Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib
Abstract
1. Introduction
2. Clinical Predictive/Prognostic Markers
2.1. Barcelona Clinic Liver Cancer Staging and Child-Pugh Cirrhosis Classifications
2.2. Viral Status
2.3. Diabetes and Use of Oral Antidiabetics
2.4. Adverse Events Due to Sorafenib
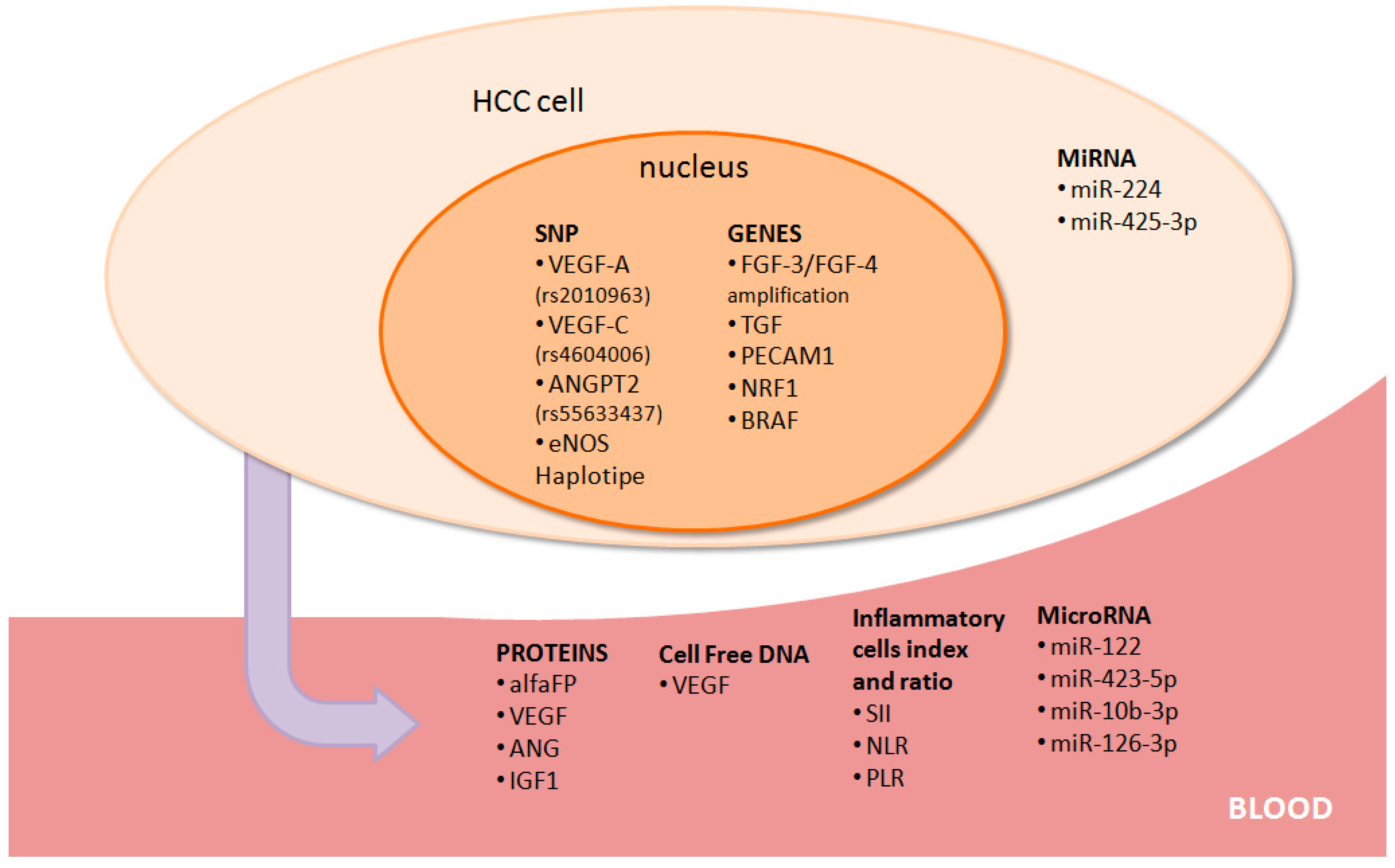
3. Biological Predictive Markers
3.1. Alpha-Fetoprotein
3.2. Angiogenetic Markers
3.3. Inflammatory Cells, Proteins, and Index
3.4. Growth Factors and Other Targets
3.5. MiRNAs
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations/Nomenclature
Appendix A
| Clinical/Biological Biomarker | Study Model | Mechanisms/Results | Reference |
|---|---|---|---|
| Clinical Biomarkers | |||
| Child-Pugh | Observational registry study | CP-A patients mOS > CP-A patients mOS (13.6 months and 5.2 months, respectively) | [11] |
| BCLC stadiation | Pooled analysis and observational trials | BCLC B achieved a better response compared to BCLC C (mOS of 14.5 months and 9.7 months, respectively) (mOS: 20.6 months and 8.4 months, p < 0.0001, respectively). BCLC B had a better survival than those with BCLC C HCC patients (HR = 1.59; p = 0.02) | [2,12,13] |
| Viral status | Pooled analysis | Non-HCV related HCC had a worse OS (HR = 0.7, p = 0.02), while HBV infection did not achieve a significant difference in patients treated with sorafenib (HR = 1.128, p = 0.4538) | [13] |
| Diabetes | Retrospective study | Metformin reduced sorafenib activity in HCC patients with type II diabetes mellitus with mPFS of 2.6 months and 5.0 months and mOS of 10.4 months and 15.1 months for patients chronically treated with or without metformin, respectively. | [18] |
| Adverse events due to sorafenib. | Observational study | mOS of HCC patients with any grade of toxicities related to sorafenib (HFSR, hypertension, diarrhea) was significantly improved compared to patients without adverse events (8.8 months vs. 5.4 months, respectively, IQR 2.7–8.8, log-rank p = 0.004) | [20] |
| HFSR—sorafenib related | Observational study | Early HFSR displayed better OS compared to patients who did not show this adverse event (18.2 months vs. 10.1 months, respectively, p = 0.009) | [21] |
| HFSR—sorafenib related | Metanalysis of 12 cohort studies | Early HFSR displayed better OS compared to patients who did not show this adverse event (pooled HR for mOS of 0.45,95% CI 0.36, 0.55, p < 0.00001, I2 = 35%) and TTP of 0.41 (95% CI 0.28, 0.60, p < 0.00001; I2 = 0%) | [22] |
| Hypertension—sorafenib related | Observational study | Patients who developed this side effect 15 days after beginning sorafenib compared to others who had better mPFS (6.0 months vs. 2.5 months, p < 0.001) and mOS (14.6 months vs. 3.9 months, p = 0.003). | [23] |
| Diarrhea—sorafenib related | Observational study | Significant correlation between the grade of this symptom and mOS (grade 2–3 vs. 0–1: 11.8 months vs.4.2 months—95% CI 6.9–16.6 vs. 95% CI 0.0–9.1, respectively, p = 0.009) | [25] |
| Biological Biomarkers | |||
| Alpha-fetoprotein | Retrospective analysis | Early AFP responding patients with a reduction of more than 20% from baseline of serum levels after two to four weeks of treatment. Responders were compared with non-responders with a significantly improved ORR (33% vs. 8%, p = 0.037) and disease control rate (DCR) (83% vs. 35%, p = 0.002), respectively. | [29] |
| Alpha-fetoprotein | Pooled analysis | APF is a positive predictive marker of response to sorafenib in a multivariate analysis (p = 0.002), with mOSs of 18 months and 10 months (p = 0.004) for responding and non-responding patients, respectively. | [30] |
| VEGF concentrations | Observational analysis | A decrease of plasma VEGF concentrations with sorafenib treatment after eight weeks was a predictor of better mOS than others (30.9 months vs. 14.4 months, p=0.038). | [33] |
| VEGF-A gene amplification | Observational analysis | mOSs were 10 months and not achieved for patients with negative (47 patients) and positive (7 patients) VEGF-A gene amplification, respectively (p = 0.029). | [34] |
