Adrenal Function in Adolescence is Related to Intrauterine and Postnatal Growth
Abstract
1. Introduction
2. Materials and Methods
2.1. Statement of Ethics
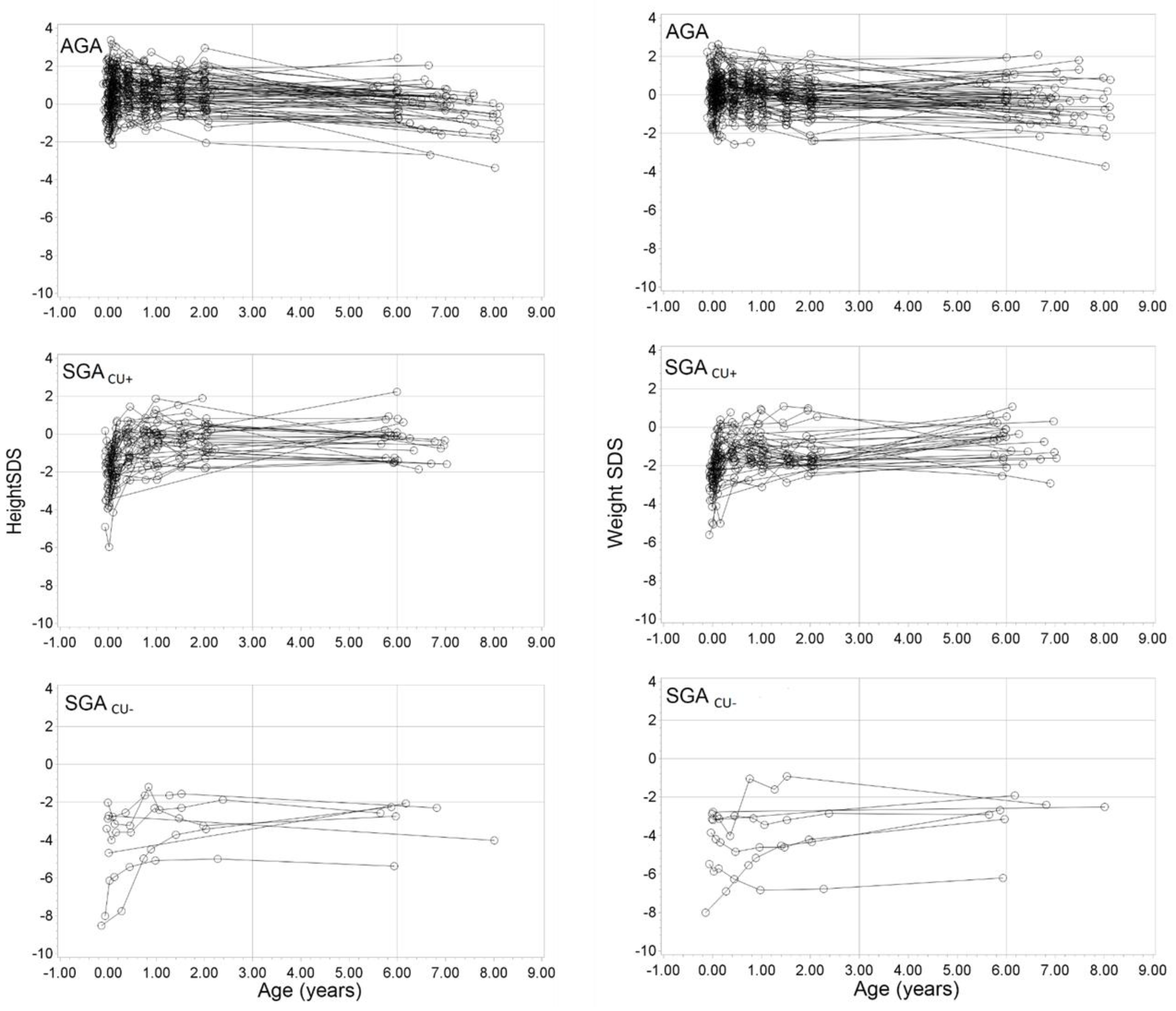
2.2. Study Population
2.3. Study Design
2.4. Hormonal Measurements
2.5. Statistical Analyses
3. Results
3.1. Study Population
3.2. Factors Influencing Adrenal Hormone Levels
3.2.1. DHEAS
Relationship to Size at Birth and Presence/Absence of Catch-Up Growth
Relationship to Sex
Relationship to Birth Characteristics
Relationship to Postnatal Growth
3.2.2. Cortisol
Relationship to Size at Birth and Catch-Up Growth
Relationship to Sex
Relationship to Birth Characteristics
Relationship to Postnatal Growth
3.2.3. Cardiovascular Parameters
4. Discussion
4.1. Principle Findings
4.1.1. DHEAS Association with Postnatal Growth
4.1.2. Secondary Sexual Characteristics and DHEAS Secretion
4.1.3. Cortisol Secretion and Cardiovascular Function
4.1.4. Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Saenger, P.; Czernichow, P.; Hughes, I.; Reiter, E.O. Small for gestational age: Short stature and beyond. Endocr. Rev. 2007, 28, 219–251. [Google Scholar] [CrossRef]
- Clayton, P.E.; Cianfarani, S.; Czernichow, P.; Johannsson, G.; Rapaport, R.; Rogol, A. Management of the child born small for gestational age through to adulthood: A consensus statement of the International Societies of Pediatric Endocrinology and the Growth Hormone Research Society. J. Clin. Endocrinol. Metab. 2007, 92, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Barker, D.J.; Winter, P.D.; Osmond, C.; Margetts, B.; Simmonds, S.J. Weight in infancy and death from ischaemic heart disease. Lancet 1989, 2, 577–580. [Google Scholar] [CrossRef]
- Osterholm, E.A.; Hostinar, C.E.; Gunnar, M.R. Alterations in stress responses of the hypothalamic-pituitary-adrenal axis in small for gestational age infants. Psychoneuroendocrinology 2012, 37, 1719–1725. [Google Scholar] [CrossRef] [PubMed]
- Liyanarachchi, K.; Ross, R.; Debono, M. Human studies on hypothalamo-pituitary-adrenal (HPA) axis. Best Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 459–473. [Google Scholar] [CrossRef]
- Todorova, B.; Salonen, M.; Jaaskelainen, J.; Tapio, A.; Jaaskelainen, T.; Palvimo, J.; Turpeinen, U.; Hamalainen, E.; Rasanen, M.; Tenhola, S.; et al. Adrenocortical hormonal activity in 20-year-old subjects born small or appropriate for gestational age. Horm. Res. Paediatr. 2012, 77, 298–304. [Google Scholar] [CrossRef]
