The Effect of Oxidant Hypochlorous Acid on Platelet Aggregation and Dityrosine Concentration in Chronic Heart Failure Patients and Healthy Controls
Abstract
:1. Introduction
2. Material and Methods
2.1. Patients and Healthy Controls
2.2. Tests and Blood Sampling
2.3. HOCl Effect on Platelet Aggregation
2.4. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Patients and Controls
3.2. The Differences of Platelet Aggregation and Dityrosine Concentration between CHF and Control
3.3. The Differences of Platelet Aggregation and Dityrosine Concentration Between Chf Subgroups
3.4. Correlations among Platelet Aggregation, Dityrosine Concentration, LVEF, and NYHA Functional Class
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Palka, I.; Nessler, J.; Nessler, B.; Piwowarska, W.; Tracz, W.; Undas, A. Altered fibrin clot properties in patients with chronic heart failure and sinus rhythm: a novel prothrombotic mechanism. Heart 2010, 96, 1114–1118. [Google Scholar] [CrossRef] [Green Version]
- Paton, L.N.; Mocatta, T.J.; Richards, A.M.; Winterbourn, C.C. Increased thrombin-induced polymerization of fibrinogen associated with high protein carbonyl levels in plasma from patirnts post myocardial infarction. Free Radic. Biol. Med. 2010, 15, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Tiden, A.K.; Sjogren, T.; Svensson, M.; Berlind, A.; Senthilmohan, R.; Auchere, F.; Normsn, H.; Markgren, P.; Gustavsson, S.; Smidt, S.; et al. 2-Thioxanthines are mechanism-based inactivators of myeloperoxidase that block oxidative stress during inflammation. J. Biol. Chem. 2011, 286, 37578–37589. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Huang, Q. Haem-assisted dityrosine-cross-linking of fibrinogen under non-thermal plasma exposure: one important mechanism of facilitated blood coagulation. Sci. Rep. 2016, 6, 26982. [Google Scholar] [CrossRef]
- Nakajimaa, K.; Nakanao, T.; Tanakab, A. The oxidative modification hypothesis of atherosclerosis: the comparison of atherogenic effects on oxidised LDL and remnant lipoproteins in plasma. Clin. Chim. Acta. 2006, 367, 36–47. [Google Scholar] [CrossRef]