| cfDNA concentrations of VEGF | Observational analysis | Patients whose disease progressed with sorafenib had significantly higher cfDNA levels than the others (0.82 ng/μLvs.0.63 ng/μL, p = 0.006) | [35] |
| SNPs of VEGF | Observational analysis | Univariate analysis VEGF-A alleles C of rs25648, T of rs833061, C of rs699947, C of rs2010963, VEGF-C alleles T of rs4604006, G of rs664393, VEGFR-2 alleles C of rs2071559, C of rs2305948 were significant predictive factors of PFS and OS in sorafenib-treated HCC. In amultivariate analysis, VEGF-A rs2010963 and VEGF-C rs4604006 were independent factors influencing PFS (HR = 0.25, 95% CI: 0.19–1.02, p = 0.0376 and HR = 0.22, 95% CI: 0.14–0.81, p = 0.004, respectively) and OS (HR = 0.28, 95% CI: 0.23–0.96, p = 0.02 and HR = 0.25, 95% CI: 0.17–0.99, p = 0.04, respectively). | [36] |
| Ang-2 | Pooled analysis | Negative predictive outcome in HCC patients with high Ang-2 serum levels before sorafenib (HR = 2.51, 95% CI: 1.01–6.57, p = 0.048) | [37] |
| SNP for ANGPT2 | Observational analysis | rs55633437 GG genotype showed a significantly longer PFS (p < 0.001) and OS (p < 0.001) than those with the other genotypes (GT+TT). | [38] |
| eNOS polymorphisms | Observational analysis | In univariate and multivariate analyses, a training cohort of HCC patients homozygous for endothelial nitric oxide synthase (eNOS) haplotype (HT1:T-4b at eNOS-786/eNOS VNTR) had a worse mPFS (2.6 months vs. 5.8 months, HR = 5.43, 95% CI: 2.46–11.98, p < 0.0001) and OS (3.2 months vs. 14.6 months, HR = 2.35, 95% CI: 1.12–4.91, p = 0.024) when compared with other haplotypes. | [39] |
| HIF-1α/SNPs of HIF-1α, VEGF, and Ang2 | Observational analysis | The multivariate analysis demonstrated that rs12434438 (SNP of HIF-1α), rs2010963 (SNP of VEGF-A), and rs4604006 (SNP of VEGF-C) were independent factors and were predictive biomarkers of the sorafenib response. | [40] |
| NRL | Observational analysis | NRL ≥ 2.3 was a negative predictive biomarker of the sorafenib response in both univariate and multivariate environments (p = 0.005 and HR 1.72, 95% CI: 1.03–2.71, respectively) | [46] |
| NLR | Meta-analysis | High NLR before any treatment was predictive of a short mOS (HR: 1.54, 95% CI: 1.34 to 1.76, p < 0.001 | [48] |
| PLR | Meta-analysis | Increase of PLR predicted an unfavorable outcome in terms of mOS (HR: 1.63, 95% CI: 1.34 to 1.98, p < 0.001). | [48] |
| SII, NLR, and PLR | Multicenter case series | Patients treated with sorafenib and with SII ≥ 360 showed poorer survival outcomes compared to patients with SII < 360 in terms of mPFS (2.6 months vs. 3.9 months, respectively, p < 0.026) and mOS (5.6 months vs. 13.9 months, respectively, p = 0.027). Patients with NLR ≥ 3 compared with those with NLR < 3, had a lower mPFS (2.6 months vs. 3.3 months, p < 0.049) but no significant data were reported in terms of mOS (5.6 months vs. 13.9 months, p = 0.062) | [49] |
| IGF-1 | Observational analysis | Patients with high (i.e., levels ≥ the median level) baseline IGF-1 levels achieved a significantly higher disease control rate (DCR) when treated with anti-angiogenic therapies (including sorafenib) than those with low levels (71% vs. 39%, respectively—p = 0.003). Patients with high IGF-1 levels, when compared with those with low levels showed longer mPFS (4.3 months vs. 1.9 months, respectively—p = 0.014) and mOS (10.7 months vs. 3.9 months, respectively—p = 0.009). | [53] |
| FGF3/FGF4 amplification | Observational analysis | FGF3/FGF4 amplification was observed in 30% of HCC samples while it was not seen in 38 non-responsive patients (p = 0.006). | [54] |
| TGFa/PECAM1 and NRG1 gene | Observational analysis | TGFa and PECAM1 gene expression levels were significantly increased in non-PD patients. Moreover, mPFS of patients with high and low NRG1 expressions were 80 days and 90 days in sorafenib respondingpatients, respectively (p = 0.0497). So far, high TGFa and PECAM1 and low NRF1 gene levels could be predictors of response to sorefenib. | [55] |