- Beck Jensen, R.; Vielwerth, S.; Larsen, T.; Hilsted, L.; Cohen, A.; Hougaard, D.M.; Jensen, L.T.; Greisen, G.; Juul, A. Influence of fetal growth velocity and smallness at birth on adrenal function in adolescence. Horm. Res. Paediatr. 2011, 75, 2–7. [Google Scholar] [CrossRef]
- Ibanez, L.; Potau, N.; Marcos, M.V.; De Zegher, F. Adrenal hyperandrogenism in adolescent girls with a history of low birthweight and precocious pubarche. Clin. Endocrinol. 2000, 53, 523–527. [Google Scholar] [CrossRef]
- Christodoulaki, C.; Trakakis, E.; Pergialiotis, V.; Panagopoulos, P.; Chrelias, C.; Kassanos, D.; Sioutis, D.; Papantoniou, N.; Xirofotos, D. Dehydroepiandrosterone-sulfate, insulin resistance and ovarian volume estimation in patients with polycystic ovarian syndrome. J. Fam. Reprod. Health 2017, 11, 24–29. [Google Scholar]
- Karlberg, J.; Albertsson-Wikland, K. Growth in full-term small-for-gestational-age infants: From birth to final height. Pediatr. Res. 1995, 38, 733–739. [Google Scholar] [CrossRef]
- Hokken-Koelega, A.C.; De Ridder, M.A.; Lemmen, R.J.; Den Hartog, H.; De Muinck Keizer-Schrama, S.M.; Drop, S.L. Children born small for gestational age: Do they catch up? Pediatr. Res. 1995, 38, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Albertsson-Wikland, K.; Boguszewski, M.; Karlberg, J. Children born small-for-gestational age: Postnatal growth and hormonal status. Horm. Res. 1998, 49, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Francois, I.; de Zegher, F. Adrenarche and fetal growth. Pediatr. Res. 1997, 41, 440–442. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ibanez, L.; Lopez-Bermejo, A.; Diaz, M.; Suarez, L.; de Zegher, F. Low-birth weight children develop lower sex hormone binding globulin and higher dehydroepiandrosterone sulfate levels and aggravate their visceral adiposity and hypoadiponectinemia between six and eight years of age. J. Clin. Endocrinol. Metab. 2009, 94, 3696–3699. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, J.; Boguszewski, M.; Rosberg, S.; Albertsson-Wikland, K. Adrenal steroid hormones in short children born small for gestational age. Clin. Endocrinol. 1998, 49, 353–361. [Google Scholar] [CrossRef]
- Boonstra, V.H.; Mulder, P.G.; de Jong, F.H.; Hokken-Koelega, A.C. Serum dehydroepiandrosterone sulfate levels and pubarche in short children born small for gestational age before and during growth hormone treatment. J. Clin. Endocrinol. Metab. 2004, 89, 712–717. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Radetti, G.; Renzullo, L.; Gottardi, E.; D’Addato, G.; Messner, H. Altered thyroid and adrenal function in children born at term and preterm, small for gestational age. J. Clin. Endocrinol. Metab. 2004, 89, 6320–6324. [Google Scholar] [CrossRef][Green Version]
