Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics, Study Design and Participants
2.2. Sample Size and Data Analysis
2.3. Body Composition Assessment
2.4. Individualized Nutrition Intervention Program
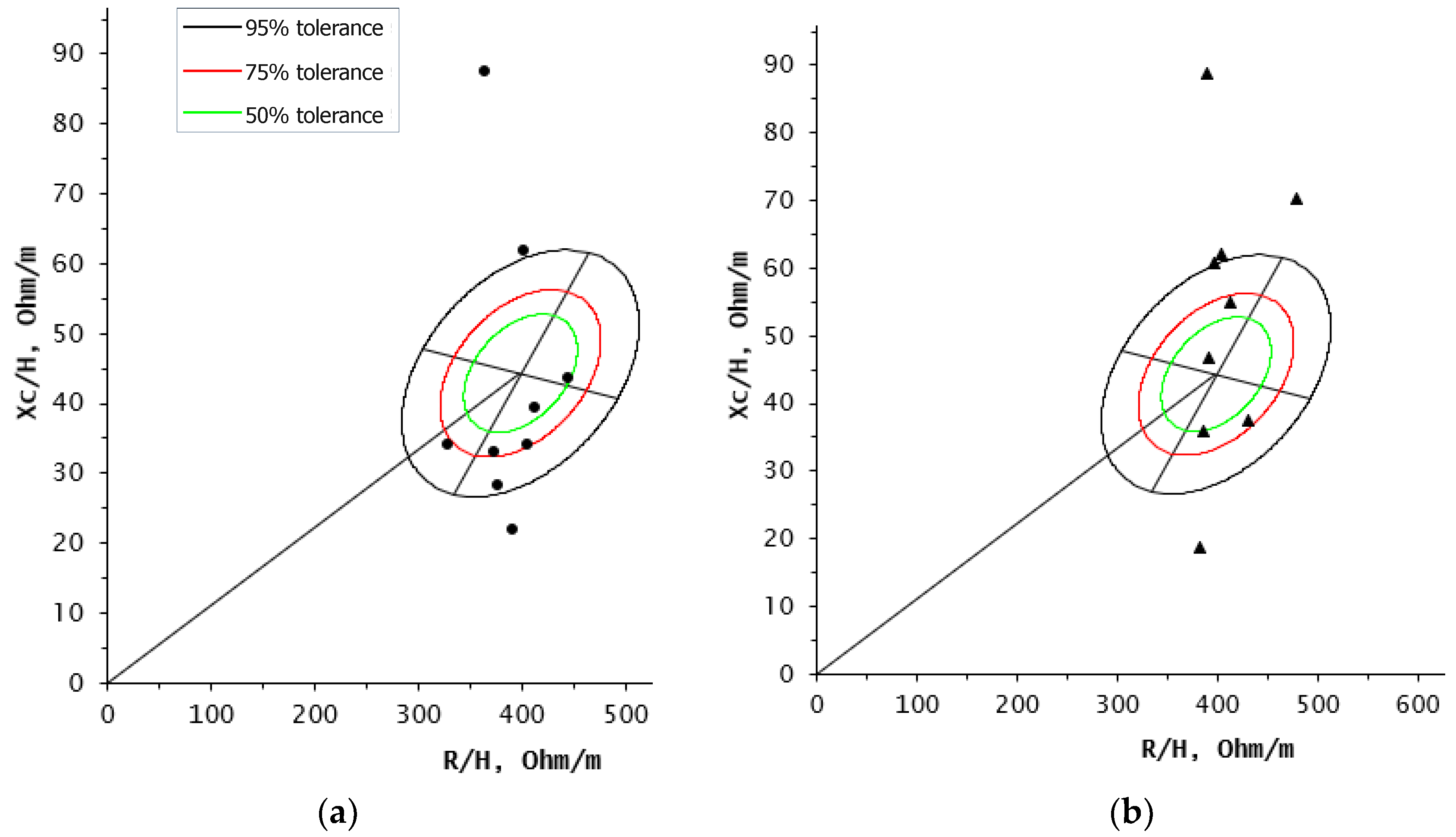
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Demark-Wahnefried, W.; Campbell, K.L.; Hayes, S.C. Weight management and its role in breast cancer rehabilitation. Cancer 2012, 118, 2277–2287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deluche, E.; Leobon, S.; Desport, J.C.; Venat-Bouvet, L.; Usseglio, J.; Tubiana-Mathieu, N. Impact of body composition on outcome in patients with early breast cancer. Support Care Cancer 2018, 26, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Caan, B.J.; Cespedes, E.M.; Prado, C.M.; Alexeeff, S.; Kroenke, C.H.; Bradshaw, P.; Quesenberry, C.P.; Weltzien, E.K.; Castillo, A.L.; Olobatuyi, T.A.; et al. Association of Muscle and Adiposity Measured by Computed Tomography with Survival in Patients With Nonmetastatic Breast Cancer. JAMA Oncol. 2018, 4, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dietary Guidelines for Breast Cancer Patients: A Critical Review. Adv. Nutr. 2017, 8, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Cuevas, M.L.; Rivas-Rodríguez, L.; González-Medina, E.C.; Atilano-Carsi, X.; Miranda-Alatriste, P.; Correa-Rotter, R. Bioimpedance vector analysis for body composition in Mexican population. Rev. Invest. Clin. 2007, 59, 15–24. [Google Scholar]
- Birnbaum, J.K.; Duggan, C.; Anderson, B.O.; Etzioni, R. Early detection and treatment strategies for breast cancer in low-income and upper middle-income countries: A modelling study. Lancet Glob. Health 2018, 6, e885–e893. [Google Scholar] [CrossRef]
