Effects of Neck Taping in the Treatment of Hemispatial Neglect in Chronic Stroke Patients: A Pilot, Single Blind, Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
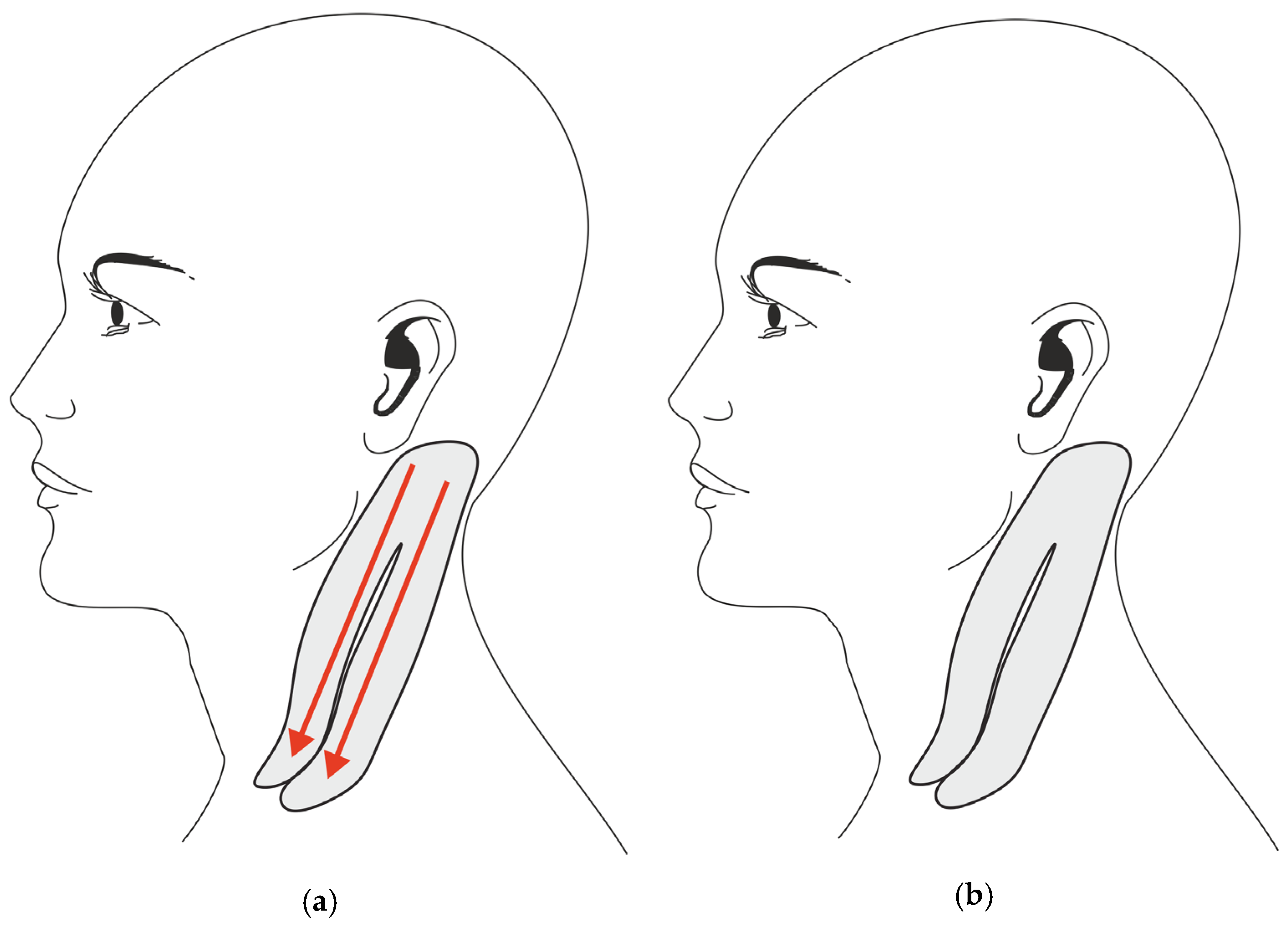
2.1. Treatment Procedures
2.2. Testing Procedures
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Mensah, G.A.; Norrving, B.; Murray, C.J.; Roth, G.A.; GBD 2013 Stroke Panel Experts Group. Atlas of the Global Burden of Stroke (1990–2013): The GBD 2013 Study. Neuroepidemiology 2015, 45, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.E.; Borazanci, A.P. Stroke rehabilitation. Neurol. Res. 2009, 31, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.; Hazelton, C.; Pollock, A.; Lincoln, N.B. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database Syst. Rev. 2013, 1, CD003586. [Google Scholar] [CrossRef]
- Pedersen, P.M.; Jørgensen, H.S.; Nakayama, H.; Raaschou, H.O.; Olsen, T.S. Hemineglect in acute stroke--incidence and prognostic implications. The Copenhagen Stroke Study. Am. J. Phys. Med. Rehabil. 1997, 76, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.; McKenna, K.; Tallis, R.C. Reasons for variability in the reported rate of occurance of unilateral spatial neglect after stroke. Stroke 1999, 30, 1196–1202. [Google Scholar] [CrossRef] [PubMed]
- Chechlacz, M.; Rotshtein, P.; Humphreys, G.W. Unilateral Visual Neglect Symptoms: ALE Meta-Analysis of Lesion-Symptom Mapping. Front. Hum. Neurosci. 2012, 6, 230. [Google Scholar] [CrossRef]
- Yue, Y.; Song, W.; Huo, S.; Wang, M. Study on the occurrence and neural bases of hemispatial neglect with different reference frames. Arch. Phys. Med. Rehabil. 2012, 93, 156–162. [Google Scholar] [CrossRef]
- Heilman, K.M.; Valenstein, E. Mechanisms underlying hemispatial neglect. Ann. Neurol. 1979, 5, 166–170. [Google Scholar] [CrossRef]
- Kwon, J.C.; Ahn, S.; Kim, S.; Heilman, K.M. Ipsilesional ‘where’ with contralesional ‘what’ neglect. Neurocase 2012, 18, 415–423. [Google Scholar] [CrossRef]
- Van Nes, I.J.; Van der Linden, S.; Hendricks, H.T.; Van Kuijk, A.A.; Rulkens, M.; Verhagen, W.I.; Geurts, A.C. Is visuospatial hemineglect really a determinant of postural control following stroke? An acute-phase study. Neurorehabil. Neural Repair 2009, 23, 609–614. [Google Scholar] [CrossRef]
- Pérennou, D.A.; Leblond, C.; Amblard, B.; Micallef, J.P.; Rouget, E.; Pélissier, J. The polymodal sensory cortex is crucial for controlling lateral postural stability: Evidence from stroke patients. Brain Res. Bull. 2000, 53, 359–365. [Google Scholar] [CrossRef]