- Ong, K.K.; Potau, N.; Petry, C.J.; Jones, R.; Ness, A.R.; Honour, J.W.; de Zegher, F.; Ibanez, L.; Dunger, D.B. Avon Longitudinal Study of Parents and Children Study Team. Opposing influences of prenatal and postnatal weight gain on adrenarche in normal boys and girls. J. Clin. Endocrinol. Metab. 2004, 89, 2647–2651. [Google Scholar] [CrossRef]
- Ruys, C.A.; van der Voorn, B.; Lafeber, H.N.; van de Lagemaat, M.; Rotteveel, J.; Finken, M.J.J. Birth weight and postnatal growth in preterm born children are associated with cortisol in early infancy, but not at age 8 years. Psychoneuroendocrinology 2017, 82, 75–82. [Google Scholar] [CrossRef]
- Eyzaguirre, F.C.; Bancalari, R.; Youlton, R.; Roman, R.; Silva, R.; Garcia, H.; Mericq, V. Precocious pubarche: Experience in 173 cases. Rev. Med. Chil. 2009, 137, 31–38. [Google Scholar] [CrossRef]
- Verkauskiene, R.; Albertsson Wikland, K.; Niklasson, A. Variation in size at birth in infants born small for gestational age in Lithuania. Acta Paediatr. 2002, 91, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Niklasson, A.; Albertsson-Wikland, K. Continuous growth reference from 24th week of gestation to 24 months by gender. BMC Pediatr. 2008, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Valuniene, M.; Danylaite, A.; Kryziute, D.; Ramanauskaite, G.; Lasiene, D.T.; Lasas, L.; Verkauskiene, R. Postnatal growth in children born small and appropriate for gestational age during the first years of life. Medicina 2009, 45, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Wikland, K.A.; Luo, Z.C.; Niklasson, A.; Karlberg, J. Swedish population-based longitudinal reference values from birth to 18 years of age for height, weight and head circumference. Acta Paediatr. 2002, 91, 739–754. [Google Scholar] [CrossRef]
- Karlberg, J.; Luo, Z.C.; Albertsson-Wikland, K. Body mass index reference values (mean and SD) for Swedish children. Acta Paediatr. 2001, 90, 1427–1434. [Google Scholar] [CrossRef] [PubMed]
- Mericq, V.; Pereira, A.; Uauy, R.; Corvalan, C. Early BMI Gain and Later Height Growth Predicts Higher DHEAS Concentrations in 7-Year-Old Chilean Children. Horm. Res. Paediatr. 2017, 87, 15–22. [Google Scholar] [CrossRef]
- Incollingo Rodriguez, A.C.; Epel, E.S.; White, M.L.; Standen, E.C.; Seckl, J.R.; Tomiyama, A.J. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: A systematic review. Psychoneuroendocrinology 2015, 62, 301–318. [Google Scholar] [CrossRef]
