Experimental and Computational Studies to Characterize and Evaluate the Therapeutic Effect of Albizia lebbeck (L.) Seeds in Alzheimer’s Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Collection and Preparation of Plant Material
2.3. Physicochemical, Phytochemical and Qualitative Analysis of Seeds Powder Material
2.4. Preparation and Characterization of Albizia lebbeck Seeds Extract
2.5. Animals and Experimental Design
- (a)
- Group I: Normal control (NC);
- (b)
- Group II: Disease control (AlCl3);
- (c)
- Group III: Standard control (STD);
- (d)
- Group IV: 100 mg/kg treatment control (ALE 100);
- (e)
- Group V: 200 mg/kg treatment control (ALE 200);
- (f)
- Group VI: 300 mg/kg treatment control (ALE 300).
2.5.1. Behavioral Experiments
Morris Water Maze Test
Open Field Test
Hole Board Test
Y-Maze Test
T-Maze Test
2.5.2. Biochemical Experiments
Estimation of Oxidative Stress
Measurement of Acetylcholinesterase Activity
2.5.3. Histological Experiment:
Acute Oral Toxicity
2.5.4. Molecular Docking
2.6. Statistical Analysis
3. Results
3.1. Physicochemical, Phytochemical, and Qualitative Analysis of Powder Material
3.2. Preparation and Characterization of ALE
3.3. Behavioral Experiments
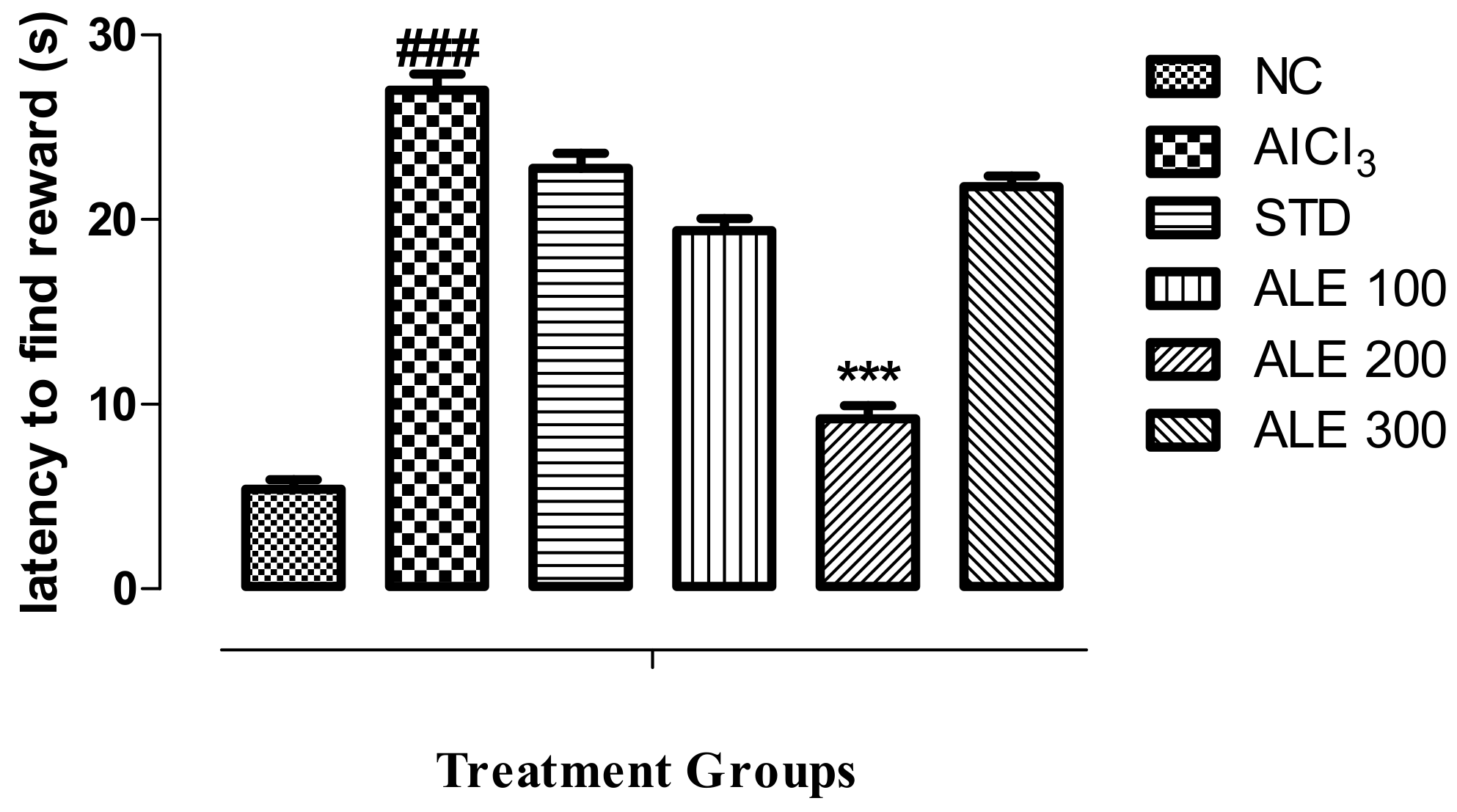
3.3.1. Morris Water Maze Test
3.3.2. Open Field Test
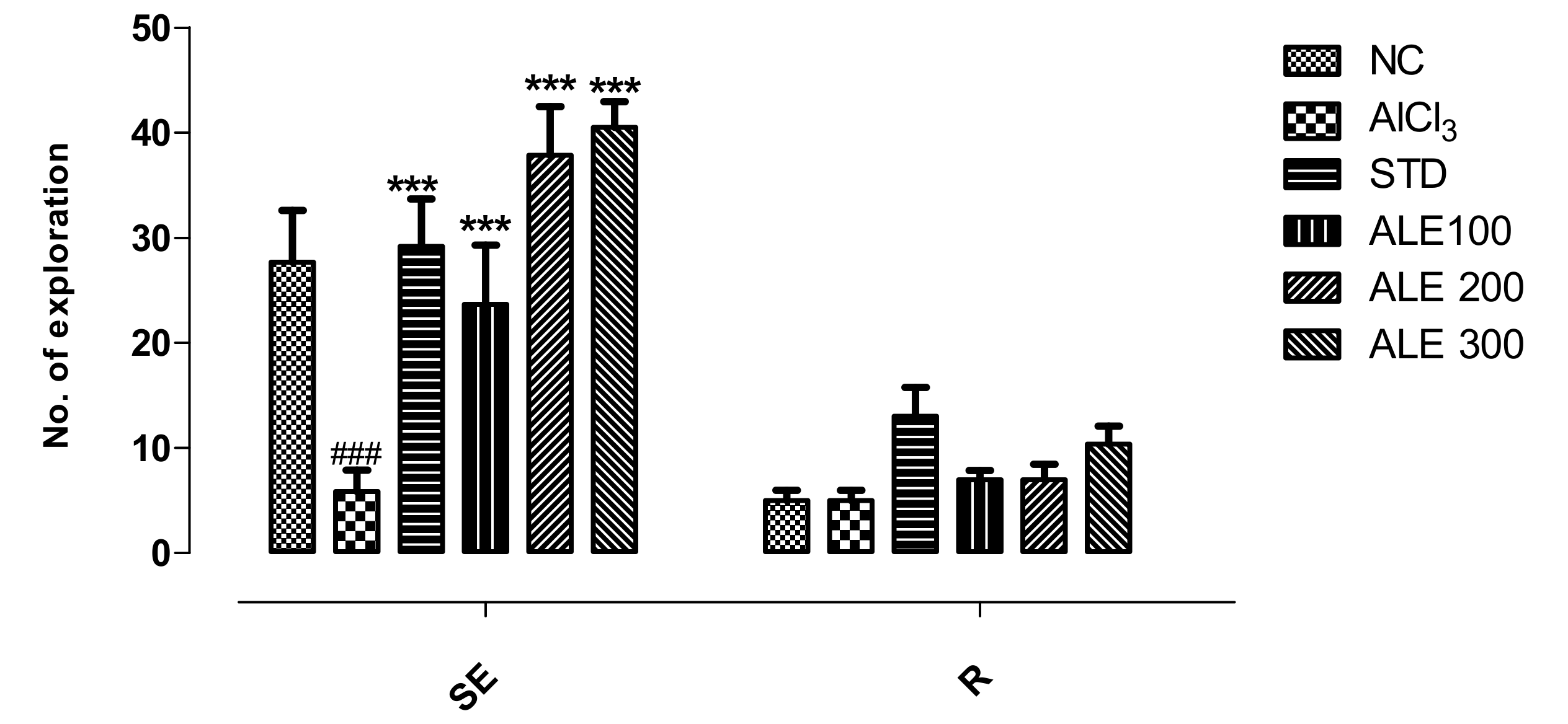
3.3.3. Hole Board Test
3.3.4. Y-Maze Test
3.3.5. T-Maze Test
3.4. Biochemical Experiment
3.4.1. Estimation of Oxidative Stress
3.4.2. Assay of Tissue Acetylcholinesterase Activity
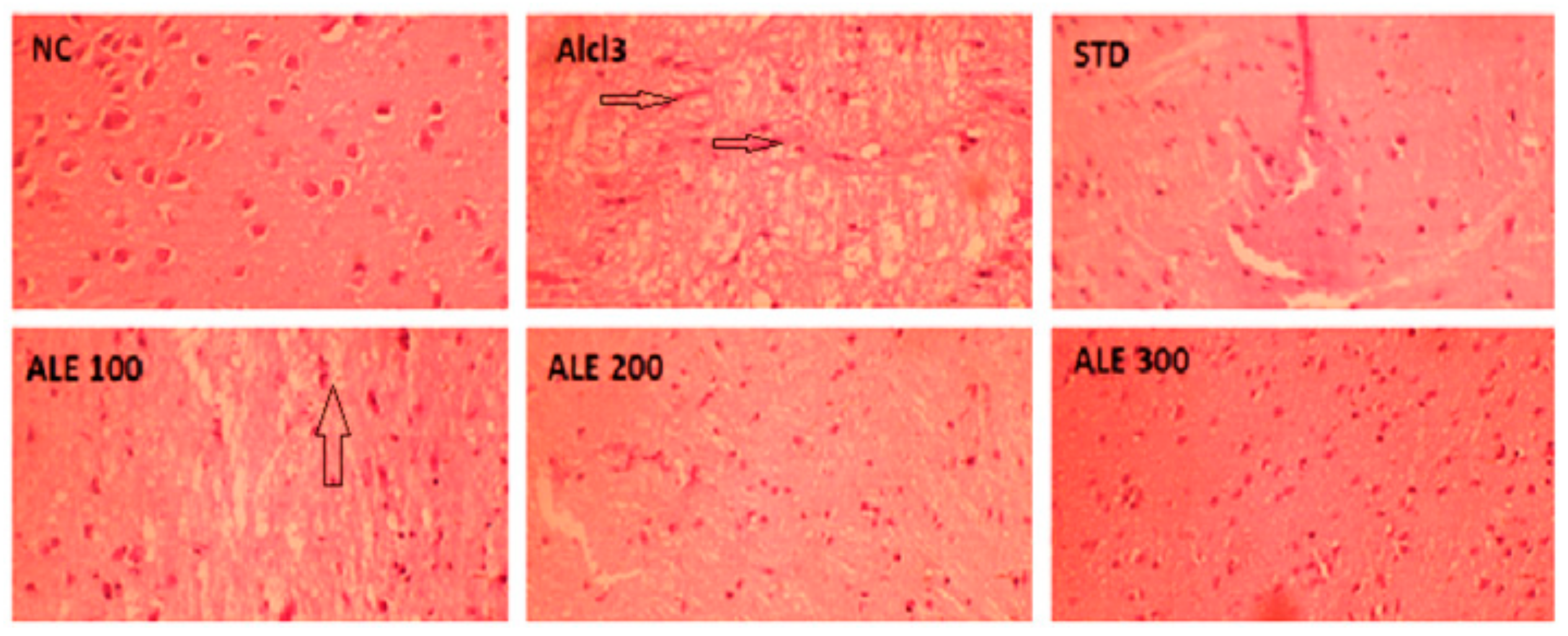
3.5. Histological Experiments
Acute Oral Toxicity
3.6. In-Silico Modeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, M.; Singh, S.; Tiwari, S.; Pandey, V.P.; Dwivedi, U.N. Computational approaches for therapeutic application of natural products in Alzheimer´s disease. In Computational Modeling of Drugs Against Alzheimer’s Disease; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Sanabria-Castro, A.; Alvarado-Echeverría, I.; Monge-Bonilla, C. Molecular pathogenesis of Alzheimer’s disease: An update. Ann. Neurosci. 2017, 24, 46–54. