A Population-Based Study of Secondary Prostate Cancer Risk after Radiotherapy in Male Patients with Rectal Cancer: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Data Sources
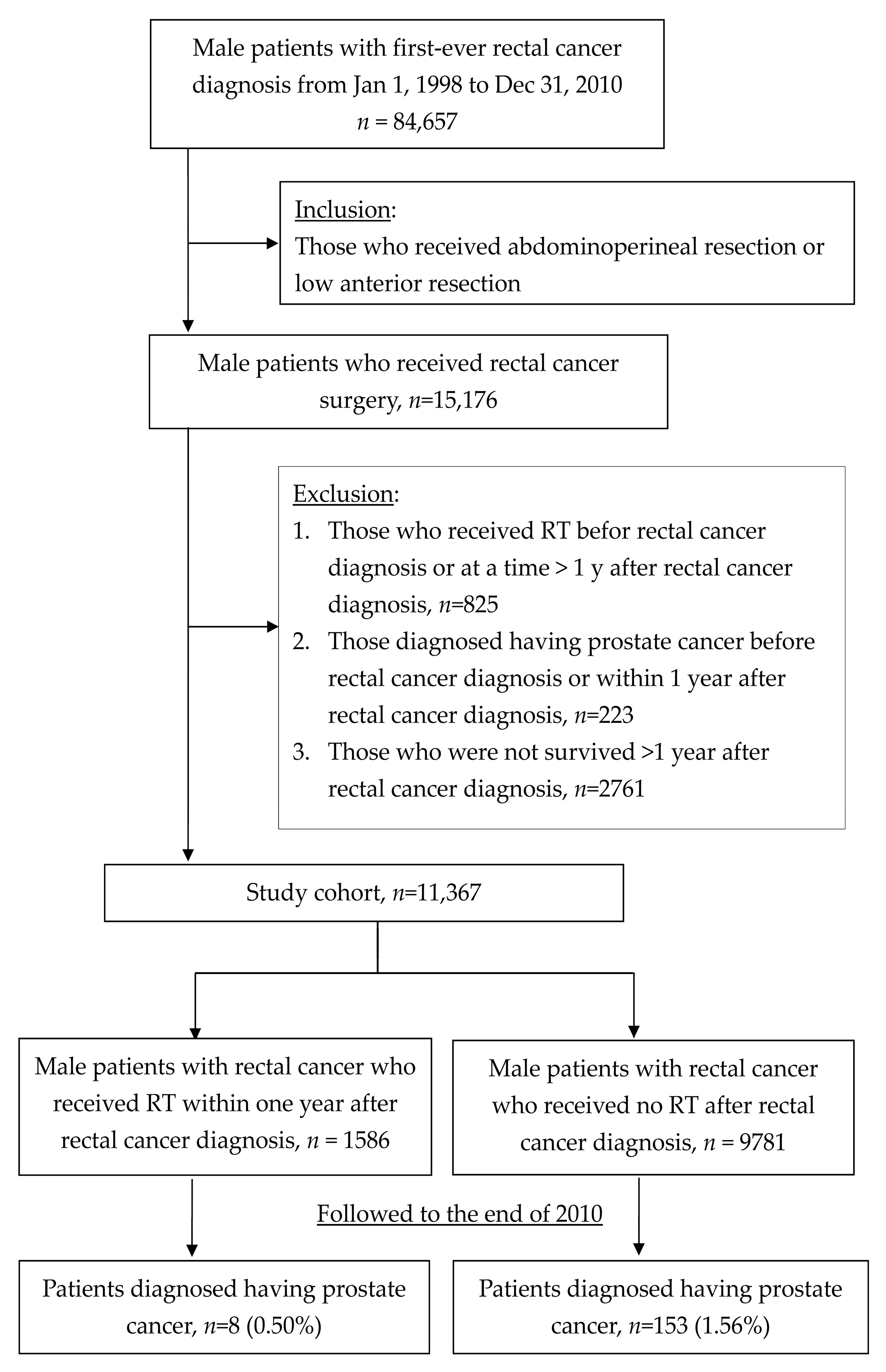
2.2. Study Cohort
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, Y.L.; Chuang, J.P.; Lee, J.C. Differences in survival between colon and rectal cancer from SEER data. PLoS ONE 2013, 8, e78709. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Colorectal cancer mortality rates in adults aged 20 to 54 years in the United States, 1970–2014. Jama 2017, 318, 572–574. [Google Scholar] [CrossRef]
- Meadows, A.T.; Friedman, D.L.; Neglia, J.P.; Mertens, A.C.; Donaldson, S.S.; Stovall, M.; Inskip, P.D. Second neoplasms in survivors of childhood cancer: Findings from the Childhood Cancer Survivor Study cohort. J. Clin. Oncol. 2009, 27, 2356–2362. [Google Scholar] [CrossRef] [PubMed]
- Travis, L.B. Therapy-associated solid tumors. Acta Oncol. 2002, 41, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Colorectal Cancer Collaborative Group. Adjuvant radiotherapy for rectal cancer: A systematic overview of 8507 patients from 22 randomised trials. Lancet 2001, 358, 1291–1304. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hsieh, C.C.; Chuang, J.P. Prognostic significance of partial tumor regression after preoperative chemoradiotherapy for rectal cancer: A meta-analysis. Dis. Colon Rectum 2013, 56, 1093–1101. [Google Scholar] [CrossRef] [PubMed]
- De Caluwé, L.; Van Nieuwenhove, Y.; Ceelen, W.P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst. Rev. 2013, 2, CD006041. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hsieh, C.C.; Li, C.Y.; Chuang, J.P.; Lee, J.C. Secondary Cancers After Radiation Therapy for Primary Prostate or Rectal Cancer. World J. Surg. 2016, 40, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Birgisson, H.; Pahlman, L.; Gunnarsson, U.; Glimelius, B. Occurrence of second cancers in patients treated with radiotherapy for rectal cancer. J. Clin. Oncol. 2005, 23, 6126–6131. [Google Scholar] [CrossRef]
- Kendal, W.S.; Nicholas, G.A. Population-based analysis of second primary cancers after irradiation for rectal cancer. Am. J. Clin. Oncol. 2007, 30, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Warschkow, R.; Guller, U.; Cerny, T.; Schmied, B.M.; Plasswilm, L.; Putora, P.M. Secondary malignancies after rectal cancer resection with and without radiation therapy: A propensity-adjusted, population-based SEER analysis. Radiotherapy and oncology. J. Eur. Soc. Ther. Radiol. Oncol. 2017, 123, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, A.J.M.; Hugen, N.; Elferink, M.A.G.; Feuth, T.; Poortmans, P.M.P.; Nagtegaal, I.D.; de Wilt, J.H.W. Incidence of second tumors after treatment with or without radiation for rectal cancer. Ann. Oncol. 2017, 28, 535–540. [Google Scholar] [CrossRef]