- Ibanez, L.; Lopez-Bermejo, A.; Suarez, L.; Marcos, M.V.; Diaz, M.; de Zegher, F. Visceral adiposity without overweight in children born small for gestational age. J. Clin. Endocrinol. Metab. 2008, 93, 2079–2083. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, L.; Ong, K.; Dunger, D.B.; de Zegher, F. Early development of adiposity and insulin resistance after catch-up weight gain in small-for-gestational-age children. J. Clin. Endocrinol. Metab. 2006, 91, 2153–2158. [Google Scholar] [CrossRef]
- Ong, K.K.; Ahmed, M.L.; Emmett, P.M.; Preece, M.A.; Dunger, D.B. Association between postnatal catch-up growth and obesity in childhood: Prospective cohort study. BMJ 2000, 320, 967–971. [Google Scholar] [CrossRef]
- Diaz, M.; Bassols, J.; Lopez-Bermejo, A.; de Zegher, F.; Ibanez, L. Metformin treatment to reduce central adiposity after prenatal growth restraint: A placebo-controlled pilot study in prepubertal children. Pediatr. Diabetes 2015, 16, 538–545. [Google Scholar] [CrossRef]
- Sebastiani, G.; Diaz, M.; Bassols, J.; Aragones, G.; Lopez-Bermejo, A.; de Zegher, F.; Ibanez, L. The sequence of prenatal growth restraint and post-natal catch-up growth leads to a thicker intima-media and more pre-peritoneal and hepatic fat by age 3–6 years. Pediatr. Obes. 2016, 11, 251–257. [Google Scholar] [CrossRef]
- Alisi, A.; Panera, N.; Agostoni, C.; Nobili, V. Intrauterine growth retardation and nonalcoholic Fatty liver disease in children. Int. J. Endocrinol. 2011, 2011, 269853. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kikuchi, T.; Nagasaki, K.; Hiura, M.; Ogawa, Y.; Uchiyama, M. Lower birth weight and visceral fat accumulation are related to hyperinsulinemia and insulin resistance in obese Japanese children. Hypertens. Res. 2005, 28, 529–536. [Google Scholar] [CrossRef]
- Biosca, M.; Rodríguez, G.; Ventura, P. Central adiposity in children born small and large for gestational age. Nutr. Hosp. 2011, 26, 971–976. [Google Scholar]
- Eriksson, M.; Tynelius, P.; Rasmussen, F. Associations of birthweight and infant growth with body composition at age 15—The COMPASS study. Paediatr. Perinat. Epidemiol. 2008, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Ghirri, P.; Bernardini, M.; Vuerich, M.; Cuttano, A.M.; Coccoli, L.; Merusi, I.; Ciulli, C.; D’Accavio, L.; Bottone, U.; Boldrini, A. Adrenarche, pubertal development, age at menarche and final height of full-term, born small for gestational age (SGA) girls. Gynecol. Endocrinol. 2001, 15, 91–97. [Google Scholar] [PubMed]
- Sloboda, D.M.; Hart, R.; Doherty, D.A.; Pennell, C.E.; Hickey, M. Age at menarche: Influences of prenatal and postnatal growth. J. Clin. Endocrinol. Metab. 2007, 92, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Chandola, T.; Brunner, E.; Kivimaki, M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2010, 95, 4415–4423. [Google Scholar] [CrossRef] [PubMed]