- Schrutka, L.; Distelmaier, K.; Hohensinner, P.; Sulzgruber, P.; Lang, I.M.; Maurer, G.; Wojta, J.; Hulsmann, M.; Niessner, A.; Koller, L. Impaired high-density lipoprotein anti-oxidative functions is associated with outcome in patients with chronic heart failure. J. Am. Heart Assoc. 2016, 5, e004169. [Google Scholar] [CrossRef] [PubMed]
- Badrnya, S.; Assinger, A.; Volf, I. Native HDL interfere with platelet activation induced by oxLDL. Int. J. Mol. Sci. 2013, 14, 10107–10121. [Google Scholar] [CrossRef] [PubMed]
- Assinger, A.; Koller, F.; Schmid, W.; Zellner, M.; Babeluk, R.; Koller, E.; Volf, I. Specific binding of hypochlorite-oxidized HDL to platelet CD 36 triggers proinflammatory and procoagulant effects. Atherosclerosis 2010, 212, 153–160. [Google Scholar] [CrossRef]
- Chan, L.; Luo, X.; Ni, H.; Shi, H.; Liu, L.; Wen, Z.; Gu, X.; Qiao, J.; Li, J. High levels of LDL-C combined with low levels of HDL-C futher increase platelet activation in hypercholesterolemic patients. Braz. J. Med. Biol. Res. 2015, 48, 167–173. [Google Scholar] [CrossRef]
- Kaplan, Z.S.; Jackson, S.P. The role of platelets in atherothrombosis. Hematology 2011, 2011, 51–61. [Google Scholar] [CrossRef]
- Magwenzi, S.; Woodward, C.; Wrait, S.; Aburima, A.; Raslan, Z.; Jones, H.; McNeil, C.; Weatcroft, S.; Yuldasheva, N.; Febbriao, M.; et al. Oxidized LDL activates blood platelets through CD36/NOX2-mediated inhibition of the cGMP/protein kinase G signalling cascade. Blood 2015, 125, 2693–2703. [Google Scholar] [CrossRef]
- Štikarova, J.; Kotlin, R.; Riedel, T.; Suttnar, J.; Pimkova, K. The effect of reagents mimicking oxidative stress on fibrinogen function. ScientificWorldJournal 2013, 2013, 359621. [Google Scholar] [CrossRef] [PubMed]
- Schildknecht, S.; van der Loo, B.; Weber, K.; Tiefenthaler, K.; Daiber, A.; Bachschmid, M. Endogenous peroxynitrite modulates PGHS-1-dependent thromboxane A2 formation and aggregation in human platelets. Free Radic. Biol. Med. 2008, 45, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Zbikowska, H.; Ponczek, M.; Kolodziejczyk, J.; Wachowicz, B. Different vulnerability of fibrinogen subunits to oxidative/nitrative modifications induced by peroxynitrite: functional consequenses. Thromb. Res. 2007, 121, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Tetik, S.; Kaya, K.; Yardimici, T. Effect of oxidized fibrinogen on hemostatic system: in vitro study. Clin. Appl. Thromb. Hemost. 