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Sokolov, V.B.; Grigoriev, V.V.; Serebryakova, O.G.; Vikhareva, E.A.; Aksinenko, A.Y.; Barreto, G.E.; Aliev, G.; et al. Conjugates of γ-Carbolines and Phenothiazine as new selective inhibitors of butyrylcholinesterase and blockers of NMDA receptors for Alzheimer disease. Sci. Rep. 2015, 5, 13164. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, V.; Verstreken, P. Autophagy in the presynaptic compartment in health and disease. J. Cell Biol. 2017, 216, 1895–1906. [Google Scholar] [CrossRef] [PubMed]
- Kuijpers, M.; Haucke, V. Presynaptic endocytic factors in autophagy and neurodegeneration. Curr. Opin. Neurobiol. 2018, 48, 153–159. [Google Scholar]
- Rollinger, J.M.; Schuster, D.; Baier, E.; Ellmerer, E.P.; Langer, T.; Stuppner, H. Taspine: Bioactivity-guided isolation and molecular ligand-target insight of a potent acetylcholinesterase inhibitor from Magnolia x soulangiana. J. Nat. Prod. 2006, 69, 1341–1346. [Google Scholar] [CrossRef]
- Jachak, S.M.; Saklani, A. Challenges and opportunities in drug discovery from plants. Curr. Sci. 2007, 92, 1251–1257. [Google Scholar]
- Srivastav Neeti, S.S.; Vijay, J.; Tiwari Brijesh, K. Anti convulsant activity of leaf extracts of Albizia lebbeck Linn in experimental rats. Int. J. Pharm. Sci. Rev. Res. 2016, 41, 173–176. [Google Scholar]
- Verma, S.C.; V, E.; Singh, R.; Kumari, A.; Meena, A.K.; Pant, P.; Bhuyan, G.C.; Padhi, M.M. A review on parts of Albizia lebbeck (L.) Benth. used as Ayurvedic drugs. Res. J. Pharm. Technol. 2013, 6, 1307–1313. [Google Scholar]
- Kasture, V.S.; Chopde, C.T.; Deshmukh, V.K. Anticonvulsive activity of Albizzia lebbeck, Hibiscus rosa sinesis and Butea monosperma in experimental animals. J. Ethnopharmacol. 2000, 71, 65–75. [Google Scholar] [CrossRef]
- Malarkodi Velraj, A.V.; Jayakumai, S.; Jayabalan, S. Antidepressant activity of the ethanolic extract of Albizia lebbeck (Linn) bark in animal models of depression. Drug Invent. Today 2009, 1, 112–115. [Google Scholar]
- Islam, S.; Shajib, M.S.; Datta, B.K.; Rashid, M.A. Neuropharmacological activities of methanol extract of Albizia lebbeck (L.) Benth. Bangladesh Pharm. J. 2018, 21, 7. [Google Scholar] [CrossRef]