- Lu, J.F.; Hsiao, W.C. Does universal health insurance make health care unaffordable? Lessons from Taiwan. Health Aff. 2003, 22, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Insurance. BoNH. Regulations Governing Contracting and Management of National Health Insurance Medical Care Institutions. Available online: http://wwwnhigovtw/English/webdata/webdataaspx?menu=11&menu_id=295&WD_ID=295&webdata_id=3284 (accessed on 4 September 2012).
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Kumar, S. Second malignant neoplasms following radiotherapy. Int. J. Environ. Res. Public Health 2012, 9, 4744–4759. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.E.; Mabuchi, K.; Ron, E.; Soda, M.; Tokunaga, M.; Ochikubo, S.; Preston, D.L. Cancer incidence in atomic bomb survivors. Part II: Solid tumors, 1958–1987. Radiat. Res. 1994, 137, S17–S67. [Google Scholar] [CrossRef] [PubMed]
- Roychoudhuri, R.; Evans, H.; Robinson, D.; Moller, H. Radiation-induced malignancies following radiotherapy for breast cancer. Br. J. Cancer 2004, 91, 868–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagars, G.K.; Pollack, A. Serum testosterone levels after external beam radiation for clinically localized prostate cancer. Int. J. Rad. Oncol. Biol. Phys. 1997, 39, 85–89. [Google Scholar] [CrossRef]
- Raynaud, J.P. Prostate cancer risk in testosterone-treated men. J. Steroid Biochem. Mol. Biol. 2006, 102, 261–266. [Google Scholar] [CrossRef]
- Michaud, J.E.; Billups, K.L.; Partin, A.W. Testosterone and prostate cancer: An evidence-based review of pathogenesis and oncologic risk. Ther. Adv. Urol. 2015, 7, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.L.; Whitton, J.; Leisenring, W.; Mertens, A.C.; Hammond, S.; Stovall, M.; Neglia, J.P. Subsequent neoplasms in 5-year survivors of childhood cancer: The Childhood Cancer Survivor Study. J. Nat. Cancer Inst. 2010, 102, 1083–1095. [Google Scholar] [CrossRef]
- Kamran, S.C.; Berrington de Gonzalez, A.; Ng, A.; Haas-Kogan, D.; Viswanathan, A.N. Therapeutic radiation and the potential risk of second malignancies. Cancer 2016, 122, 1809–1821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Discacciati, A.; Orsini, N.; Andersson, S.O.; Andren, O.; Johansson, J.E.; Wolk, A. Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: A population-based prospective study. Br. J. Cancer 2011, 105, 1061–1068. [Google Scholar] [CrossRef] [PubMed]

| With RT (n = 1586) | Without RT (n = 9781) | p-Value † | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Median age (years), range | 60, (15–93) | 66, (14–106) | <0.001 | ||
| Surgery methods | |||||
| APR | 481 | 30.3 | 1950 | 19.9 | <0.001 |
| LAR | 1105 | 69.37 | 7831 | 80.1 | |
| Follow up time (months) | |||||
| Median with inter-quartile range | 24.0 (9.7, 48.3) | 34.6 (14.4, 68.6) | <0.001 | ||