2011, 17, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Parastatidis, I.; Thomson, L.; Burke, A.; Chernysh, I.; Nagaswami, C.; Visser, J.; Stamer, S.; Liebler, D.; Koliakos, G.; Heijnen, H.; et al. Fibrinogen β-chain tyrosine nitration is a prothrombotic risk factor. J. Biol. Chem. 2008, 283, 33846–33853. [Google Scholar] [CrossRef] [PubMed]
- Dickstein, K.; Cohen-Solal, A.; Filippatos, G.; McMurray, J.V.; Ponikowski, P.; Poole-Wilson, P.A.; Stromberg, A.; van Veldhuisen, D.J.; Atar, D.; Hoes, A.V.; et al. The task orce or the diagnosis and treatment of acute and chronic heart failure 2008 of the european society of cardiology. Eur. Heart. J. 2008, 29, 2388–2442. [Google Scholar] [PubMed]
- Mongirdienė, A.; Kuršvietienė, L.; Kašauskas, A. The coagulation system changes in patients with chronic heart failure. Medicina (Kaunas) 2010, 46, 642–647. [Google Scholar] [CrossRef]
- Born, G.V.R. Aggregation of blood platelets by adenosine diphosphate and its reversal. Nature 1962, 194, 927–929. [Google Scholar] [CrossRef] [PubMed]
- Mironchuk, N.N.; Mirsaeva, G.K.; Fazlyev, M.M. Features of vascular-platelet hemostasis in patients with chronic heart failure with cardio-renal syndrome. Med. Sci. 2013, 5, 333–338. [Google Scholar]
- Mongirdienė, A. Changes in parameters of coagulation system after treatment of chronic heart failure with β-adrenoblocers, angiotenzin-converting enzyme inhibitors, calcium antagonists, diuretics, anticoagulans, antiagregants, cardiac glycosides and exercise. Medicina (Kaunas) 2011, 47, 238–243. [Google Scholar]
- Konkle, A.B. Acquired disorders of platelet function. Hematology 2011, 1, 391–396. [Google Scholar] [CrossRef]
- Gibbs, C.R.; Blann, A.D.; Watson, R.D.; Lip, G.Y. Abnormalities of hemorheological, endothelial and platelet function in patients with chronic heart failure in sinus rythm. Circulation 2001, 103, 1746–1751. [Google Scholar] [CrossRef]
- Ghatak, A.; Brar, M.J.; Agarwal, A.; Goel, N.; Rastogi, A.K.; Vaish, A.K.; Sircar, A.R.; Chandra, M. Oxy free radical system in heart failure and therapeutic role of oral vitamin E. Int. J. Cardiol. 1996, 57, 119–127. [Google Scholar] [CrossRef]