| miRNA181a-5p | Observational analysis | The miRNA181a-5p levels resulted in the unique independent factor for sorefenib-treated patients achieving a DCR in 53 patients (HR 0.139, 95% CI 0.011–0.658, p = 0.0092) | [65] |
| miRNA423-5p | In vivo/in vitro study | Sorafenib upregulated both in vitro and in vivo and its increase from baseline to evaluation at 6 months correlated with the response. In fact, 75% of patients with the miR423-5p level increase achieved a disease control. | [66] |
| miRNA-126-3p | In vivo/in vitro study | MiR-126-3p was down-regulated after sorafenib treatment in HCC celllines. Circulating miR-126-3p expression levels were significantly higher in HCC patients when compared withcontrol subjects (26.7 vs. 26.6 mean expression levels; p-value=0.0002). | [67] |
| miRNA10b-3p | Exploratory study | MiRNA10b-3p expression levels were significantly higher (fold increase = 5.8) in the subgroup of HCC patients with worse OS (p = 0.008) and with a putative prediction of short survival of sorafenib-treated patients | [68] |
| miRNA-224 | Exploratory study | High levels of HCC samples were correlated with an increase of PFS (HR = 0.28, 95% CI: 0.09–0.92, p = 0.029) and OS (HR = 0.0.24, 95% CI: 0.07–0.79, p = 0.012) in patients treated with sorafenib [69]. | [69] |
| miR-425-3p | Exploratory study | Patients with high levels of miR-425-3p in HCC tissue treated with sorafenib achieved a better PFS (HR = 0.5, 95% CI: 0.3–0.9, p = 0.007) and TTP (HR = 0.4, 95% CI: 0.2–0.7, p = 0.0008) | [70] |
References
- Chen, C.; Wang, G. Mechanisms of hepatocellular carcinoma and challenges and opportunities for molecular targeted therapy. World J. Hepatol. 2015, 7, 1964–1970. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.L.; Kang, Y.K.; Chen, Z.; Tsao, C.J.; Qin, S.; Kim, J.S.; Luo, R.; Feng, J.; Ye, S.; Yang, T.S.; et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009, 10, 25–34. [Google Scholar] [CrossRef]
- Faivre, S.; Raymond, E.; Boucher, E.; Douillard, J.; Lim, H.Y.; Kim, J.S.; Zappa, M.; Lanzalone, S.; Lin, X.; Deprimo, S.; et al. Safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma: Anopen-label, multicentre, phase II study. Lancet Oncol. 2009, 10, 794–800. [Google Scholar] [CrossRef]
- Cainap, C.; Qin, S.; Huang, W.T.; Chung, I.J.; Pan, H.; Cheng, Y.; Kudo, M.; Kang, Y.K.; Chen, P.J.; Toh, H.C.; et al. Linifanib versus Sorafenib in patients with advanced hepatocellular carcinoma: Results of a randomized phase III trial. J. Clin. Oncol. 2015, 33, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P.J.; Qin, S.; Park, J.W.; Poon, R.T.; Raoul, J.L.; Philip, P.A.; Hsu, C.H.; Hu, T.H.; Heo, J.; Xu, J.; et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: Results from the randomized phase III BRISK-FL study. J. Clin. Oncol. 2013, 31, 3517–3524. [Google Scholar] [CrossRef]
- Llovet, J.M.; Hernandez-Gea, V. Hepatocellular carcinoma: Reasons for phase III failure and novel perspectives on trial design. Clin. Cancer Res. 2014, 20, 2072–2079. [Google Scholar] [CrossRef]
- Kudo, M.; Finn, R.S.; Qin, S.; Han, K.H.; Ikeda, K.; Piscaglia, F.; Baron, A.; Park, J.W.; Han, G.; Jassem, J.; et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: A randomised phase 3 non-inferiority trial. Lancet 2018, 391, 1163–1173. [Google Scholar] [CrossRef]
- Hollebecque, A.; Cattan, S.; Romano, O.; Sergent, G.; Mourad, A.; Louvet, A.; Dharancy, S.; Boleslawski, E.; Truant, S.; Pruvot, F.R.; et al. Safety and efficacy of sorafenib in hepatocellular carcinoma: The impact of the Child-Pugh score. Aliment. Pharmacol. Ther. 2011, 34, 1193–1201. [Google Scholar] [CrossRef]
- Pressiani, T.; Boni, C.; Rimassa, L.; Labianca, R.; Fagiuoli, S.; Salvagni, S.; Ferrari, D.; Cortesi, E.; Porta, C.; Mucciarini, C.; et al. Sorafenib in patients with Child-Pugh class A and B advanced hepatocellular carcinoma: A prospective feasibility analysis. Ann. Oncol. 2013, 24, 406–411. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kudo, M.; Venook, A.P.; Ye, S.L.; Bronowicki, J.P.; Chen, X.P.; Dagher, L.; Furuse, J.; Geschwind, J.H.; de Guevara, L.L.; et al. Observational registry of sorafenib use in clinical practice across Child-Pugh subgroups: The GIDEON study. J. Hepatol. 2016, 65, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Iavarone, M.; Cabibbo, G.; Piscaglia, F.; Zavaglia, C.; Grieco, A.; Villa, E.; Cammà, C.; Colombo, M.; SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology 2011, 54, 2055–2063. [Google Scholar] [CrossRef] [PubMed]