- Pérennou, D.A.; Leblond, C.; Amblard, B.; Micallef, J.P.; Hérisson, C.; Pélissier, J.Y. Transcutaneous electric nerve stimulation reduces neglect-related postural instability after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 440–448. [Google Scholar] [CrossRef]
- Paolucci, S.; Antonucci, G.; Grasso, G.; Pizzamiglio, L. The role of unilateral spatial neglect in rehabilitation of right brain-damaged ischemic stroke patients: A matched comparison. Arch. Phys. Med. Rehabil. 2001, 82, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Jutai, J.; Bhogal, K.; Foley, N.; Bayley, M.; Teasell, W.; Speechley, M. Treatment of visual perceptual disorders post stroke. Top. Stroke Rehabil. 2003, 10, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Mackintosh, S.F.; Hill, K.D.; Doss, K.J.; Goldie, P.A.; Culham, E.G. Balance score and a history of falls in hospital predict recurrent falls in the 6 months following stroke rehabilitation. Arch. Phys. Med. Rehabil. 2006, 87, 1583–1589. [Google Scholar] [CrossRef] [PubMed]
- Pizzamiglio, L.; Guariglia, C.; Antonucci, G.; Zoccolotti, P. Development of a rehabilitative program for unilateral neglect. Restor. Neurol. Neurosci. 2006, 24, 337–345. [Google Scholar] [PubMed]
- Antonucci, G.; Guariglia, C.; Judica, A.; Magnotti, L.; Paolucci, S.; Pizzamiglio, L.; Zoccolotti, P. Effectiveness of neglect rehabilitation in a randomized group study. J. Clin. Exp. Neuropsychol. 1995, 17, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Luauté, J.; Halligan, P.; Rode, G.; Rossetti, Y.; Boisson, D. Visuo-spatial neglect: A systematic review of current interventions and their effectiveness. Neurosci. Biobehav. Rev. 2006, 30, 961–982. [Google Scholar] [CrossRef]
- Ianes, P.; Varalta, V.; Gandolfi, M.; Picelli, A.; Corno, M.; Di Matteo, A.; Fiaschi, A.; Smania, N. Stimulating visual exploration of the neglected space in the early stage of stroke by hemifield eye-patching: A randomized controlled trial in patients with right brain damage. Eur. J. Phys. Rehabil. Med. 2012, 48, 189–196. [Google Scholar]
- Smania, N.; Fonte, C.; Picelli, A.; Gandolfi, M.; Varalta, V. Effect of eye patching in rehabilitation of hemispatial neglect. Front. Hum. Neurosci. 2013, 7, 527. [Google Scholar] [CrossRef]
- Varalta, V.; Picelli, A.; Fonte, C.; Montemezzi, G.; La Marchina, E.; Smania, N. Effects of contralesional robot-assisted hand training in patients with unilateral spatial neglect following stroke: A case series study. J. Neuroeng. Rehabil. 2014, 11, 160. [Google Scholar] [CrossRef]
- Guariglia, C.; Coriale, G.; Cosentino, T.; Pizzamiglio, L. TENS modulates spatial reorientation in neglect patients. Neuroreport 2000, 11, 1945–1948. [Google Scholar] [CrossRef]