- Mallat, Z.; Philip, I.; Lebret, M.; Chatel, D.; Maclouf, J.; Tedgui, A. Elevated levels of 8-iso-prostaglandin F2alpha in pericardial fluid of patients with heart failure: a potential role for in vivo oxidant stress in ventricular dilatation and progression to heart failure. Circulation 1998, 97, 1536–1539. [Google Scholar] [CrossRef] [PubMed]
- Banfi, C.; Brioschi, M.; Barcella, S.; Veglia, F.; Biglioli, P.; Tremoli, E.; Agostoni, P. Oxidised proteins in plasma of patients with heart failure: role in endothelial damage. Eur. J. Heart Fail. 2008, 10, 244–251. [Google Scholar] [CrossRef]
- Nergiz-Unal, R.; Lamers, M.M.; van Kruchten, R.; Luiken, J.J.; Cosemans, J.M.; Glatz, J.F.; Kuijpers, M.J.; Heemskerk, J.W. Signaling role of CD36 in platelet activation and thrombus formation on immobilized thrombospondin or oxidized low-density lipoprotein. J. Thromb. Haemost. 2011, 9, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Farady, N.; Schapf, R.B.; Doddo-o, J.M.; Martinez, E.A.; Rosenfeld, B.A.; Dorman, T. Leikocytes can enhance platelet-mediated aggregation and thromboxane release via interaction of P-selectin glykoprotein ligand 1 with P-selectin. Anesthesiology 2001, 94, 145–151. [Google Scholar] [CrossRef]
- Dalle-Donne, I.; Guistarini, D.; Colombo, R.; Rossi, R.; Milzani, A. Protein carbonilation in human diseases. Trends Mol. Med. 2003, 9, 169–176. [Google Scholar] [CrossRef]
- Azizova, O.A.; Maksyanina, E.V.; Romanov, Y.A.; Aseichev, A.V.; Scheglovitova, O.N. Fibrinogen and its oxidised form induce interleukin-8 production in cultured endothelial cells of human vessels. Bull. Exp. Biol. Med. 2004, 137, 358–360. [Google Scholar] [CrossRef] [PubMed]
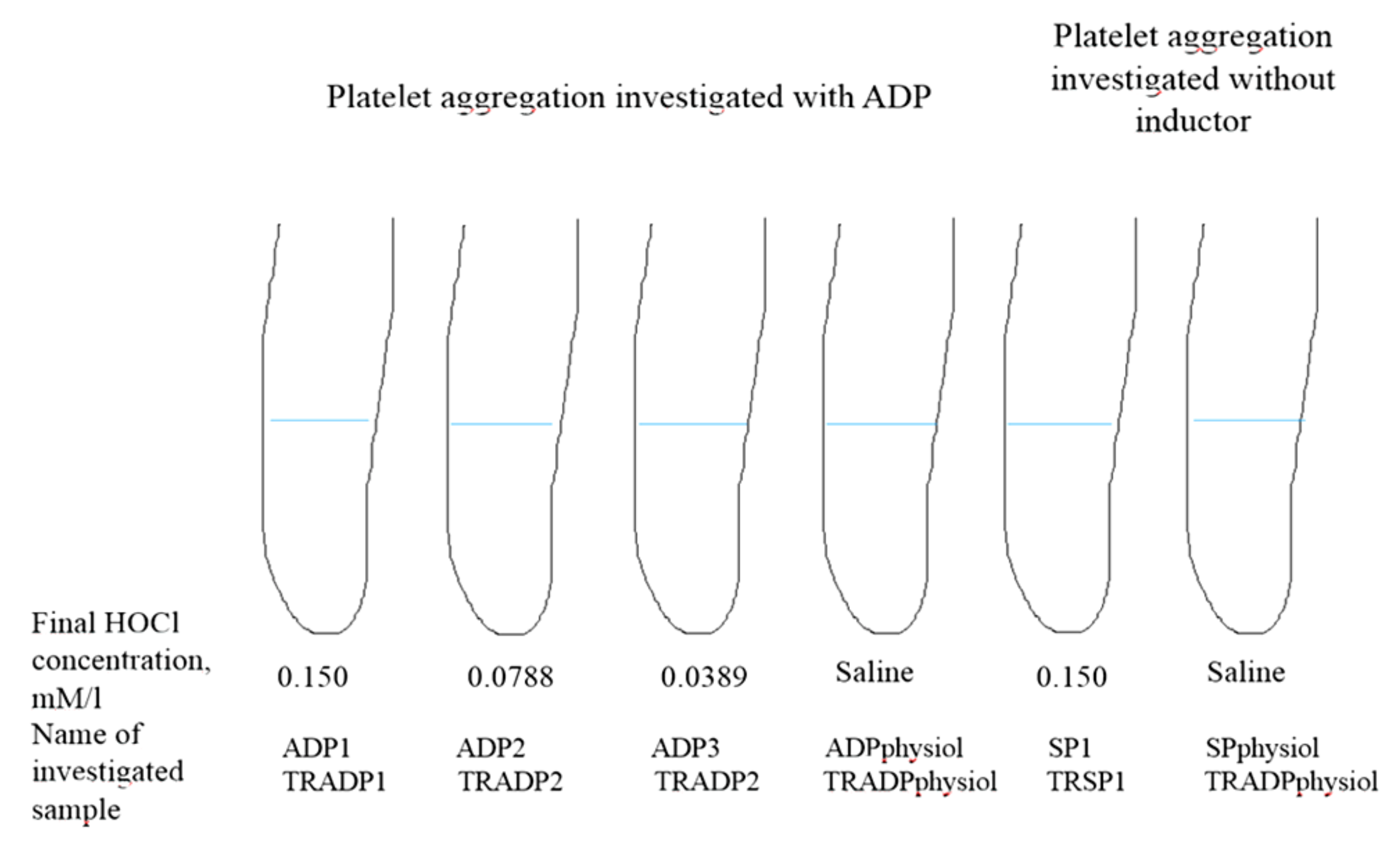
| Clinical Variables | II NYHA (n = 21) | III NYHA (n = 24) | IV NYHA (n = 22) | Healthy Controls (n = 31) | p-Value |
|---|---|---|---|---|---|
| Age, years, mean ± SD | 65.19 ± 13.23‡ | 65.0 ± 13.24‡ | 61.39 ± 13.57 | 55.42 ± 8.88 | 0.024 |
| Left ventricular ejection fraction, %, mean ± SD | 44.19 ± 5.81ǂ | 39.00 ± 10.86* | 26.50 ± 13.73 | 56.94 ± 3.08‡ | <0.001 |
| 6-min walking test, m, mean ± SD | 334 ± 41ǂ | 324 ± 39+ | 222 ± 79* | 573 ± 85‡ | <0.001 |