- Bruix, J.; Cheng, A.L.; Meinhardt, G.; Nakajima, K.; De Sanctis, Y.; Llovet, J. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: Analysis of two phase III studies. J. Hepatol. 2017, 67, 999–1008. [Google Scholar] [CrossRef]
- Park, J.; Cho, J.; Lim, J.H.; Lee, M.H.; Kim, J. Relative Efficacy of Systemic Treatments for Patients with Advanced Hepatocellular Carcinoma According to Viral Status: A Systematic Review and Network Meta-Analysis. Target. Oncol. 2019, 14, 395–403. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Hampel, H.; Javadi, F. The association between diabetes and hepatocellular carcinoma: A systematic review of epidemiologic evidence. Clin. Gastroenterol. Hepatol. 2006, 4, 369–380. [Google Scholar] [CrossRef]
- Gandini, S.; Puntoni, M.; Heckman-Stoddard, B.M.; Dunn, B.K.; Ford, L.; De Censi, A.; Szabo, E. Metformin and cancer risk and mortality: A systematic review and meta-analysis taking into account biases and confounders. Cancer Prev. Res. 2014, 7, 867–885. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Kim, M.S.; Lim, J.S.; Yoo, H.J.; Seo, Y.S.; Han, C.J.; Park, S.C.; Kay, C.S.; Kim, M.; Jang, H.S.; et al. Survival Advantage Associated with Metformin Usage in Hepatocellular Carcinoma Patients Receiving Radiotherapy: A Propensity Score Matching Analysis. Anticancer Res. 2015, 35, 5047–5054. [Google Scholar]
- Casadei-Gardini, A.; Marisi, G.; Scarpi, E.; Scartozzi, M.; Faloppi, L.; Silvestris, N.; Masi, G.; Vivaldi, C.; Brunetti, O.; Tamberi, S.; et al. Effects of metformin on clinical outcome in diabetic patients with advanced HCC receiving sorafenib. Expert Opin. Pharmacother. 2015, 16, 2719–2725. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Faloppi, L.; De Matteis, S.; Foschi, F.G.; Silvestris, N.; Tovoli, F.; Palmieri, V.; Marisi, G.; Brunetti, O.; Vespasiani-Gentilucci, U.; et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur. J. Cancer 2017, 86, 106–114. [Google Scholar] [CrossRef]
- Howell, J.; Pinato, D.J.; Ramaswami, R.; Bettinger, D.; Arizumi, T.; Ferrari, C.; Yen, C.; Gibbin, A.; Burlone, M.E.; Guaschino, G.; et al. On-target sorafenib toxicity predicts improved survival in hepatocellular carcinoma: A multi-centre, prospective study. Aliment. Pharmacol. Ther. 2017, 45, 1146–1155. [Google Scholar] [CrossRef]
- Reig, M.; Torres, F.; Rodriguez-Lope, C.; Forner, A.; LLarch, N.; Rimola, J.; Darnell, A.; Ríos, J.; Ayuso, C.; Bruix, J. Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J. Hepatol. 2014, 61, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Tan, G.; Zhu, M.; Li, W.; Zhai, B.; Sun, X. Hand-foot skin reaction is a beneficial indicator of sorafenib therapy for patients with hepatocellular carcinoma: A systemic review and metaanalysis. Expert Rev. Gastroenterol. Hepatol. 2018, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Lee, Y.J. Correlation of skin toxicity and hypertension with clinical benefit in advanced hepatocellular carcinoma patients treated with sorafenib. Int. J. Clin. Pharmacol. Ther. 2013, 51, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, T.; Eguchi, Y.; Kawazoe, S.; Yanagita, K.; Ario, K.; Kitahara, K.; Kawasoe, H.; Kato, H.; Mizuta, T.; Saga Liver Cancer Study Group; et al. Skin toxicities and survival in advanced hepatocellular carcinoma patients treated with sorafenib. Hepatol. Res. 2012, 42, 879–886. [Google Scholar] [CrossRef] [PubMed]
- Koschny, R.; Gotthardt, D.; Koehler, C.; Jaeger, D.; Stremmel, W.; Ganten, T.M. Diarrhea is a positive outcome predictor for sorafenib treatment of advanced hepatocellular carcinoma. Oncology 2013, 84, 6–13. [Google Scholar] [CrossRef]