- Pitzalis, S.; Spinelli, D.; Vallar, G.; Di Russo, F. Transcutaneous electrical nerve stimulation effects on neglect: A visual-evoked potential study. Front. Hum. Neurosci. 2013, 7, 111. [Google Scholar] [CrossRef]
- Vallar, G.; Rusconi, M.L.; Barozzi, S.; Bernardini, B.; Ovadia, D.; Papagno, C.; Cesarani, A. Improvement of left visuo-spatial hemineglect by left-sided transcutaneous electrical stimulation. Neuropsychologia 1995, 33, 73–82. [Google Scholar] [CrossRef]
- Kalron, A.; Bar-Sela, S. A systematic review of the effectiveness of Kinesio Taping--fact or fashion? Eur. J. Phys. Rehabil. Med. 2013, 49, 699–709. [Google Scholar]
- Kase, K.; Wallis, J.; Kase, T. Clinical Therapeutic Applications of the Kinesio Taping Method, 2nd ed.; Kase, K., Wallis, J., Kase, T., Eds.; Ken Ika: Tokyo, Japan, 2003. [Google Scholar]
- Tamburella, F.; Scivoletto, G.; Molinari, M. Somatosensory inputs by application of KinesioTaping: Effects on spasticity, balance, and gait in chronic spinal cord injury. Front. Hum. Neurosci. 2014, 8, 367. [Google Scholar] [CrossRef]
- Alexander, C.M.; Stynes, S.; Thomas, A.; Lewis, J.; Harrison, P.J. Does tape facilitate or inhibit the lower fibres of trapezius? Man. Ther. 2003, 8, 37–41. [Google Scholar] [CrossRef]
- Alexander, C.M.; McMullan, M.; Harrison, P.J. What is the effect of taping along or across a muscle on motoneurone excitability? A study using triceps surae. Man. Ther. 2008, 13, 57–62. [Google Scholar] [CrossRef]
- Wilson, B.; Cockburn, J.; Halligan, P.W. The Behavioural Inattention Test; Thames Valley Test Company: Bury St. Edmunds, UK, 1987. [Google Scholar]
- Magni, E.; Binetti, G.; Padovani, A.; Cappa, S.F.; Bianchetti, A.; Trabucchi, M. The Mini-Mental State Examination in Alzheimer’s disease and multi-infarct dementia. Int. Psychogeriatr. 1996, 8, 127–134. [Google Scholar] [CrossRef]
- Clarkson, P.M.; Hubal, M.J. Exercise-induced muscle damage in humans. Am. J. Phys. Med. Rehabil. 2002, 81, S52–S69. [Google Scholar] [CrossRef]
- McIntosh, R.D.; Brodie, E.E.; Beschin, N.; Robertson, I.H. Improving the clinical diagnosis of personal neglect: A reformulated comb and razor test. Cortex 2000, 36, 289–292. [Google Scholar] [CrossRef]
- Revel, M.; Andre-Deshays, C.; Minguet, M. Cervicocephalic kinesthetic sensibility in patients with cervical pain. Arch. Phys. Med. Rehabil. 1991, 72, 288–289. [Google Scholar] [PubMed]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Pelosin, E.; Avanzino, L.; Marchese, R.; Stramesi, P.; Bilanci, M.; Trompetto, C.; Abbruzzese, G. Kinesiotaping reduces pain and modulates sensory function in patients with focal dystonia: A randomized crossover pilot study. Neurorehabil. Neural Repair 2013, 27, 722–731. [Google Scholar] [CrossRef]