- Scafoglieri, A.; Clarys, J.P.; Bauer, J.M.; Verlaan, S.; Van Malderen, L.; Vantieghem, S.; Cederholm, T.; Sieber, C.C.; Mets, T.; Bautmans, I.; et al. Predicting appendicular lean and fat mass with bioelectrical impedance analysis in older adults with physical function decline—The PROVIDE study. Clin. Nutr. 2017, 36, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, A.; Pillon, L.; Dumler, F. Impedance vector distribution by sex, race, body mass index, and age in the United States: Standard reference intervals as bivariate Z scores. Nutrition 2002, 18, 153–167. [Google Scholar] [CrossRef]
- Monroy-Cisneros, K.; Astiazarán-García, H.; Esparza-Romero, J.; Guevara-Torres, A.G.; Valencia-Juillerat, M.E.; Méndez-Estrada, R.O.; Tortoledo-Ortiz, O.; Pacheco-Moreno, B.I. Antineoplastic treatment impact on nutritional status in patients with breast cancer. Nutr. Hosp. 2014, 30, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Limon-Miro, A.T.; Lopez-Teros, V.; Astiazaran-Garcia, H. Dynamic Macronutrient Meal-Equivalent Menu Method: Towards Individual Nutrition Intervention Programs. Methods Protoc. 2019, 2, 78. [Google Scholar] [CrossRef] [PubMed]
- Valencia, M.E. Chapter 5: Energía. In Recomendaciones de Ingestión de Nutrimentos para la Población Mexicana: Bases Fisiológicas, 1st ed.; Bourges, H., Casanueva, E., Rosado, J.L., Eds.; Editorial Médica Panamericana: Mexico City, Mexico, 2009; Volume 1, pp. 57–63. [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Continuous Update Project Expert Report 2018. Diet, Nutrition, Physical Activity, and Breast Cancer. Available online: dietandcancerreport.org (accessed on 21 January 2019).