- Di Costanzo, G.G.; CasadeiGardini, A.; Marisi, G.; Foschi, F.G.; Scartozzi, M.; Granata, R.; Faloppi, L.; Cascinu, S.; Silvestris, N.; Brunetti, O.; et al. Validation of a Simple Scoring System to Predict Sorafenib Effectiveness in Patients with Hepatocellular Carcinoma. Target. Oncol. 2017, 12, 795–803. [Google Scholar] [CrossRef]
- Silvestris, N.; Ciliberto, G.; De Paoli, P.; Apolone, G.; Lavitrano, M.L.; Pierotti, M.A.; Stanta, G.; On the behalf of the “dynamic medicine OECI group”. On the behalf of the “dynamic medicine OECI group”. Liquid dynamic medicine and N-of-1 clinical trials: A change of perspective in oncology research. J. Exp. Clin. Cancer Res. 2017, 36, 128. [Google Scholar] [CrossRef]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Lin, Z.Z.; Hsu, C.; Shen, Y.C.; Hsu, C.H.; Cheng, A.L. Early alpha-fetoprotein response predicts treatment efficacy of antiangiogenic systemic therapy in patients with advanced hepatocellular carcinoma. Cancer 2010, 116, 4590–4596. [Google Scholar] [CrossRef]
- Sánchez, A.I.P.; Roces, L.V.; García, I.Z.; López, E.L.; Hernandez, M.A.C.; Parejo, M.I.B.; Peña-Díaz, J. Value of α-fetoprotein as an early biomarker for treatment response to sorafenib therapy in advanced hepatocellular carcinoma. Oncol. Lett. 2018, 15, 8863–8870. [Google Scholar]
- Nakazawa, T.; Hidaka, H.; Takada, J.; Okuwaki, Y.; Tanaka, Y.; Watanabe, M.; Shibuya, A.; Minamino, T.; Kokubu, S.; Koizumi, W. Early increase in α-fetoprotein for predicting unfavorable clinical outcomes in patients with advanced hepatocellular carcinoma treated with sorafenib. Eur. J. Gastroenterol Hepatol. 2013, 25, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Brunetti, O.; Gnoni, A.; Cascinu, S.; Gasparini, G.; Lorusso, V.; Ribatti, D.; Silvestris, N. Angiogenesis in pancreatic ductal adenocarcinoma: A controversial issue. Oncotarget 2016, 7, 58649–58658. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, K.; Asahina, Y.; Matsuda, S.; Muraoka, M.; Nakata, T.; Suzuki, Y.; Tamaki, N.; Yasui, Y.; Suzuki, S.; Hosokawa, T.; et al. Changes in plasma vascular endothelial growth factor at 8 weeks after sorafenib administration as predictors of survival for advanced hepatocellular carcinoma. Cancer 2014, 120, 229–237. [Google Scholar] [CrossRef]
- Horwitz, E.; Stein, I.; Andreozzi, M.; Nemeth, J.; Shoham, A.; Pappo, O.; Schweitzer, N.; Tornillo, L.; Kanarek, N.; Quagliata, L.; et al. Human and mouse VEGFA-amplified hepatocellular carcinomas are highly sensitive to sorafenib treatment. Cancer Discov. 2014, 4, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Oh, C.R.; Kong, S.Y.; Im, H.S.; Kim, H.J.; Kim, M.K.; Yoon, K.A.; Cho, E.H.; Jang, J.H.; Lee, J.; Kang, J.; et al. Genome-wide copy number alteration and VEGFA amplification of circulating cell-free DNA as a biomarker in advanced hepatocellular carcinoma patients treated with Sorafenib. BMC Cancer 2019, 19, 292. [Google Scholar] [CrossRef]
- Scartozzi, M.; Faloppi, L.; Svegliati Baroni, G.; Loretelli, C.; Piscaglia, F.; Iavarone, M.; Toniutto, P.; Fava, G.; De Minicis, S.; Mandolesi, A.; et al. VEGF and VEGFR genotyping in the prediction of clinical outcome for HCC patients receiving sorafenib: The ALICE-1 study. Int. J. Cancer 2014, 135, 1247–1256. [Google Scholar] [CrossRef]
- Miyahara, K.; Nouso, K.; Tomoda, T.; Kobayashi, S.; Hagihara, H.; Kuwaki, K.; Toshimori, J.; Onishi, H.; Ikeda, F.; Miyake, Y.; et al. Predicting the treatment effect of sorafenib using serum angiogenesis markers in patients with hepatocellular carcinoma. J. Gastroenterol. Hepatol. 2011, 26, 1604–1611. [Google Scholar] [CrossRef]
- Marisi, G.; Petracci, E.; Raimondi, F.; Faloppi, L.; Foschi, F.G.; Lauletta, G.; Iavarone, M.; Canale, M.; Valgiusti, M.; Neri, L.M.; et al. ANGPT2 and NOS3 Polymorphisms and Clinical Outcome in Advanced Hepatocellular Carcinoma Patients Receiving Sorafenib. Cancers 2019, 11, 1023. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Marisi, G.; Faloppi, L.; Scarpi, E.; Foschi, F.G.; Iavarone, M.; Lauletta, G.; Corbelli, J.; Valgiusti, M.; Facchetti, F.; et al. eNOS polymorphisms andclinical outcome in advanced HCC patients receiving sorafenib: Final results of the ePHAS study. Oncotarget 2016, 7, 27988–27999. [Google Scholar]
