Evaluation of Atrial Electromechanical Delay in Children with Obesity
Abstract
:1. Introduction
2. Materials and Method
2.1. Study Population
2.2. Electrocardiography (ECG) and P Wave Dispersion (PWD) Measurement
2.3. Standard Echocardiography
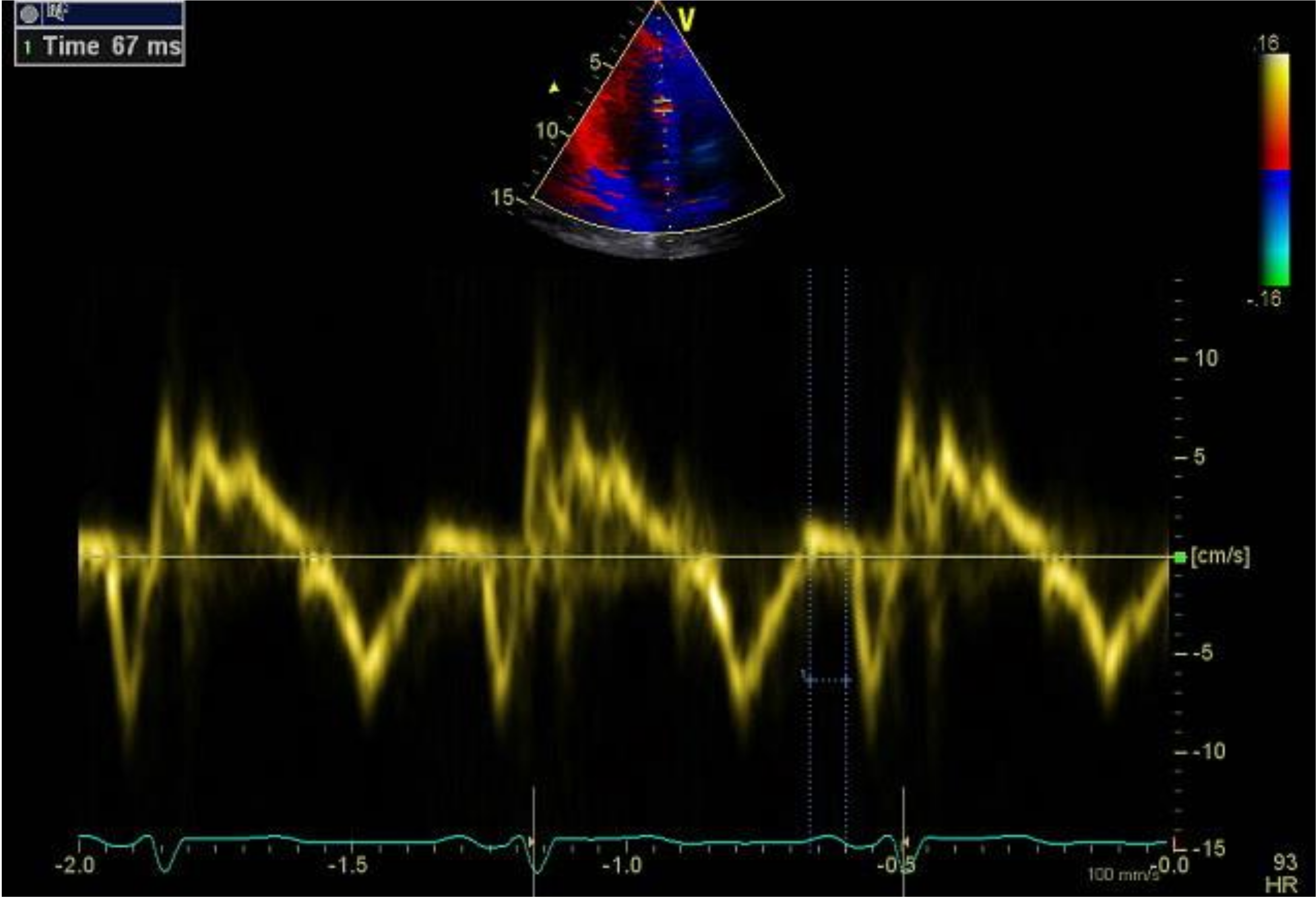
2.4. Tissue Doppler Echocardiography (TDE)
2.5. Ethics Statement
2.6. Statistical Analysis
3. Results
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ogden, C.L.; Carroll, M.D.; Kit, B.K.; Flegal, K.M. Prevalence of childhood and adult obesity in the United States. JAMA 2014, 311, 806–814. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.S.; Bradley, L.J.; Brolin, R.E. Gastric bypass surgery in adolescents with morbid obesity. J. Pediatr. 2001, 138, 499–504. [Google Scholar] [CrossRef]
- Roberto, C.A.; Swinburn, B.; Hawkes, C.; Huang, T.K.; Costa, S.A.; Ashe, M.; Zwicker, M.; Cawley, J.H.; Brownell, K. Patchy progress on obesity prevention: Emerging examples, entrenched barriers, and new thinking. Lancet 2015, 385, 2400–2409. [Google Scholar] [CrossRef]
- Singh, G.M.; Danaei, G.; Farzadfar, F.; Stevens, G.A.; Woodward, M.; Wormser, D.; Kaptoge, S.; Whitlock, G.; Qiao, Q.; Lewington, S.; et al. The age-specific quantitative effects of metabolic risk factors on cardiovasculardiseases and diabetes: A pooled analysis. PLoS ONE 2013, 8, 65174. [Google Scholar] [CrossRef]
- Emerging Risk Factors Collaboration; Wormser, D.; Kaptoge, S.; Di Angelantonio, E.; Wood, A.M.; Pennels, L.; Thompson, A.; Sarwar, N.; Kizer, J.R.; Lawlor, D.A.; et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: Collaborative analysis of 58 prospective studies. Lancet 2011, 377, 1085–1095. [Google Scholar] [CrossRef]
- Lauby-Secretan, B.; Scoccianti, C.; Loomis, D.; Grosse, Y.; Bianchini, F.; Straif, K. Body fatness and cancer — viewpoint of the IARC Working Group. N. Engl. J. Med. 2016, 375, 794–798. [Google Scholar] [CrossRef]
- Jiang, L.; Rong, J.; Wang, Y.; Hu, F.; Bao, C.; Li, X.; Zhao, Y. The relationship between body mass index and hip osteoarthritis: A systematic review and meta-analysis. Jt. Bone Spine 2011, 78, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Tian, W.; Wang, Y.; Rong, J.; Bao, C.; Liu, Y.; Zhao, Y.; Wang, C. Body mass index and susceptibility to knee osteoarthritis: A systematic review and metaanalysis. Jt. Bone Spine 2012, 79, 291–297. [Google Scholar] [CrossRef]