- Knutsson, U.; Dahlgren, J.; Marcus, C.; Rosberg, S.; Bronnegard, M.; Stierna, P.; Albertsson-Wikland, K. Circadian cortisol rhythms in healthy boys and girls: Relationship with age, growth, body composition, and pubertal development. J. Clin. Endocrinol. Metab. 1997, 82, 536–540. [Google Scholar] [CrossRef]
- Kouda, K.; Ohara, K.; Fujita, Y.; Nakamura, H.; Iki, M. Trunk-to-peripheral fat ratio predicts subsequent blood pressure levels in pubertal children with relatively low body fat- three-year follow-up study. Circ. J. 2016, 80, 1838–1845. [Google Scholar] [CrossRef] [PubMed]


| Total (n = 102) | SGA Subgroups | Boys (n = 47) | Girls (n = 55) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SGA (n = 47) | AGA (n = 55) | P Value | SGACU− (n = 7) | P Value | SGACU+ (n = 40) | P Value | SGA (n = 24) | AGA (n = 23) | P Value | SGA (n = 23) | AGA (n = 32) | P Value | |
| Gender, boys/girls (%) | 51.1/48.9 | 41.8/58.2 | 0.353 * | 42.9/57.1 | 0.969 | 52.5/47.5 | 0.400 | ||||||
| At Birth | |||||||||||||
| Gestational age (weeks) | 38.7 ± 0.3 | 39.2 ± 0.2 | 0.134 | 38.7 ± 0.6 | 0.412 | 38.9 ± 0.2 | 0.340 | 38.5 ± 0.4 | 39.0 ± 0.4 | 0.385 | 39.0 ± 0.3 | 39.4 ± 0.2 | 0.274 |
| WeightSDS | −3.09 ± 0.16 | −0.08 ± 0.13 | <0.001 | −4.19 ± 0.73 | <0.001 | −2.89 ± 0.13 | <0.001 | −3.41 ± 0.27 | 0.06 ± 0.23 | <0.001 | −2.75 ± 0.16 | −0.18 ± 0.15 | <0.001 |
| LengthSDS | −2.62 ± 0.24 | −0.07 ± 0.16 | <0.001 | −4.60 ± 1.0 | <0.001 | −2.27 ± 0.18 | <0.001 | −2.86 ± 0.34 | −0.10 ± 0.26 | <0.001 | −2.37 ± 0.34 | −0.04 ± 0.21 | <0.001 |
| Lean massSDS | −1.82 ± 0.17 | −0.04 ± 0.14 | <0.001 | −1.89 ± 0.86 | <0.001 | −1.80 ± 0.14 | <0.001 | −2.13 ± 0.23 | 0.18 ± 0.23 | <0.001 | −1.49 ± 0.24 | −0.21 ± 0.17 | <0.001 |
| BMI (kg/m2) | 11.1 ± 0.2 | 13.7 ± 0.2 | <0.001 | 10.8 ± 0.7 | <0.001 | 11.1 ± 0.16 | <0.001 | 10.9 ± 0.2 | 14.0 ± 0.3 | <0.001 | 11.3 ± 0.2 | 13.5 ± 0.2 | <0.001 |
| Ponderal index (kg/m3) | 2.37 ± 0.03 | 2.70 ± 0.03 | <0.001 | 2.52 ± 0.12 | 0.051 | 2.35 ± 0.03 | <0.001 | 2.33 ± 0.03 | 2.75 ± 0.06 | <0.001 | 2.42 ± 0.06 | 2.67 ± 0.04 | 0.001 |
| At the Time of Investigation | |||||||||||||
| Age (years) | 12.3 ± 0.1 | 12.6 ± 0.1 | 0.096 | 11.6 ± 0.34 | 0.056 | 12.4 ± 0.15 | 0.356 | 12.2 ± 0.2 | 12.4 ± 0.2 | 0.366 | 12.4 ± 0.2 | 12.7 ± 0.2 | 0.215 |
| Height (cm) | 152.1 ± 1.5 | 158.8 ± 1.0 | <0.001 | 137.6 ± 4.4 | <0.001 | 154.6 ± 1.2 | 0.016 | 151.3 ± 2.0 | 159.3 ± 1.8 | 0.005 | 152.9 ± 2.3 | 158.5 ± 1.2 | 0.023 |