- Faloppi, L.; Casadei Gardini, A.; Masi, G.; Silvestris, N.; Loretelli, C.; Ulivi, P.; Bianconi, M.; Gianpieri, R.; Bittoni, A.; Andrikou, K.; et al. Angiogenesis polymorphisms profile in the prediction of clinical outcome of advanced HCC patients receiving sorafenib: Combined analysis of VEGF and HIF-1α. Final results of the ALICE-2 study. J. Clin. Oncol. 2016, 34 (Suppl. 4), 280. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Faloppi, L.; Aprile, G.; Brunetti, O.; Caparello, C.; Corbelli, J.; Chessa, L.; Bruno, D.; Ercolani, G.; Leonetti, A.; et al. Multicenter Prospective Study of Angiogenesis Polymorphism Validation in HCC Patients Treated with Sorafenib. An INNOVATE Study Protocol. Tumori. J. 2017, 104, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Gnoni, A.; Casadei-Gardini, A.; Pisconti, S.; Licchetta, A.; Scartozzi, M.; Memeo, R.; Palmieri, V.; Aprile, G.; Santini, D.; et al. Immunotherapeutic approaches for hepatocellular carcinoma. Oncotarget 2017, 8, 33897–33910. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.; Garattini, S.K.; Bonotto, M.; Ongaro, E.; Casagrande, M.; Cattaneo, M.; Fanotto, V.; De Carlo, E.; Loupakis, F.; Urbano, F.; et al. Immunotherapy for colorectal cancer: Where are we heading? Expert Opin. Biol. Ther. 2017, 17, 709–721. [Google Scholar] [CrossRef] [PubMed]
- FarajzadehValilou, S.; Keshavarz-Fathi, M.; Silvestris, N.; Argentiero, A.; Rezaei, N. The role of inflammatory cytokines and tumor associated macrophages (TAMs) in microenvironment of pancreatic cancer. Cytokine Growth Factor Rev. 2018, 39, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Yang, X.R.; Xu, Y.; Sun, Y.F.; Sun, C.; Guo, W.; Zhang, X.; Wang, W.M.; Qiu, S.J.; Zhou, J.; et al. Systemic immune-inflammationindex predicts prognosis of patients after curative resection for hepatocellular carcinoma. Clin. Cancer Res. 2014, 20, 6212–6222. [Google Scholar] [CrossRef]
- Lué, A.; Serrano, M.T.; Bustamante, F.J.; Iñarrairaegui, M.; Arenas, J.I.; Testillano, M.; Lorente, S.; Gil, C.; de la Torre, M.; Gomez, A.; et al. Neutrophil-to-lymphocyte ratio predicts survival in European patients with hepatocellular carcinoma administered sorafenib. Oncotarget 2017, 8, 103077–103086. [Google Scholar] [CrossRef]
- Hong, Y.M.; Yoon, K.T.; Hwang, T.H.; Heo, J.; Woo, H.Y.; Cho, M. Changes in the neutrophil-to-lymphocyte ratio predict the prognosis of patients with advanced hepatocellular carcinoma treated with sorafenib. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1250–1255. [Google Scholar] [CrossRef]
- Zheng, J.; Cai, J.; Li, H.; Zeng, K.; He, L.; Fu, H.; Zhang, J.; Chen, L.; Yao, J.; Zhang, Y.; et al. Neutrophil to Lymphocyte Ratio and Platelet to Lymphocyte Ratio as Prognostic Predictors for Hepatocellular Carcinoma Patients with Various Treatments: A Meta-Analysis and Systematic Review. Cell Physiol. Biochem. 2017, 44, 967–981. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Scarpi, E.; Faloppi, L.; Scartozzi, M.; Silvestris, N.; Santini, D.; de Stefano, G.; Marisi, G.; Negri, F.V.; Foschi, F.G.; et al. Immune inflammation indicators and implication for immune modulation strategies in advanced hepatocellular carcinoma patients receiving sorafenib. Oncotarget 2016, 7, 67142–67149. [Google Scholar]
- Xu, D.; Xu, M.; Jeong, S.; Qian, Y.; Wu, H.; Xia, Q.; Kong, X. The Role of Nrf2 in liver disease: Novel molecular mechanisms and therapeutic approaches. Front. Pharmacol. 2019, 9, 1428. [Google Scholar] [CrossRef]
- Raghunath, A.; Sundarraj, K.; Arfuso, F.; Sethi, G.; Perumal, E. Dysregulation of Nrf2 in hepatocellular carcinoma: Role in cancer progression and chemoresistance. Cancers 2018, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Ye, W.; Duan, X.; Zhang, M.; Wang, J. The noncytotoxic dose of sorafenib sensitizes Bel-7402/5-FU cells to 5-FU by down-regulating 5-FU-induced Nrf2 expression. Dig. Dis. Sci. 2013, 58, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.Y.; Huang, C.C.; Lin, S.D.; Hsu, C.H.; Cheng, A.L. Serum insulinlike growth factor-1 levels predict outcomes of patients with advanced hepatocellular carcinoma receiving antiangiogenic therapy. Clin. Cancer Res. 2012, 18, 3992–3997. [Google Scholar] [CrossRef] [PubMed]