- Cho, H.Y.; Kim, E.H.; Kim, J.; Yoon, Y.W. Kinesio taping improves pain, range of motion, and proprioception in older patients with knee osteoarthritis: A randomized controlled trial. Am. J. Phys. Med. Rehabil. 2015, 94, 192–200. [Google Scholar] [CrossRef]
- Yoshida, A.; Kahanov, L. The effect of kinesio taping on lower trunk range of motions. Res. Sports Med. 2007, 15, 103–112. [Google Scholar] [CrossRef]
- Miyai, I.; Blau, A.D.; Reding, M.J.; Volpe, B.T. Patients with stroke confined to basal ganglia have diminished response to rehabilitation efforts. Neurology 1997, 48, 95–101. [Google Scholar] [CrossRef]
- Karnath, H.O. Subjective body orientation in neglect and the interactive contribution of neck muscle proprioception and vestibular stimulation. Brain 1994, 117, 1001–1012. [Google Scholar] [CrossRef]
- Karnath, H.O. Optokinetic stimulation influences the disturbed perception of body orientation in spatial neglect. J. Neurol. Neurosurg. Psychiatry 1996, 60, 217–220. [Google Scholar] [CrossRef]
- Lin, J.J.; Hung, C.J.; Yang, P.L. The effects of scapular taping on electromyographic muscle activity and proprioception feedback in healthy shoulders. J. Orthop. Res. 2011, 29, 53–57. [Google Scholar] [CrossRef]
- Simoneau, G.G.; Degner, R.M.; Kramper, C.A.; Kittleson, K.H. Changes in ankle joint proprioception resulting from strips of athletic tape applied over the skin. J. Athl. Train. 1997, 32, 141–147. [Google Scholar]
- Halseth, T.; McChesney, J.W.; Debeliso, M.; Vaughn, R.; Lien, J. The effects of kinesio™ taping on proprioception at the ankle. J. Sports Sci. Med. 2004, 3, 1–7. [Google Scholar]
- Kilbreath, S.L.; Perkins, S.; Crosbie, J.; McConnell, J. Gluteal taping improves hip extension during stance phase of walking following stroke. Aust. J. Physiother. 2006, 52, 53–56. [Google Scholar] [CrossRef]
- Hsu, Y.H.; Chen, W.Y.; Lin, H.C.; Wang, W.T.; Shih, Y.F. The effects of taping on scapular kinematics and muscle performance in baseball players with shoulder impingement syndrome. J. Electromyogr. Kinesiol. 2009, 19, 1092–1099. [Google Scholar] [CrossRef]
- Riemann, B.L.; Lephart, S.M. The sensorimotor system, part I: The physiologic basis of functional joint stability. The Sensorimotor System, Part II: The Role of Proprioception in Motor Control and Functional Joint Stability. J. Athl. Train. 2002, 37, 80–84. [Google Scholar]