| Platelet count, × 109/L, mean ± SD | 199 ± 54.21 | 199.38 ± 47.30 | 208.44 ± 61.33 | 217.61 ± 47.7 | 0.033 |
| Platelet volume, fl, mean ± SD | 10.57 ± 1.1 | 11.24 ± 1.03 | 11.03 ± 1.0 | 8.7 ± 1.01‡ | <0.001 |
| Diuretics, n | 9 | 18 | 17 | 0 | |
| Beta-blockers, n | 16 | 21 | 11 | 0 | |
| ACE-inhibitors, n | 15 | 21 | 14 | 0 | |
| Nitrates, n | 5 | 11 | 11 | 0 | |
| Digoxin, n | 1 | 3 | 4 | 0 | |
| Statines, n | 12 | 11 | 7 | 0 | |
| Heparine, n | 6 | 11 | 11 | 0 | |
| Calcium channel blockers, n | 1 | 0 | 1 | 0 | |
| Thrombosis, n | 0 | 0 | 3 | 0 | |
| Aflt/Afib, n | 7 | 14 | 13 | 0 | |
| Renal failure, n | 2 | 5 | 12 | 0 | |
| Obesity, n | 11 | 10 | 11 | 0 | |
| Dyslipidemia, n | 11 | 10 | 9 | 0 | |
| Cardiovascular diseases, n | 10 | 11 | 12 | 0 |
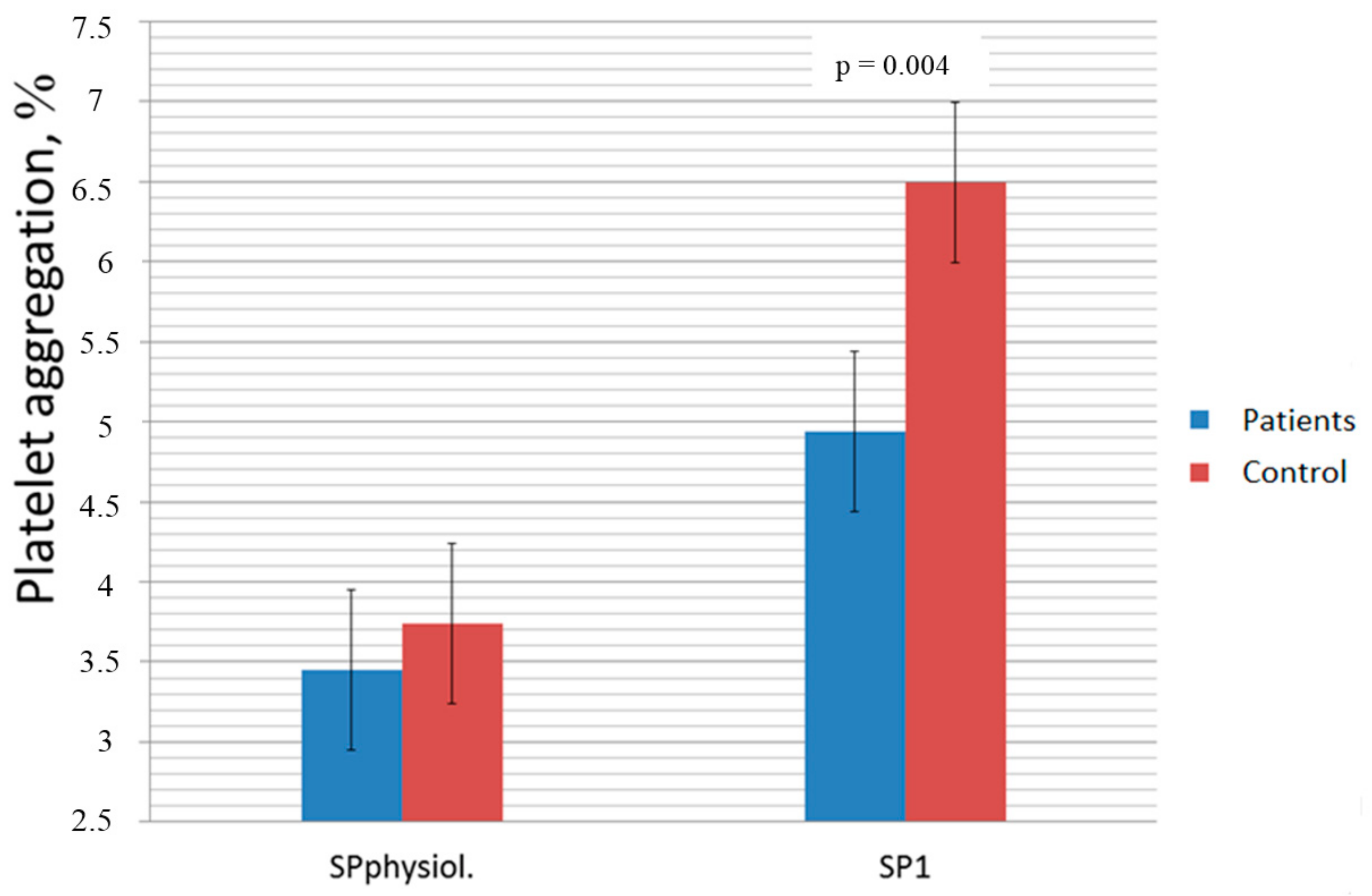
| Used Oxidant Concentration, mmol/L | CHF Patients (n = 67) | Healthy Controls (n = 31) | ||||
|---|---|---|---|---|---|---|
| Platelet Aggregation, Induced with ADP (%, mean ± SD) | Increase of Platelet Aggregation in Samples with Oxidant, % | p-Value | Platelet Aggregation, Induced with ADP (%, Mean ± SD) | Increase of Platelet Aggregation in Samples with Oxidant, % | p-Value | |
| Saline | 57.52 ± 19.77 | 66.3 ± 9.63 | ||||
| 0.0389 (III) | 66.55 ± 21.56 | 15 | <0.001 | 78.94 ± 14.31 | 14 | 0.005 |
| 0.0778 (II) | 58.64 ± 18.54 | 2 | 0.74 | 67.16 ± 10.96 | 2 | 0.45 |
| 0.15 (1) | 69.10 ± 21.06 | 19 | <0.001 | 81.61 ± 13.26 | 20 | 0.005 |
| Used Oxidant Concentration, mmol/L or Saline | Dityrosine Concentration in Plasma (Relative Units of Fluorescence) (Mean ± SD) | p-Value | |
|---|---|---|---|
| CHF Patients (n = 67) | Healthy Controls (n = 31) | ||
| Saline | 1.54 ± 0.48 | 1.27 ± 0.53 | 0.049 |
| 0.0389 (III) | 1.56 ± 0.49 | 1.34 ± 0.40 | 0.032 |
| 0.0778 (II) | 1.55 ± 0.51 | 1.55 ± 0.38 | 0.310 |
| 0.15 (1) | 1.73 ± 0.54 | 1.82 ± 0.48 | 0.025 |
| Used Oxidant Concentration, mmol/L or Saline | Platelet Aggregation, Induced with ADP in Samples with Oxidant or Saline Added (mean ± SD) | Reliability of Data Differences between Samples of Patients Subgroups (p-Value) | ||||
|---|---|---|---|---|---|---|
| NYHA II | NYHA III | NYHA IV | NYHA II and NNYHA III | NYHA III and NYHA IV | NYHA II and NYHA IV | |