- Arao, T.; Ueshima, K.; Matsumoto, K.; Nagai, T.; Kimura, H.; Hagiwara, S.; Sakurai, T.; Haji, S.; Kanazawa, A.; Hidaka, H.; et al. FGF3/FGF4 amplification and multiple lung metastases in responders to sorafenib in hepatocellular carcinoma. Hepatology 2013, 57, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Sakai, K.; Takeda, H.; Nishijima, N.; Orito, E.; Joko, K.; Uchida, Y.; Izumi, N.; Nishio, K.; Osaki, Y. Targeted DNA and RNA sequencing of fine-needle biopsy FFPE specimens in patients with unresectable hepatocellular carcinoma treated with sorafenib. Oncotarget 2015, 6, 21636–21644. [Google Scholar] [CrossRef] [PubMed]
- Santini, D.; Spoto, C.; Loupakis, F.; Vincenzi, B.; Silvestris, N.; Cremolini, C.; Canestrari, E.; Graziano, F.; Galluccio, N.; Salvatore, L.; et al. High concordance of BRAF status between primary colorectal tumours and related metastatic sites: Implications for clinical practice. Ann. Oncol. 2010, 21, 1565. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.; Rosania, R.; Franke, S.; Haybaeck, J.; Canbay, A.; Venerito, M. The BRAF Status May Predict Response to Sorafenib in Gastrointestinal Stromal Tumors Resistant to Imatinib, Sunitinib, and Regorafenib: Case Series and Review of the Literature. Digestion 2019, 99, 179–184. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Chiadini, E.; Faloppi, L.; Marisi, G.; Delmonte, A.; Scartozzi, M.; Loretelli, C.; Lucchesi, A.; Oboldi, D.; Dubini, A.; et al. Efficacy of sorafenib in BRAF-mutated non-small-cell lung cancer (NSCLC) and no response in synchronous BRAF wild type-hepatocellular carcinoma: A case report. BMC Cancer 2016, 16, 429. [Google Scholar] [CrossRef]
- Nedaeinia, R.; Manian, M.; Jazayeri, M.H.; Ranjbar, M.; Salehi, R.; Sharifi, M.; Mohaghegh, F.; Goli, M.; Jahednia, S.H.; Avan, A.; et al. Circulating exosomes and exosomal microRNAs as biomarkers in gastrointestinal cancer. Cancer Gene Ther. 2017, 24, 48–56. [Google Scholar] [CrossRef]
- Brunetti, O.; Russo, A.; Scarpa, A.; Santini, D.; Reni, M.; Bittoni, A.; Azzariti, A.; Aprile, G.; Delcuratolo, S.; Signorile, M.; et al. MicroRNA in pancreatic adenocarcinoma: Predictive/prognostic biomarkers or therapeutic targets? Oncotarget 2015, 6, 23323–23341. [Google Scholar] [CrossRef]
- Gnoni, A.; Santini, D.; Scartozzi, M.; Russo, A.; Licchetta, A.; Palmieri, V.; Lupo, L.; Faloppi, L.; Palasciano, G.; Memeo, V.; et al. Hepatocellular carcinoma treatment over sorafenib: Epigenetics, microRNAs and microenvironment. Is there a light at the end of the tunnel? Expert Opin. Ther. Targets 2015, 19, 1623–1635. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Dong, X.; Zhai, B.; Jiang, X.; Dong, D.; Li, B.; Jiang, H.; Xu, S.; Sun, X. MiR-21 mediates sorafenib resistance of hepatocellular carcinoma cells by inhibiting autophagy via the PTEN/Akt pathway. Oncotarget 2015, 6, 28867–28881. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, J.; Ma, L.; Shan, J.; Shen, J.; Yang, Z.; Liu, L.; Luo, Y.; Yao, C.; Qian, C. MicroRNA-122 confers sorafenib resistance to hepatocellular carcinoma cells by targeting IGF-1R to regulate RAS/RAF/ERK signaling pathways. Cancer Lett. 2016, 371, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Turato, C.; Fornari, F.; Pollutri, D.; Fassan, M.; Quarta, S.; Villano, G.; Ruvoletto, M.; Bolondi, L.; Gramantieri, L.; Pontisso, P. MiR-122 Targets SerpinB3 and Is Involved in Sorafenib Resistance in Hepatocellular Carcinoma. J. Clin. Med. 2019, 8, 171. [Google Scholar] [CrossRef] [PubMed]