| HeightSDS | −0.38 ± 0.19 | 0.36 ± 0.13 | 0.001 | 2.03 ± 0.67 | <0.001 | −0.10 ± 0.15 | 0.038 | −0.34 ± 0.26 | 0.64 ± 0.18 | 0.004 | −0.43 ± 0.7 | 0.16 ± 0.18 | 0.072 |
| Weight (kg) | 42.2 ± 1.4 | 49.3 ± 1.5 | 0.001 | 29.2 ± 2.7 | <0.001 | 44.5 ± 1.3 | 0.021 | 41.9 ± 1.9 | 48.8 ± 1.8 | 0.012 | 42.5 ± 2.1 | 49.6 ± 2.2 | 0.029 |
| WeightSDS | −0.36 ± 0.25 | 0.59 ± 0.21 | 0.004 | −2.53 ± 0.67 | <0.001 | 0.02 ± 0.23 | 0.074 | −0.33 ± 0.36 | 0.73 ± 0.29 | 0.028 | −0.40 ± 0.36 | 0.48 ± 0.29 | 0.062 |
| BMI (kg/m2) | 18.0 ± 0.4 | 19.4 ± 0.5 | 0.032 | 15.2 ± 0.6 | 0.011 | 18.5 ± 0.4 | 0.164 | 18.1±0.6 | 19.2 ± 0.6 | 0.209 | 17.9 ± 0.6 | 19.6 ± 0.7 | 0.094 |
| BMISDS | −0.17 ± 0.20 | 0.31 ± 0.17 | 0.069 | −1.52 ± 0.49 | 0.001 | 0.06 ± 0.20 | 0.353 | −0.05 ± 0.28 | 0.41 ± 0.24 | 0.212 | −0.30 ± 0.29 | 0.23 ± 0.24 | 0.165 |
| Subscapular skinfold thickness *** | 11.1 ± 0.6 | 9.0 ± 0.5 | 0.004 | 13.1 ± 1.8 | 0.043 | 11.0 ± 0.7 | 0.041 | 8.8 ± 0.5 | 7.2 ± 0.5 | 0.098 | 13.2 ± 1.0 | 10.5 ± 0.8 | 0.051 |
| Limb skinfold thickness *** | 31.2 ± 1.3 | 28.1 ± 1.2 | 0.089 | 33.9 ± 3.9 | 0.138 | 30.8 ± 1.4 | 0.100 | 27.3 ± 1.6 | 24.9 ± 1.7 | 0.330 | 33.8 ± 2.1 | 31.5 ± 1.7 | 0.408 |
| Waist circumference *** | 66.1 ± 0.5 | 66.3 ± 0.4 | 0.912 | 66.8 ± 1.4 | 0.762 | 66.0 ± 0.5 | 0.786 | 66.6 ± 0.6 | 66.8 ± 0.6 | 0.957 | 66.1 ± 0.7 | 65.5 ± 0.6 | 0.510 |
| Waist to height ratio *** | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.013 | 0.45 ± 0.01 | <0.001 | 0.43 ± 0.01 | 0.071 | 0.43 ± 0.01 | 0.42 ± 0.01 | 0.009 | 0.43 ± 0.01 | 0.41 ± 0.01 | 0.060 |
| Pubic hair ** | 2 [1,2] | 2 [1,2] | 0.820 | 3 [3,4] | 3 [2,3,4] | 0.964 | |||||||
| Tanner stages of breast development in girls | - | - | - | 3 [2,3] | 3 [2,3] | 0.694 | |||||||
| Post-menarche (%) | - | - | - | 27.3 | 50 | 0.158 * | |||||||
| Age at menarche (years) | - | - | - | 12.0 ± 0.3 | 13.0 ± 0.3 | 0.048 | |||||||
| Tanner stages of external genitalia development | 1 [1,2,3] | 2 [1,2,3] | 0.204 | - | - | - | |||||||
| Testicular volume (mL) ** | 4 [2,3,4,5,6] | 6 [2,3,4,5,6,7,8,9,10] | 0.120 | - | - | - | |||||||
| Group | N | SGA | N | AGA | P Value | |
|---|---|---|---|---|---|---|
| DHEAS (µmol/L) | Total | 47 | 4.65 ± 0.32 | 55 | 3.36 ± 0.29 | 0.007 |
| SGACU− | 7 | 4.45 ± 0.91 | 0.232 | |||
| SGACU+ | 40 | 4.68 ± 0.34 | 0.008 | |||
| Boys | 24 | 4.90 ± 0.51 | 23 | 3.54 ± 0.53 | 0.141 | |
| Girls | 23 | 4.22 ± 0.41 | 32 | 3.36 ± 0.35 | 0.112 |
| Variable | Standardized Coefficient β | B | 95% CI for B | P Value |
|---|---|---|---|---|
| Size at birth | ||||
| WeightSDS | 0.664 | 0.083 | −0.048 to 0.214 | 0.200 |