- Fu, M.R.; Cleland, C.M.; Guth, A.A.; Kayal, M.; Haber, J.; Cartwright, F.; Kleinman, R.; Kang, Y.; Scagliola, J.; Axelrod, D. L-dex ratio in detecting breast cancer-related lymphedema: Reliability, sensitivity, and specificity. Lymphology 2013, 46, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Nwosu, A.C.; Mayland, C.R.; Mason, S.; Cox, T.F.; Varro, A.; Stanley, S.; Ellershaw, J. Bioelectrical impedance vector analysis (BIVA) as a method to compare body composition differences according to cancer stage and type. Clin. Nutr. ESPEN 2019, 30, 59–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Małecka-Massalska, T.; Chara, K.; Smolen, A.; Kurylcio, A.; Polkowski, W.; Lupa-Zatwarnicka, K. Bioimpedance vector pattern in women with breast cancer detected by bioelectric impedance vector analysis. Preliminary observations. Ann. Agric. Environ. Med. 2012, 19, 697–700. [Google Scholar] [PubMed]
- Gupta, D.; Lammersfeld, C.A.; Vashi, P.G.; King, J.; Dahlk, S.L.; Grutsch, J.F.; Lis, C.G. Bioelectrical impedance phase angle as a prognostic indicator in breast cancer. BMC Cancer 2008, 8, 249. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lis, C.G.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Lammersfeld, C.A. Bioelectrical impedance phase angle as a prognostic indicator in advanced pancreatic cancer. Br. J. Nutr. 2004, 92, 957–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, D.; Lammersfeld, C.A.; Burrows, J.L.; Dahlk, S.L.; Vashi, P.G.; Grutsch, J.F.; Hoffman, S.; Lis, C.G. Bioelectrical impedance phase angle in clinical practice: Implications for prognosis in advanced colorectal cancer. Am. J. Clin. Nutr. 2004, 80, 1634–1638. [Google Scholar] [CrossRef] [PubMed]


| n = 9 | |
|---|---|
| Surgery | |
| Quadrantectomy | 4 |
| Mastectomy | 5 |
| Breast cancer stage | |
| I | 2 |
| IIA | 4 |
| IIB | 3 |
| Histological subtype | |
| Invasive ductal carcinoma | 7 |
| Invasive lobular carcinoma | 2 |
| Molecular subtypes | |
| Luminal A | 3 |
| Luminal B | 2 |
| HER2 | 1 |
| Triple Negative | 3 |
| Chemotherapy cycles | |
| 0–6 | 3 |
| 7–8 | 6 |
| Radiotherapy | |
| 5000 cGy in 25 fractions | 7 |
| None | 2 |
| Baseline | Postintervention | Δ 1 | p2 | |
|---|---|---|---|---|
| Median (IQR) | ||||
| Height, m | 1.6 (0.1–0.2) | 1.6 (0.1–0.2) | 0 | 0.95 |
| Body weight, kg | 79.2 (10–27) | 73.4 (13–22) | −5.8 | <0.05 |
| BMI 3, kg/m2 | 30.7 (7–11) | 29.5 (7–9) | −1.2 | <0.05 |
| Fat mass, kg | 33 (9–20) | 29.2 (10–17) | −3.8 | <0.05 |
| Fat-free mass, kg | 43 (5–8) | 41.6 (7–9) | −1.4 | <0.05 |
| TASM 4, kg | 15.5 (2–4) | 15.3 (3–6) | −0.2 | 0.4 |
| Resistance 50 kHz, ohm | 639 (79–128) | 639 (90–167) | 0 | 0.01 |
| Reactance 50 kHz, ohm | 60 (35–103) | 87 (45–117) | +27 | 0.2 |
| Phase angle | 5.5 (3–10) | 7.6 (4–10) | +2.1 | 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Limon-Miro, A.T.; Valencia, M.E.; Lopez-Teros, V.; Guzman-Leon, A.E.; Mendivil-Alvarado, H.; Astiazaran-Garcia, H. Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice. Medicina 2019, 55, 663. https://doi.org/10.3390/medicina55100663
Limon-Miro AT, Valencia ME, Lopez-Teros V, Guzman-Leon AE, Mendivil-Alvarado H, Astiazaran-Garcia H. Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice. Medicina. 2019; 55(10):663. https://doi.org/10.3390/medicina55100663
Chicago/Turabian StyleLimon-Miro, Ana Teresa, Mauro E. Valencia, Veronica Lopez-Teros, Alan Eduardo Guzman-Leon, Herminia Mendivil-Alvarado, and Humberto Astiazaran-Garcia. 2019. "Bioelectric Impedance Vector Analysis (BIVA) in Breast Cancer Patients: A Tool for Research and Clinical Practice" Medicina 55, no. 10: 663. https://doi.org/10.3390/medicina55100663