- Nishida, N.; Arizumi, T.; Hagiwara, S.; Ida, H.; Sakurai, T.; Kudo, M. MicroRNAs for the Prediction of Early Response to Sorafenib Treatment in Human Hepatocellular Carcinoma. Liver Cancer 2017, 6, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Stiuso, P.; Potenza, N.; Lombardi, A.; Ferrandino, I.; Monaco, A.; Zappavigna, S.; Vanacore, D.; Mosca, N.; Castiello, F.; Porto, S.; et al. MicroRNA-423-5p Promotes Autophagy in Cancer Cells and Is Increased in Serum From Hepatocarcinoma Patients Treated with Sorafenib. Mol. Ther. Nucleic Acids 2015, 4, e233. [Google Scholar] [CrossRef] [PubMed]
- Faranda, T.; Grossi, I.; Manganelli, M.; Marchina, E.; Baiocchi, G.; Portolani, N.; Crosatti, M.; De Petro, G.; Salvi, A. Differential expression profiling of long non-coding RNA GAS5 and miR-126-3p in human cancer cells in response to sorafenib. Sci. Rep. 2019, 9, 9118. [Google Scholar] [CrossRef]
- Yoon, E.L.; Yeon, J.E.; Ko, E.; Lee, H.J.; Je, J.H.; Yoo, Y.J.; Kang, S.H.; Suh, S.J.; Kim, J.H.; Seo, Y.S.; et al. An Explorative Analysis for the Role of Serum miR-10b-3p Levels in Predicting Response to Sorafenib in Patients with Advanced Hepatocellular Carcinoma. J. Korean Med. Sci. 2017, 32, 212–220. [Google Scholar] [CrossRef]
- Gyöngyösi, B.; Végh, É.; Járay, B.; Székely, E.; Fassan, M.; Bodoky, G.; Schaff, Z.; Kiss, A. Pretreatment MicroRNA Level and Outcome in Sorafenib-treated Hepatocellular Carcinoma. J. Histochem. Cytochem. 2014, 62, 547–555. [Google Scholar] [CrossRef]
- Vaira, V.; Roncalli, M.; Carnaghi, C.; Faversani, A.; Maggioni, M.; Augello, C.; Rimassa, L.; Pressiani, T.; Spagnuolo, G.; Di Tommaso, L.; et al. MicroRNA-425-3p predicts response to sorafenib therapy in patients with hepatocellular carcinoma. Liver Int. 2015, 35, 1077–1086. [Google Scholar] [CrossRef]
- Kim, H.Y.; Lee, D.H.; Lee, J.H.; Cho, Y.Y.; Cho, E.J.; Yu, S.J.; Kim, Y.J.; Yoon, J.H. Novel biomarker-based model for the prediction of sorafenib response and overall survival in advanced hepatocellular carcinoma: A prospective cohort study. BMC Cancer 2018, 18, 307. [Google Scholar] [CrossRef] [PubMed]
- Gavini, J.; Dommann, N.; Jakob, M.O.; Keogh, A.; Bouchez, L.C.; Karkampouna, S.; Julio, M.K.; Medova, M.; Zimmer, Y.; Schläfli, A.M.; et al. Verteporfin-induced lysosomal compartment dysregulation potentiates the effect of sorafenib in hepatocellular carcinoma. Cell Death Dis. 2019, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Gane, E.; Assenat, E.; Siebler, J.; Galle, P.R.; Merle, P.; Hourmand, I.O.; Cleverly, A.; Zhao, Y.; Gueorguieva, I.; et al. A phase 2 study of Galunisertib (TGF-β1 Receptor Type I Inhibitor) and Sorafenib in patients with advanced hepatocellular carcinoma. Clin. Transl. Gastroenterol. 2019, 7, e00056. [Google Scholar] [CrossRef] [PubMed]
- Abou-Alfa, G.K.; Miksad, R.A.; Tejani, M.A.; Williamson, S.; Gutierrez, M.E.; Olowokure, O.O.; Sharma, M.R.; El Dika, I.; Sherman, M.L.; Pandya, S.S. A phase Ib, open-label study of Dalantercept, an Activin Receptor-Like Kinase 1 Ligand Trap, plus Sorafenib in advanced hepatocellular carcinoma. Oncologist 2019, 24, 161.e70. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brunetti, O.; Gnoni, A.; Licchetta, A.; Longo, V.; Calabrese, A.; Argentiero, A.; Delcuratolo, S.; Solimando, A.G.; Casadei-Gardini, A.; Silvestris, N. Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Medicina 2019, 55, 707. https://doi.org/10.3390/medicina55100707
Brunetti O, Gnoni A, Licchetta A, Longo V, Calabrese A, Argentiero A, Delcuratolo S, Solimando AG, Casadei-Gardini A, Silvestris N. Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Medicina. 2019; 55(10):707. https://doi.org/10.3390/medicina55100707
Chicago/Turabian StyleBrunetti, Oronzo, Antonio Gnoni, Antonella Licchetta, Vito Longo, Angela Calabrese, Antonella Argentiero, Sabina Delcuratolo, Antonio Giovanni Solimando, Andrea Casadei-Gardini, and Nicola Silvestris. 2019. "Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib" Medicina 55, no. 10: 707. https://doi.org/10.3390/medicina55100707
APA StyleBrunetti, O., Gnoni, A., Licchetta, A., Longo, V., Calabrese, A., Argentiero, A., Delcuratolo, S., Solimando, A. G., Casadei-Gardini, A., & Silvestris, N. (2019). Predictive and Prognostic Factors in HCC Patients Treated with Sorafenib. Medicina, 55(10), 707. https://doi.org/10.3390/medicina55100707