- Baker, J.L.; Olsen, L.W.; Sørensen, T.I.A. Childhood body-mass indexand the risk of coronary heart disease in adulthood. N. Engl. J. Med. 2007, 357, 2329–2337. [Google Scholar]
- Must, A.; Jacques, P.F.; Dallal, G.E.; Bajema, C.J.; Dietz, W.H. Long-term morbidity and mortality of overweight adolescents—a follow-up of the Harvard Growth Study of 1922 to 1935. N. Engl. J. Med. 1992, 327, 1350–1355. [Google Scholar] [CrossRef]
- Franks, P.W.; Hanson, R.L.; Knowler, W.C.; Sievers, M.L.; Bennett, P.H.; Looker, H.C. Childhood obesity, other cardiovascular risk factors, and premature death. N. Engl. J. Med. 2010, 362, 485–493. [Google Scholar] [CrossRef]
- Engeland, A.; Bjørge, T.; Søgaard, A.J.; Tverdal, A. Body mass index in adolescence in relation to total mortality: 32-year follow-up of of 227,000 Norwegian boys and girls. Am. J. Epidemiol. 2003, 157, 517–523. [Google Scholar] [CrossRef]
- Schmidt, M.; Bøtker, H.E.; Pedersen, L.; Sørensen, H.T. Young adulthood obesity and risk of acute coronary syndromes, stable angina pectoris, and congestive heart failure: A 36-year cohort study. Ann. Epidemiol. 2014, 24, 356–361. [Google Scholar] [CrossRef]
- Sommer, A.; Twig, G. The impact of childhood and adolescent obesity on cardiovascular risk in adulthood: A systematic review. Curr. Diab. Rep. 2018, 18, 91. [Google Scholar] [CrossRef]
- El-Assaad, I.; Al-Kindi, S.G.; Saarel, E.V.; Aziz, P.F. Lone pediatric atrial fibrillation in the United States: Analysis of over 1500 cases. Pediatr. Cardiol. 2017, 38, 1004–1009. [Google Scholar] [CrossRef]
- Pérez-Riera, A.R.; de Abreu, L.C.; Barbosa-Barros, R.; Grindler, J.; Fernandes-Cardoso, A.; Baranchuk, A. P-wave dispersion: An update. Indian Pacing Electrophysiol. J. 2016, 16, 126–133. [Google Scholar] [CrossRef]
- Yasar, A.S.; Bilen, E.; Bilge, M.; Ipek, G.; Ipek, E.; Kirbas, O. P-wave duration and dispersion in patients with metabolic syndrome. Pacing Clin. Electrophysiol. 2009, 32, 1168–1172. [Google Scholar] [CrossRef]
- Yazici, M.; Ozdemir, K.; Altunkeser, B.B.; Kayrak, M.; Duzenli, M.A.; Vatankulu, M.A.; Soylu, A.; Ulgen, M.S. The effect of diabetes mellitus on the P-wave dispersion. Circ. J. 2007, 71, 880–883. [Google Scholar] [CrossRef]