- Saleem, U.; Hussain, K.; Ahmad, M.; Bukhari, N.I.; Malik, A.; Ahmad, B. Report: Physicochemical and phytochemical analysis of Euphorbia helioscopia (L.). Pak. J. Pharm. Sci. 2014, 27, 577–585. [Google Scholar]
- Aminjafari, A.; Miroliaei, M.; Angelova, V.T.; Emamzadeh, R.; Djukic, M.M.; Djuric, A.; Saso, L. Antioxidant activity and protective role on protein glycation of synthetic aminocoumarins. Electron. J. Biotechnol. 2016, 24, 43–48. [Google Scholar] [CrossRef]
- Tokusoglu, O.; Unal, M.K.; Yildirim, Z. HPLC-UV and GC-MS characterization of the flavonol aglycons quercetin, kaempferol, and myricetin in tomato pastes and other tomato-based products. Acta Chromatogr. 2003, 13, 196–207. [Google Scholar]
- Prema, A.; Thenmozhi, A.J.; Manivasagam, T.; Essa, M.M.; Akbar, M.D.; Akbar, M. Fenugreek seed powder nullified aluminium chloride induced memory loss, biochemical changes, Aβ burden and apoptosis via regulating Akt/GSK3β signaling pathway. PLoS ONE 2016, 11, e0165955. [Google Scholar] [CrossRef]
- Justin Thenmozhi, A.; Raja, T.R.; Janakiraman, U.; Manivasagam, T. Neuroprotective effect of hesperidin on aluminium chloride induced Alzheimer’s disease in Wistar rats. Neurochem. Res. 2015, 40, 767–776. [Google Scholar] [CrossRef]
- Barua, C.C.; Talukdar, A.; Begum, S.A.; Borah, P.; Lahkar, M. Anxiolytic activity of methanol leaf extract of Achyranthes aspera Linn in mice using experimental models of anxiety. Indian J. Pharmacol. 2012, 44, 63–67. [Google Scholar] [CrossRef]
- Harquin Simplice, F.; David Emery, T.; Herve Herve, N.A. Enhancing spatial memory: Anxiolytic and antidepressant effects of Tapinanthus dodoneifolius (DC) Danser in mice. Neurol. Res. Int. 2014, 2014, 974308. [Google Scholar] [CrossRef]
- Yassin Nemat, A.Z.; El-Shenawy, S.M.A.; Mahdy, K.A.; Gouda, N.A.M.; Marrie, A.E.-F.H.; Farrag, A.R.H.; Bassant, M.M. Ibrahim. Effect of Boswellia serrata on Alzheimer’s disease induced in rats. J. Arab Soc. Med. ResJ Arab Soc. Med. Res. 2013, 8, 1–11. [Google Scholar] [CrossRef]