| Saline | 56.13 ± 12.57 | 61.75 ± 22.44 | 53.11 ± 21.04 | 0.001 | NS | 0.056 |
| 0.0389 (III) | 64.88 ± 12.36 | 71.5 ± 23.49 | 61.44 ± 24.75 | <0.001 | NS | 0.012 |
| 0.0778 (II) | 55.69 ± 11.22 | 64.21 ± 20.44 | 53.83 ± 20.0 | <0.001 | NS | 0.028 |
| 0.15 (1) | 66.81 ± 11.79 | 74.58 ± 23.31 | 63.83 ± 23.52 | <0.001 | NS | 0.012 |
| Used Oxidant Concentration, mmol/L or Saline | Dityrosine Concentration in Plasma with Oxidant or Saline Added (mean ± SD) | Reliability of Data Differences between Samples of Patients Subgroups (p-Value) | ||||
|---|---|---|---|---|---|---|
| NYHA II | NYHA III | NYHA IV | NYHA II and NNYHA III | NYHA III and NYHA IV | NYHA II and NYHA IV | |
| Saline | 1.50 ± 0.51 | 1.47 ± 0.49 | 1.71 ± 0.46 | NS | 0.237 | 0.037 |
| 0.0389 (III) | 1.56 ± 0.51 | 1.42 ± 0.40 | 1.63 ± 0.52 | NS | 0.260 | 0.680 |
| 0.0778 (II) | 1.45 ± 0.39 | 1.38 ± 0.44 | 1.09 ± 0.65 | NS | 0.023 | 0.048 |
| 0.15 (1) | 1.67 ± 0.57 | 1.58 ± 0.58 | 2.11 ± 0.43 | NS | 0.014 | 0.066 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mongirdienė, A.; Laukaitienė, J.; Skipskis, V.; Kašauskas, A. The Effect of Oxidant Hypochlorous Acid on Platelet Aggregation and Dityrosine Concentration in Chronic Heart Failure Patients and Healthy Controls. Medicina 2019, 55, 198. https://doi.org/10.3390/medicina55050198
Mongirdienė A, Laukaitienė J, Skipskis V, Kašauskas A. The Effect of Oxidant Hypochlorous Acid on Platelet Aggregation and Dityrosine Concentration in Chronic Heart Failure Patients and Healthy Controls. Medicina. 2019; 55(5):198. https://doi.org/10.3390/medicina55050198
Chicago/Turabian StyleMongirdienė, Aušra, Jolanta Laukaitienė, Vilius Skipskis, and Artūras Kašauskas. 2019. "The Effect of Oxidant Hypochlorous Acid on Platelet Aggregation and Dityrosine Concentration in Chronic Heart Failure Patients and Healthy Controls" Medicina 55, no. 5: 198. https://doi.org/10.3390/medicina55050198
APA StyleMongirdienė, A., Laukaitienė, J., Skipskis, V., & Kašauskas, A. (2019). The Effect of Oxidant Hypochlorous Acid on Platelet Aggregation and Dityrosine Concentration in Chronic Heart Failure Patients and Healthy Controls. Medicina, 55(5), 198. https://doi.org/10.3390/medicina55050198