- Callaghan, M.J.; McKie, S.; Richardson, P.; Oldham, J.A. Effects of patellar taping on brain activity during knee joint proprioception tests using functional magnetic resonance imaging. Phys. Ther. 2012, 92, 821–830. [Google Scholar] [CrossRef]
- Kim, B.J.; Lee, J.H.; Kim, C.T.; Lee, S.M. Effects of ankle balance taping with kinesiology tape for a patient with chronic ankle instability. J. Phys. Ther. Sci. 2015, 27, 2405–2406. [Google Scholar] [CrossRef]
- Leanderson, J.; Ekstam, S.; Salomonsson, C. Taping of the ankle: The effect on postural sway during perturbation, before and after a training session. Knee Surg. Sports Traumatol. Arthrosc. 1996, 4, 53–56. [Google Scholar] [CrossRef]
- Karlsson, J.; Andreasson, G.O. The effect of external ankle support in chronic lateral ankle joint instability. An electromyographic study. Am. J. Sports Med. 1992, 20, 257–261. [Google Scholar] [CrossRef]
- Johansson, B.B. Brain plasticity and stroke rehabilitation. The Willis lecture. Stroke 2000, 31, 223–230. [Google Scholar] [CrossRef]

| TG Group | CG Group | p-Value | |
|---|---|---|---|
| Gender male/female | 2/5 | 4/1 | |
| Stroke ischemic/hemorrhagic | 5/2 | 2/3 | |
| Age (years) mean (SD) | 65.5 ± 10.2 | 67.0 (11.5) | 0.830 |
| Education (years) mean (SD) | 9.0 (3.4) | 7.8 (3.9) | 0.599 |
| Time from onset (months) mean (SD) | 19.7 (27.7) | 28.0 (40.6) | 0.908 |
| BI modified (0–100) mean (SD) | 77.4 (34.3) | 57.0 (15.5) | 0.247 |
| MMSE (0–30) mean (SD) | 26.4 (2.1) | 25.4 (1.9) | 0.406 |
| Star Cancellation Test (0–50) mean (SD) | 39.1 (14.9) | 34.0 (5.4) | 0.575 |
| AROM Left Rotation (degrees) mean (SD) | 14.5 (2.3) | 17.2 (4.1) | 0.176 |
| Outcome Measures | TG vs. CG Comparison | p-Value | Effect Size (r) |
|---|---|---|---|
| Star Cancellation Test (0–50) | 0.387 | ‒0.29 | |
| Letters Cancellation Test (0–40) | 0.379 | ‒0.25 | |
| Comb and Razor Test (%) | 0.772 | ‒0.10 | |
| AROM (degrees) | Rotation right | 0.049 * | ‒0.56 |
| Rotation left | 0.432 | ‒0.21 | |
| Inclination right | 0.330 | 0.29 | |
| Inclination left | 0.499 | 0.20 | |
| Flexion | 0.107 | ‒0.47 | |
| Extension | 0.548 | 0.19 | |
| CJPET (degrees) | 0.025 * | ‒0.73 |
| Outcome | Group | T0 | T1 | Within-Group Comparisons |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p-Value (95% CI) | ||
| Star Cancellation Test | TG | 39.14 (14.98) | 41.71 (13.44) | 0.203 (‒6.970; 1.827) |
| CG | 34 (15.42) | 43.2 (3.27) | 0.242 (‒27.813; 9.413) | |
| Letters Cancellation Test | TG | 26.29 (11.31) | 29.14 (12.13) | 0.118 (‒6.686; 0.972) |
| CG | 23.60 (11.61) | 29.40 (10.00) | 0.137 (‒14.465; 2.865) | |
| Comb and Razor Test | TG | ‒0.17 (0.46) | ‒0.10 (0.13) | 0.604 (‒0.618; 0.401) |