- Hira, S.; Anwar, F.; Ahmad, B.; Saleem, U. Antioxidants attenuate isolation- and L-DOPA-induced aggression in mice. Front. Pharmacol. Front. Pharmacol. 2018, 8, 945. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, B.V.; Sudhakar, M.; Prakash, K.S. Protective effect of selenium against aluminum chloride-induced Alzheimer’s disease: Behavioral and biochemical alterations in rats. Biol. Trace Elem. Res. 2015, 165, 67–74. [Google Scholar] [CrossRef]
- Akhila, J.S.; Shyamjith, D.; Alwar, M. Acute toxicity studies and determination of median lethal dose. Curr. Sci. 2007, 20, 917–920. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Nogara, P.A.; Saraiva, R.A.; Caeran Bueno, D.; Lissner, L.J.; Lenz Dalla Corte, C.; Braga, M.M.; Rosemberg, D.B.; Rocha, J.B. Virtual screening of acetylcholinesterase inhibitors using the Lipinski’s rule of five and ZINC databank. BioMed Res. Int. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Seyedi, S.S.; Shukri, M.; Hassandarvish, P.; Oo, A.; Shankar, E.M.; Abubakar, S.; Zandi, K. Computational approach towards exploring potential Anti-Chikungunya activity of selected flavonoids. Sci. Rep. 2016, 6, 24027. [Google Scholar] [CrossRef] [PubMed]
- Chandel, H.S.; Pathak, A.K.; Tailang, M. Standardization of some herbal antidiabetic drugs in polyherbal formulation. Pharmacogn. Res. 2011, 3, 49–56. [Google Scholar] [CrossRef]
- Poojary, M.M.; Vishnumurthy, K.A.; Vasudeva Adhikari, A. Extraction, characterization and biological studies of phytochemicals from Mammea suriga. J. Pharm. Anal. 2015, 5, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, C.; Kanjilal, S.; Gupta, A.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu AYU (An Int. Q. J. Res. Ayurveda) 2012, 33, 10. [Google Scholar] [CrossRef]
- Thakur, L.; Ghodasra, U.; Patel, N.; Dabhi, M. Novel approaches for stability improvement in natural medicines. Pharmacogn. Rev. 2011, 5, 48–54. [Google Scholar] [CrossRef]
