019) | ||
| Parasympathetic activation | 0 | 3.55 ± 0.40 | 3.42 ± 0.38 | −0.849 (0.424) |
| 4 | 4.49 ± 0.36 | 3.58 ± 0.40 | −4.003 (0.001) | |
| 8 | 4.96 ± 0.28 | 3.50 ± 0.46 | −4.162 (0.001) | |
| X2 (p) ** | 22.000 (0.001) | 2.923 (0.232) | ||
| Item (Units) | Groups | |||
|---|---|---|---|---|
| Week | LBVG (n = 11) | nLBVG (n = 13) | Z (p) * | |
| Extensor peak torque (Nm) | 0 | 77.82 ± 12.91 | 70.00 ± 6.53 | −2.311 (0.022) |
| 4 | 123.09 ± 35.18 | 100.15 ± 33.83 | −1.574 (0.119) | |
| 8 | 178.73 ± 27.45 | 110.23 ± 53.88 | −3.229 (0.001) | |
| X2 (p) ** | 13.273 (0.001) | 1.385 (0.500) | ||
| Flexor peak torque (Nm) | 0 | 52.36 ± 5.24 | 47.08 ± 10.47 | −1.196 (0.252) |
| 4 | 63.91 ± 6.24 | 43.08 ± 16.27 | −3.286 (0.001) | |
| 8 | 68.00 ± 10.26 | 50.23 ± 19.43 | −2.052 (0.041) | |
| X2 (p) ** | 17.636 (0.001) | 3.231 (0.199) | ||
| Range of motion (°) | 0 | 94.55 ± 16.05 | 86.00 ± 15.35 | −1.279 (0.207) |
| 4 | 121.73 ± 14.36 | 89.62 ± 17.47 | −3.604 (0.001) | |
| 8 | 127.82 ± 12.46 | 92.38 ± 27.65 | −2.970 (0.002) | |
| X2 (p) ** | 19.860 (0.001) | 1.385 (0.500) | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-M.; Park, S.; Jee, Y.-S. Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction. Medicina 2019, 55, 659. https://doi.org/10.3390/medicina55100659
Park J-M, Park S, Jee Y-S. Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction. Medicina. 2019; 55(10):659. https://doi.org/10.3390/medicina55100659
Chicago/Turabian StylePark, Jung-Min, Sihwa Park, and Yong-Seok Jee. 2019. "Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction" Medicina 55, no. 10: 659. https://doi.org/10.3390/medicina55100659
APA StylePark, J.-M., Park, S., & Jee, Y.-S. (2019). Rehabilitation Program Combined with Local Vibroacoustics Improves Psychophysiological Conditions in Patients with ACL Reconstruction. Medicina, 55(10), 659. https://doi.org/10.3390/medicina55100659