| Variables | n | Person-Years (×100) | No. of Event | Incidence Rate † | Adjusted HR ‡ | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | p-Value | ||||
| Radiation | ||||||||
| No | 9781 | 359.52 | 153 | 0.43 | 0.36–0.50 | 1.00 | ||
| Yes | 1586 | 45.23 | 8 | 0.18 | 0.08–0.34 | 0.41 | 0.20–0.83 | <0.013 |
| Age (yrs) | ||||||||
| <50 | 1599 | 62.74 | 6 | 0.10 | 0.04–0.20 | 1.00 | ||
| 50– < 60 | 2404 | 86.76 | 21 | 0.24 | 0.15–0.36 | 2.48 | 1.00–6.15 | 0.05 |
| 60– < 70 | 3213 | 123.46 | 48 | 0.39 | 0.29–0.51 | 3.73 | 1.60–8.70 | <0.002 |
| ≥70 | 4151 | 131.79 | 86 | 0.65 | 0.53–0.80 | 5.21 | 2.28–11.90 | <0.001 |
| Total | 11,367 | 404.75 | 161 | |||||
| Variables | n | Person-Years (×100) | No. of Event | Incidence Rate † | Adjusted HR ‡ | |||
|---|---|---|---|---|---|---|---|---|
| Estimate | 95% CI | Estimate | 95% CI | p-Value | ||||
| ≤2 years | ||||||||
| Radiation | ||||||||
| No | 2105 | 10.65 | 32 | 1.02 | 0.71–1.42 | 1.00 | ||
| Yes | 473 | 2.83 | 4 | 0.60 | 0.19–1.46 | 0.94 | 0.33–2.71 | 0.91 |
| ≤3 years | ||||||||
| Radiation | ||||||||
| No | 3746 | 26.31 | 57 | 0.68 | 0.52–0.88 | 1.00 | ||
| Yes | 802 | 6.23 | 4 | 0.23 | 0.08–0.58 | 0.53 | 0.19–1.48 | 0.23 |
| ≤4 years | ||||||||
| Radiation | ||||||||
| No | 5043 | 42.24 | 79 | 0.53 | 0.42–0.66 | 1.00 | ||
| Yes | 1025 | 8.75 | 4 | 0.14 | 0.04–0.34 | 0.39 | 0.14–1.06 | 0.07 |
| ≤5 years | ||||||||
| Radiation | ||||||||
| No | 6020 | 55.64 | 103 | 0.47 | 0.38–0.56 | 1.00 | ||
| Yes | 1197 | 11.47 | 6 | 0.15 | 0.06–0.30 | 0.45 | 0.20–1.03 | 0.06 |
| >5 years | ||||||||
| Radiation | ||||||||
| No | 3761 | 46.04 | 50 | 0.18 | 0.13–0.23 | 1.00 | ||
| Yes | 389 | 5.67 | 2 | 0.07 | 0.01–0.23 | 0.32 | 0.05–2.28 | 0.26 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, J.-P.; Lee, Y.-C.; Lee, J.-C.; Lu, C.-L.; Li, C.-Y. A Population-Based Study of Secondary Prostate Cancer Risk after Radiotherapy in Male Patients with Rectal Cancer: A Retrospective Cohort Study. Medicina 2019, 55, 104. https://doi.org/10.3390/medicina55040104
Chuang J-P, Lee Y-C, Lee J-C, Lu C-L, Li C-Y. A Population-Based Study of Secondary Prostate Cancer Risk after Radiotherapy in Male Patients with Rectal Cancer: A Retrospective Cohort Study. Medicina. 2019; 55(4):104. https://doi.org/10.3390/medicina55040104
Chicago/Turabian StyleChuang, Jen-Pin, Yen-Chien Lee, Jenq-Chang Lee, Chin-Li Lu, and Chung-Yi Li. 2019. "A Population-Based Study of Secondary Prostate Cancer Risk after Radiotherapy in Male Patients with Rectal Cancer: A Retrospective Cohort Study" Medicina 55, no. 4: 104. https://doi.org/10.3390/medicina55040104
APA StyleChuang, J.-P., Lee, Y.-C., Lee, J.-C., Lu, C.-L., & Li, C.-Y. (2019). A Population-Based Study of Secondary Prostate Cancer Risk after Radiotherapy in Male Patients with Rectal Cancer: A Retrospective Cohort Study. Medicina, 55(4), 104. https://doi.org/10.3390/medicina55040104