- Ballesteros, L.; Teixeira, J.; Mussatto, S. Selection of the solvent and extraction conditions for maximum recovery of antioxidant phenolic compounds from coffee silverskin. Food Bioprocess Technol. 2014, 7, 1322–1332. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of antioxidant potential of plants and its relevance to therapeutic applications. Int. J. Biol. Sci. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Swarts, S.G.; Yin, L.; Liu, C.; Tian, Y.; Cao, Y.; Swarts, M.; Yang, S.; Zhang, S.B.; Zhang, K. Antioxidant properties of quercetin. Adv. Exp. Med. Biol. 2011, 701, 283–290. [Google Scholar] [PubMed]
- Badhani, B.; Sharma, N.; Kakkar, R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. RSC Adv. 2015, 5, 27540–27557. [Google Scholar] [CrossRef]
- Niciforovic, N.; Abramovic, H. Sinapic acid and its derivatives natural sources and bioactivity. Compr. Rev. Food Sci. Food Saf. 2014, 13, 34–51. [Google Scholar] [CrossRef]
- Kim, J.S. Antioxidant activities of selected berries and their free, esterified, and insoluble-bound phenolic acid contents. Prev. Nutr. Food Sci. 2018, 23, 35–45. [Google Scholar] [CrossRef]
- Huang, W.J.; Zhang, X.; Chen, W.W. Role of oxidative stress in Alzheimer’s disease. Biomed. Rep. 2016, 4, 519–522. [Google Scholar] [CrossRef]
- Smith, G.P.A.D.C.M.A. Alzheimer disease and oxidative stress. J. Biomed. Biotechnol. 2002, 2, 120–123. [Google Scholar]
- Feng, Y.; Wang, X. Antioxidant therapies for Alzheimer’s disease. Oxid. Med. Cell. Longev. 2012, 2012, 472932. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, R.A.; Assi, A.-A.A.; Kostandy, B.B. Rivastigmine reverses aluminum-induced behavioral changes in rats. Eur. J. Pharmacol. 2011, 659, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Nampoothiri, M.; John, J.; Kumar, N.; Mudgal, J.; Nampurath, G.K.; Chamallamudi, M.R. Modulatory Role of simvastatin against aluminium chloride-induced behavioural and biochemical changes in rats. Behav. Neurol. Behav. Neurol. 2015, 2015, 1–9. [Google Scholar] [CrossRef]
- Gortz, N.; Lewejohann, L.; Tomm, M.; Ambree, O.; Keyvani, K.; Paulus, W.; Sachser, N. Effects of environmental enrichment on exploration, anxiety, and memory in female TgCRND8 Alzheimer mice. Behav. Brain Res. 2008, 191, 43–48. [Google Scholar] [CrossRef]