- Kosar, F.; Aksoy, Y.; Ari, F.; Keskin, L.; Sahin, I. P-wave duration and dispersion in obese subjects. Ann. Noninvasive Electrocardiol. 2008, 13, 3–7. [Google Scholar] [CrossRef]
- Gunes, H.; Sokmen, A.; Kaya, H.; Gungor, O.; Kerkutluoglu, M.; Guzel, F.B.; Sokmen, G. Evaluation of atrial electromechanical delay to predict atrial fibrillation in hemodialysis patients. Medicina 2018, 54, E58. [Google Scholar] [CrossRef]
- Rago, A.; Russo, V.; Papa, A.A.; Ciardiello, C.; Pannone, B.; Mayer, M.C.; Cimmino, G.; Nigro, G. The role of the atrial electromechanical delay in predicting atrial fibrillation in beta-thalassemia major patients. J. Interv. Card Electrophysiol. 2017, 48, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.E. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120, 164–192. [Google Scholar] [CrossRef]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar] [PubMed]
- Bilgin, M.; Yıldız, B.S.; Tülüce, K.; Gül, İ.; Alkan, M.B.; Sayın, A.; İslamlı, A.; Efe, N.T.H.; Alihanoğlu, Y.İ.; Zoghi, M.; et al. Evaluating functional capacity, and mortality effects in the presence of atrial electromechanical conduction delay in patients with systolic heart failure. Anatol. J. Cardiol. 2016, 16, 579–586. [Google Scholar] [CrossRef]
- Sokmen, A.; Acar, G.; Sokmen, G.; Akcay, A.; Akkoyun, M.; Koroglu, S.; Nacar, A.B.; Ozkaya, M. Evaluation of atrial electromechanical delay and diastolic functions in patients with hyperthyroidism. Echocardiography 2013, 30, 1194–1201. [Google Scholar] [CrossRef] [PubMed]
- Tomás, E.; Lin, Y.S.; Dagher, Z.; Saha, A.; Luo, Z.; Ido, Y.; Ruderman, N.B. Hyperglycemia and insulin resistance: Possible mechanisms. Ann. N.Y. Acad. Sci. 2002, 967, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Bergmeier, W. Sugar makes neutrophils RAGE: Linking diabetes-associated hyperglycemia to thrombocytosis and platelet reactivity. J. Clin. Invest. 2017, 127, 2040–2043. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.J.; Parise, H.; Levy, D.; D’Agostino, R.B., Sr.; Wolf, P.A.; Vasan, R.S.; Benjamin, E.J. Obesity and the risk of new onset atrial fibrillation. JAMA 2004, 292, 2471–2477. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.; Hune, L.J.; Vestergaard, P. Overweight and obesity as risk factors for atrial fibrillation or flutter: The Danish Diet, Cancer, and Health Study. Am. J. Med. 2005, 118, 489–495. [Google Scholar] [CrossRef]