| CG | ‒0.31 (0.33) | ‒0.22 (0.14) | 0.196 (‒0.505; 0.159) | |
| Neck AROM | Rotation right | |||
| TG | 14.07 (3.27) | 11.79 (2.67) | 0.011 (0.732; 3.839) * | |
| CG | 13.80 (3.27) | 13.60 (3.11) | 0.772 (‒1.589; 1.989) | |
| Rotation left | ||||
| TG | 14.50 (2.36) | 12.64 (2.62) | 0.012 (0.585; 3.130) * | |
| CG | 17.20 (4.08) | 17.20 (4.62) | 1.000 (‒7.306; 7.306) | |
| Inclination right | ||||
| TG | 15.29 (2.50) | 17.21 (2.23) | 0.032 (‒3.626; ‒0.231) * | |
| CG | 19.20 (2.59) | 20.10 (2.30) | 0.255 (‒2.783; 0.983) | |
| Inclination left | ||||
| TG | 14.71 (2.69) | 17.57 (2.30) | 0.025 (‒5.211; ‒0.504) * | |
| CG | 18.30 (1.72) | 20.10 (3.88) | 0.198 (‒5.044; 1.444) | |
| Flexion | ||||
| TG | 2.34 (0.94) | 1.57 (0.79) | 0.028 (0.118; 1.425) * | |
| CG | 1.60 (0.90) | 1.50 (0.50) | 0.704 (‒0.580; 0.780) | |
| Extension | ||||
| TG | 12.36 (1.4) | 13.36 (2.36) | 0.212 (‒2.751; 0.751) | |
| CG | 14.90 (2.35) | 15.30 (2.11) | 0.495 (‒1.882; 1.082) | |
| CJPET | TG | 10.43 (5.91) | 5.14 (4.26) | 0.037 (0.429; 10.142) * |
| CG | 9.00 (8.00) | 10.40 (6.99) | 0.263 (‒4.390; 1.590) | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varalta, V.; Munari, D.; Pertile, L.; Fonte, C.; Vallies, G.; Chemello, E.; Gandolfi, M.; Modenese, A.; Smania, N.; Picelli, A. Effects of Neck Taping in the Treatment of Hemispatial Neglect in Chronic Stroke Patients: A Pilot, Single Blind, Randomized Controlled Trial. Medicina 2019, 55, 108. https://doi.org/10.3390/medicina55040108
Varalta V, Munari D, Pertile L, Fonte C, Vallies G, Chemello E, Gandolfi M, Modenese A, Smania N, Picelli A. Effects of Neck Taping in the Treatment of Hemispatial Neglect in Chronic Stroke Patients: A Pilot, Single Blind, Randomized Controlled Trial. Medicina. 2019; 55(4):108. https://doi.org/10.3390/medicina55040108
Chicago/Turabian StyleVaralta, Valentina, Daniele Munari, Lucrezia Pertile, Cristina Fonte, Gabriella Vallies, Elena Chemello, Marialuisa Gandolfi, Angela Modenese, Nicola Smania, and Alessandro Picelli. 2019. "Effects of Neck Taping in the Treatment of Hemispatial Neglect in Chronic Stroke Patients: A Pilot, Single Blind, Randomized Controlled Trial" Medicina 55, no. 4: 108. https://doi.org/10.3390/medicina55040108
APA StyleVaralta, V., Munari, D., Pertile, L., Fonte, C., Vallies, G., Chemello, E., Gandolfi, M., Modenese, A., Smania, N., & Picelli, A. (2019). Effects of Neck Taping in the Treatment of Hemispatial Neglect in Chronic Stroke Patients: A Pilot, Single Blind, Randomized Controlled Trial. Medicina, 55(4), 108. https://doi.org/10.3390/medicina55040108