- Leussis, M.P.; Bolivar, V.J. Habituation in rodents: A review of behavior, neurobiology, and genetics. Neurosci. Biobehav. Rev. 2006, 30, 1045–1064. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, H.; Fuentes, S.; Nadal, R.; Armario, A. Previous exposure to immobilisation and repeated exposure to a novel environment demonstrate a marked dissociation between behavioral and pituitary-adrenal responses. Behav. Brain Res. 2008, 187, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Duzel, E.; Habib, R.; Guderian, S.; Heinze, H.J. Four types of novelty-familiarity responses in associative recognition memory of humans. Eur. J. Neurosci. 2004, 19, 1408–1416. [Google Scholar] [CrossRef]
- Schiller, D.; Eichenbaum, H.; Buffalo, E.A.; Davachi, L.; Foster, D.J.; Leutgeb, S.; Ranganath, C. Memory and space: Towards an understanding of the cognitive map. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 13904–13911. [Google Scholar] [CrossRef]
- Jheng, S.S.; Pai, M.C. Cognitive map in patients with mild Alzheimer’s disease: A computer-generated arena study. Behav. Brain Res. 2009, 200, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Prakash, A.; Kumar, A. Effect of N-Acetyl Cysteine against aluminium-induced cognitive dysfunction and oxidative damage in rats. Basic Clin. Pharmacol. Toxicol. 2009, 105, 98–104. [Google Scholar] [CrossRef]
- Shim, S.Y.; Kim, H.S. Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J. Pediatr. 2013, 56, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Mackey, M.M.; Diaz, A.A.; Cox, D.P. Hydroxyl radical is produced via the Fenton reaction in submitochondrial particles under oxidative stress: Implications for diseases associated with iron accumulation. Redox Rep. Redox Rep. 2009, 14, 102–108. [Google Scholar] [CrossRef]
- Pavun, L.; Dimitric Markovic, J.M.; Ðurdevic, P.; Jelikic Stankov, M.; Ðikanovic, D.; Ciric, A.; Malesev, D. Development and validation of a fluorometric method for the determination of hesperidin in human plasma and pharmaceutical forms. J. Serb. Chem. Soc. 2012, 77, 1625–1640. [Google Scholar] [CrossRef]
- Choi, D.Y.; Lee, Y.J.; Hong, J.T.; Lee, H.J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer’s disease. Brain Res. Bull. 2012, 87, 144–153. [Google Scholar] [CrossRef]