| LengthSDS | −0.593 | −0.057 | −0.141 to 0.028 | 0.178 |
| Postnatal growth | ||||
| 0–6 yr. ∆ BMI (kg/m2) | 0.657 | 0.069 | 0.011 to 0.128 | 0.022 |
| 1–2 yr. ∆ height (cm) | 0.412 | 0.041 | 0.008 to 0.075 | 0.018 |
| 1–2 yr. ∆ limb skinfold thickness (mm) | −0.405 | −0.022 | −0.041 to −0.004 | 0.018 |
| 0–1 yr. ∆ weight (kg) | −0.270 | −0.00006 | −0.00014 to 0.00002 | 0.139 |
| Controlling factors | ||||
| Current Age (y) | 0.529 | 0.142 | 0.031 to 0.253 | 0.015 |
| Sex | −0.429 | −0.224 | −0.412 to −0.037 | 0.021 |
| Current pubertal stage | −0.163 | −0.037 | −0.135 to 0.060 | 0.433 |
| Current BMISDS | −0.093 | −0.019 | −0.111 to 0.074 | 0.682 |
| Group | N | SGA | N | AGA | P Value | |
|---|---|---|---|---|---|---|
| Cortisol (nmol/L) | Total | 45 | 308.9 ± 17.4 | 55 | 301.9 ± 15.0 | 0.758 |
| SGACU− | 6 | 423.5 ± 49.2 | 0.023 | |||
| SGACU+ | 39 | 294.0 ± 17.9 | 0.736 | |||
| Boys | 24 | 318.8 ± 24.8 | 23 | 292.4 ± 26.9 | 0.567 | |
| Girls | 21 | 320.8 ± 23.1 | 32 | 296.6 ± 16.9 | 0.324 |
| Parameter | Standardized Coefficient β | B | 95% CI for B | P Value |
|---|---|---|---|---|
| Postnatal growth | ||||
| 2–12 yr. ∆ subscapular skinfold thickness (mm) | 0.840 | 0.028 | 0.012 to 0.044 | 0.001 |
| 1–2 yr. ∆ BMI (kg/m2) | −0.459 | −0.004 | −0.007 to −0.001 | 0.010 |
| 0–2 mo. ∆ height (cm) | −0.160 | −0.015 | −0.043 to 0.014 | 0.311 |
| Controlling factors | ||||
| Current BMISDS | −0.596 | −0.088 | −0.158 to −0.017 | 0.016 |
| Current age | 0.169 | 0.034 | −0.051 to 0.118 | 0.423 |
| Sex | −0.245 | −0.095 | −0.232 to 0.042 | 0.167 |
| Current pubertal stage | −0.136 | −0.023 | −0.098 to 0.052 | 0.538 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petraitienė, I.; Valūnienė, M.; Albertsson-Wikland, K.; Verkauskienė, R. Adrenal Function in Adolescence is Related to Intrauterine and Postnatal Growth. Medicina 2019, 55, 167. https://doi.org/10.3390/medicina55050167
Petraitienė I, Valūnienė M, Albertsson-Wikland K, Verkauskienė R. Adrenal Function in Adolescence is Related to Intrauterine and Postnatal Growth. Medicina. 2019; 55(5):167. https://doi.org/10.3390/medicina55050167
Chicago/Turabian StylePetraitienė, Indrė, Margarita Valūnienė, Kerstin Albertsson-Wikland, and Rasa Verkauskienė. 2019. "Adrenal Function in Adolescence is Related to Intrauterine and Postnatal Growth" Medicina 55, no. 5: 167. https://doi.org/10.3390/medicina55050167
APA StylePetraitienė, I., Valūnienė, M., Albertsson-Wikland, K., & Verkauskienė, R. (2019). Adrenal Function in Adolescence is Related to Intrauterine and Postnatal Growth. Medicina, 55(5), 167. https://doi.org/10.3390/medicina55050167