- Dai, J.; Buijs, R.M.; Kamphorst, W.; Swaab, D.F. Impaired axonal transport of cortical neurons in Alzheimer’s disease is associated with neuropathological changes. Brain Res. 2002, 948, 1–2. [Google Scholar] [CrossRef]
- Geula, C.; Mesulam, M.M. Cholinesterases and the pathology of Alzheimer disease. Alzheimer Dis. Assoc. Disord. 1995, 9 (Suppl. 2), 23–28. [Google Scholar] [CrossRef]
- Zatta, P.; Ibn-Lkhayat-Idrissi, M.; Zambenedetti, P.; Kilyen, M.; Kiss, T. In vivo and in vitro effects of aluminum on the activity of mouse brain acetylcholinesterase. Brain Res. Bull. 2002, 59, 41–45. [Google Scholar] [CrossRef]
- Zatta, P.; Zambenedetti, P.; Bruna, V.; Filippi, B. Activation of acetylcholinesterase by aluminium(III): The relevance of the metal species. Neuroreport 1994, 5, 1777–1780. [Google Scholar] [CrossRef]
- Karimi, R. Biomedical & Pharmaceutical Sciences with Patient Care Correlations; Jones & Bartlett Learning: Burlington, MA, USA, 2015. [Google Scholar]






| Moisture Content | Water-Soluble Extractives | Alcohol-Soluble Extractives | Total Ash Content | Water-Insoluble Ash | Acid-Insoluble Ash | Sulphated Ash Content |
|---|---|---|---|---|---|---|
| 8.5 | 7 | 4 | 11 | 91 | 18.18 | 40 |
| Primary Metabolites | Total protein content % | 2.277 ± 0.007 |
| Total glycosaponins % | 68.033 ± 0.606 | |
| Secondary Metabolites | Total alkaloids content % | 1.27 ± 0.002 |
| Total polyphenolics % | 88.6 ± 0.032 | |
| Total flavonoids % | 36.327 ± 0.049 |
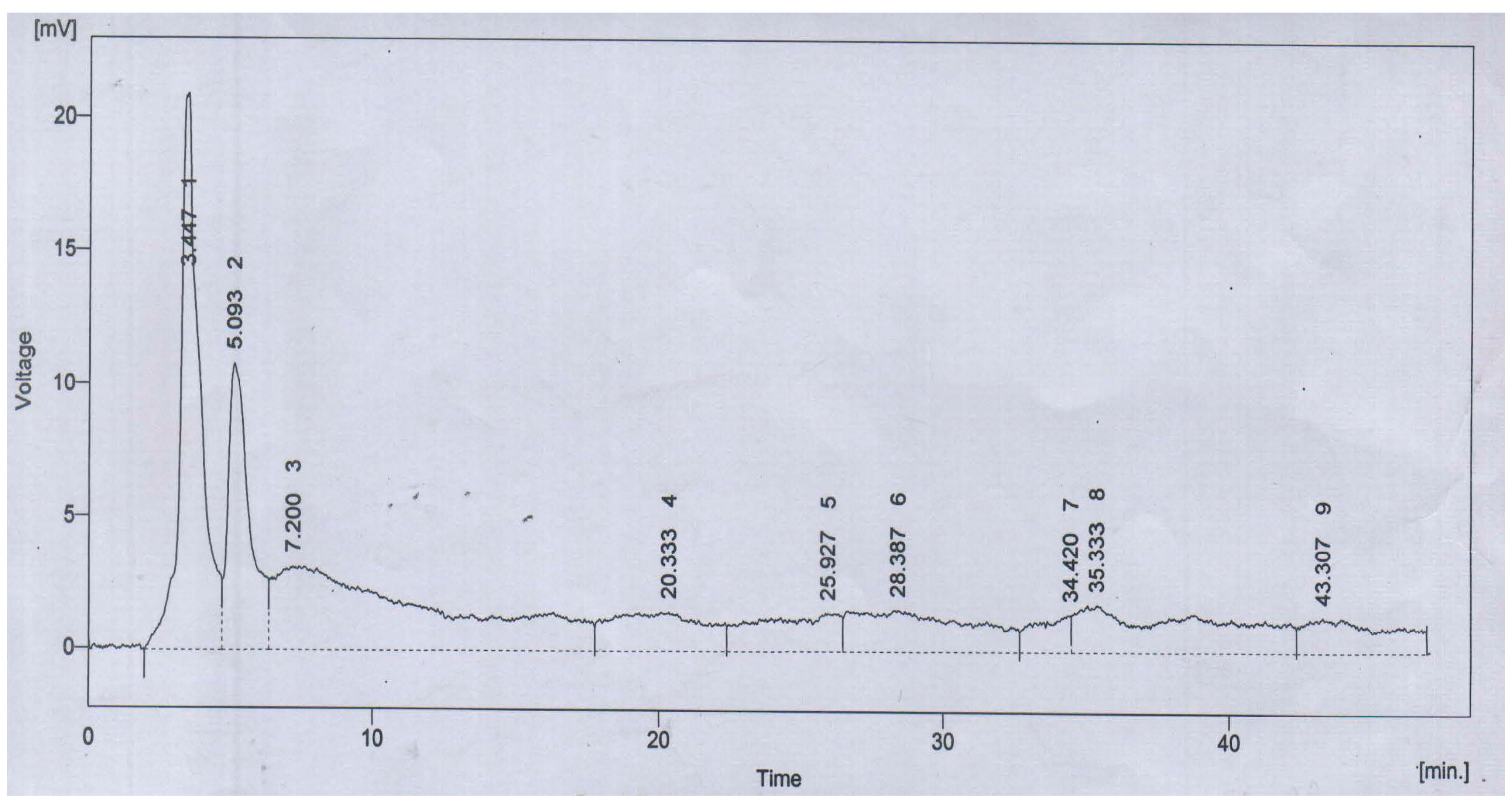
| Peak | Retention Time (Min.) | % Area | Compounds |
|---|---|---|---|
| 1 | 3.447 | 20.6 | Quercetin |
| 2 | 5.093 | 11.7 | Gallic acid |
| 4 | 20.333 | 6.9 | m-Coumaric acid |
| 5 | 25.927 | 5.9 | Sinapic acid |
| Groups | Treatment | DOSE (mg/kg) | GSH (µg/mg of Brain Tissue) | SOD (µg/mg of Brain Tissue) | CAT (µg/mg of Brain Tissue) |
|---|---|---|---|---|---|
| I | Normal control | N/A | 79.26 ± 1.09 | 27.49 ± 0.35 | 23.34 ± 0.83 |
| II | Disease control (AlCl3) | 100 | 21.01 ± 0.89 | 12.05 ± 0.61 | 4.39 ± 0.53 |
| III | Standard (STD) | 0.8 | 79.68 ± 2.27 *** | 24.68 ± 1.76 *** | 12.46 ± 1.09 *** |
| IV | ALE 100 | 100 | 65.56 ± 3.58 *** | 23.82 ± 0.98 *** | 9.64 ± 1.65 * |
| V | ALE 200 | 200 | 76.56 ± 1.06 *** | 24.73 ± 0.25 *** | 9.85 ± 0.67 * |
| VI | ALE 300 | 300 | 80.14 ± 2.55 *** | 26.43 ± 0.68 *** | 22.60 ±0.62 *** |
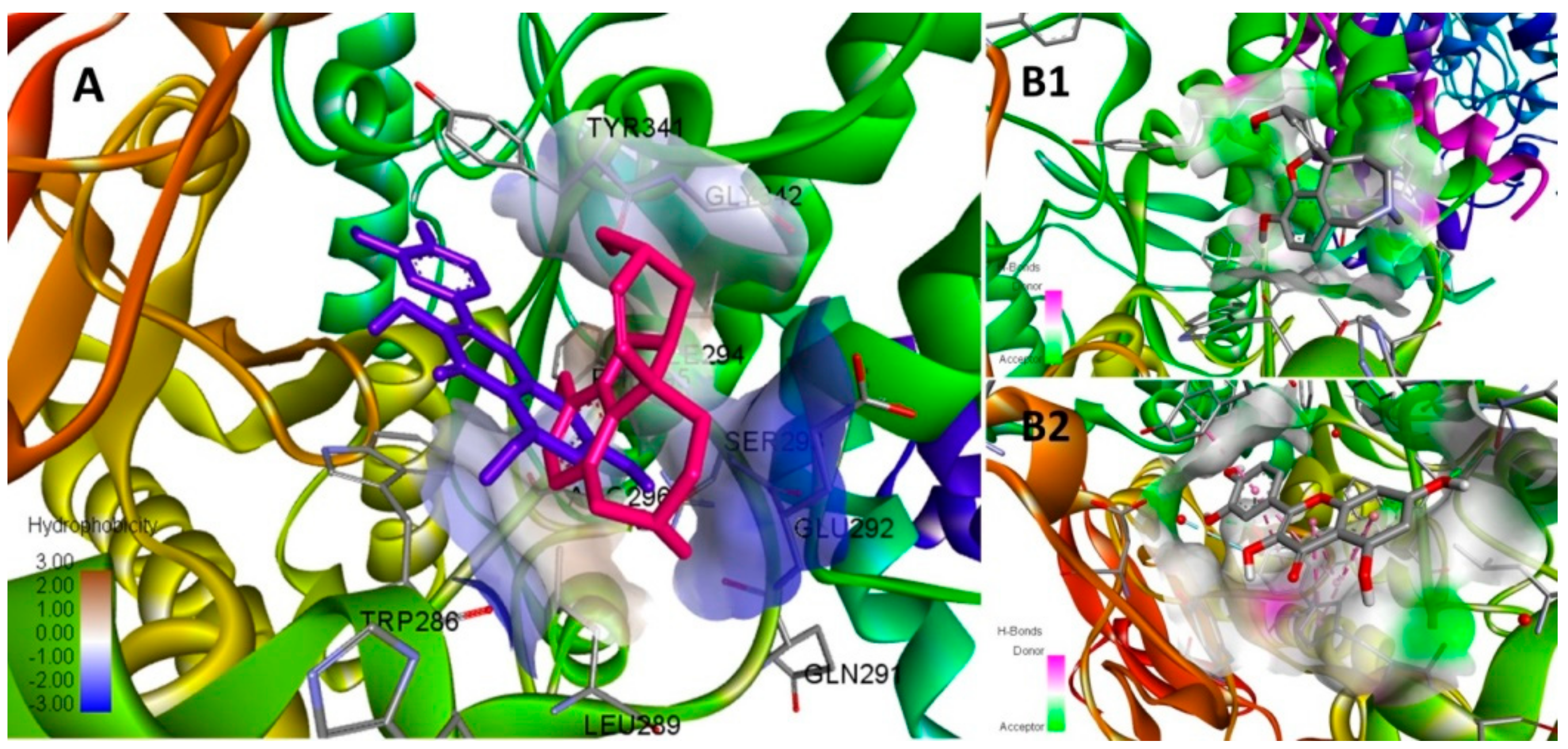
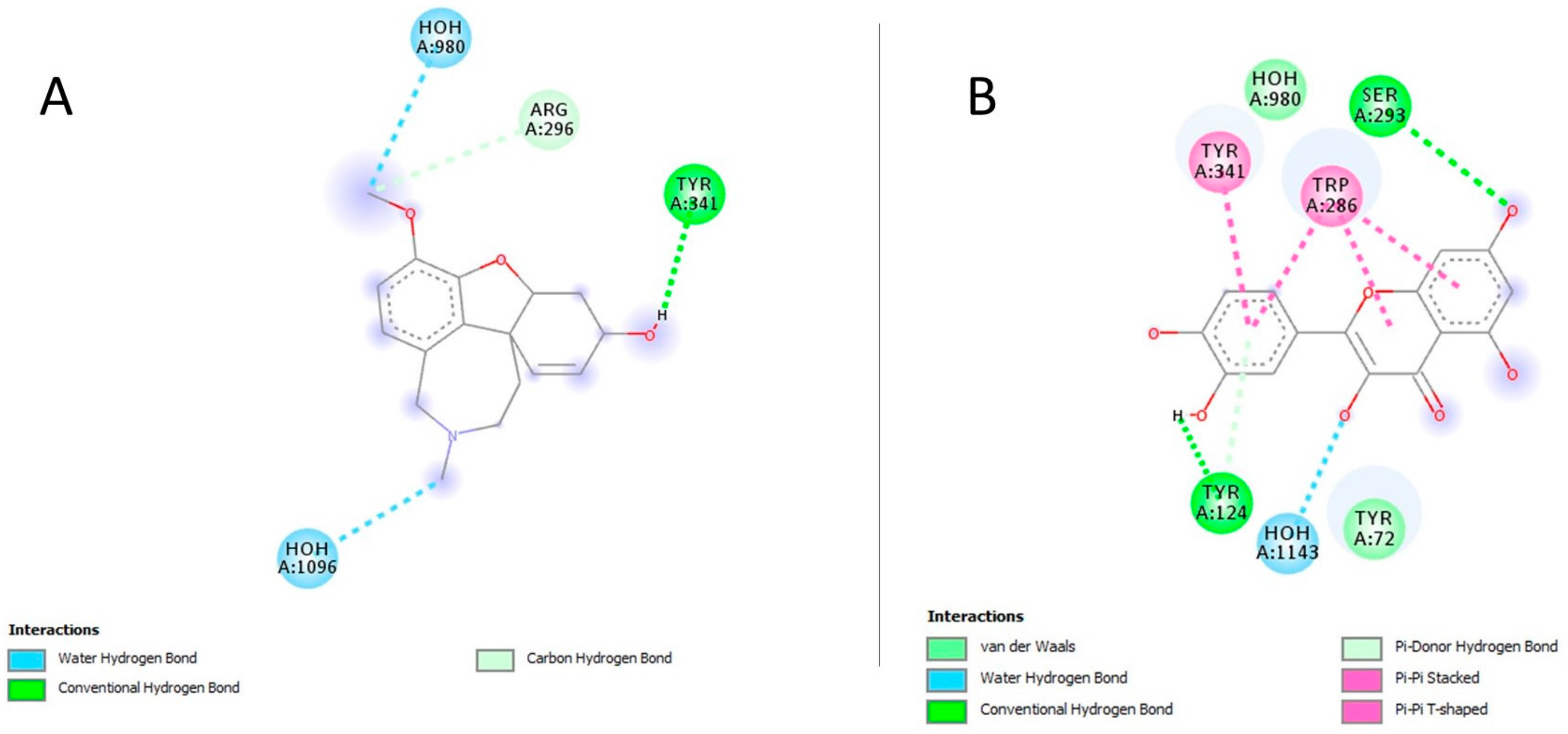
| Compound | Binding Energy (Kcal/mol) | Ki (mM) | Interacting Residues | Interaction Types |
|---|---|---|---|---|
| Galantamine | −5.8 | 0.056 | TYR341, ARG269 | H-Bonding |
| Quercetin | −8.3 | 0.000824 | TYR341, TRP286, SER293, TYR124, TYR72 | H-Bonding, π-π Stacked, van der Waals |
| m-Coumaric acid | −5.7 | 0.066 | LEU540, PRO537, LEU536, ASN533 | H-bonding, π-Alkyl |
| Gallic acid | −5.4 | 0.11 | TYR341, SER293 | H-Bonding |
| Sinapic acid | −5.3 | 0.13 | ASP349, SER347, GLY345, VAL343, GLY342 | H-bonding |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saleem, U.; Raza, Z.; Anwar, F.; Ahmad, B.; Hira, S.; Ali, T. Experimental and Computational Studies to Characterize and Evaluate the Therapeutic Effect of Albizia lebbeck (L.) Seeds in Alzheimer’s Disease. Medicina 2019, 55, 184. https://doi.org/10.3390/medicina55050184
Saleem U, Raza Z, Anwar F, Ahmad B, Hira S, Ali T. Experimental and Computational Studies to Characterize and Evaluate the Therapeutic Effect of Albizia lebbeck (L.) Seeds in Alzheimer’s Disease. Medicina. 2019; 55(5):184. https://doi.org/10.3390/medicina55050184
Chicago/Turabian StyleSaleem, Uzma, Zohaib Raza, Fareeha Anwar, Bashir Ahmad, Sundas Hira, and Tahir Ali. 2019. "Experimental and Computational Studies to Characterize and Evaluate the Therapeutic Effect of Albizia lebbeck (L.) Seeds in Alzheimer’s Disease" Medicina 55, no. 5: 184. https://doi.org/10.3390/medicina55050184
APA StyleSaleem, U., Raza, Z., Anwar, F., Ahmad, B., Hira, S., & Ali, T. (2019). Experimental and Computational Studies to Characterize and Evaluate the Therapeutic Effect of Albizia lebbeck (L.) Seeds in Alzheimer’s Disease. Medicina, 55(5), 184. https://doi.org/10.3390/medicina55050184