- Mahajan, R.; Lau, D.H.; Sanders, P. Impact of obesity on cardiac metabolism, fibrosis, and function. Trends Cardiovasc. Med. 2014, 14, 1050–1738. [Google Scholar] [CrossRef]
- Walsh, E.P.; Cecchin, F. Arrhythmias in adult patients with congenital heart disease. Circulation 2007, 115, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Botker, H.E.; Pedersen, L.; Sorensen, H.T. Comparison of the frequency of atrial fibrillation in young obese versus young nonobese men undergoing examination for fitness for military service. Am. J. Cardiol. 2014, 113, 822–826. [Google Scholar] [CrossRef] [PubMed]
- Cohoon, K.P.; Mazur, M.; McBane, R.D.; Ketha, S.; Ammash, N.; Wysokinski, W.E. The impact of gender and left atrial blood stasis on adiponectin levels in non-valvular atrial fibrillation. Int. J. Cardiol. 2015, 181, 207–212. [Google Scholar] [CrossRef] [PubMed]
- Kirchhof, P.; Benussi, S.; Kotecha, D.; Ahlsson, A.; Atar, D.; Casadei, B.; Castella, M.; Diener, H.C.; Heidbuchel, H.; Hendriks, J.; et al. ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur. Heart J. 2016, 37, 2893–2962. [Google Scholar] [CrossRef] [PubMed]
- Erdem, F.H.; Ozturk, S.; Baltaci, D.; Donmez, I.; Alçelik, A.; Ayhan, S.; Yaz, M. Detection of atrial electromechanical dysfunction in obesity. Acta Cardiol. 2015, 70, 678–684. [Google Scholar] [CrossRef]
- Yagmur, J.; Cansel, M.; Acikgoz, N.; Ermis, N.; Yagmur, M.; Atas, H.; Tasolar, H.; Karakus, Y.; Pekdemir, H.; Ozdemir, R. Assessment of atrial electromechanical delay by tissue Doppler echocardiography in obese subjects. Obesity 2011, 19, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Üner, A.; Doğan, M.; Epcacan, Z.; Epçaçan, S. The effect of childhood obesity on cardiac functions. J. Pediatr. Endocrinol. Metab. 2014, 27, 261–271. [Google Scholar] [CrossRef]

| Obese Subjects (n = 59) | Non-Obese Subjects (n = 38) | p | |
|---|---|---|---|
| Age, mean ± SD, years | 13.4 ± 2,7 | 13.4 ± 2.9 | 0.963 |
| Male/female, n | 22/37 | 7/31 | 0.079 |
| Body mass index, mean ± SD | 31.5 ± 8.5 | 19.6 ± 4.3 | <0.001 |
| Heart rate, mean ± SD, beats/min | 73 ± 8 | 72 ± 6 | 0.132 |
| Blood glucose, mean ± SD, mg/dL | 88 ± 8 | 84 ± 8 | 0.016 |
| ALT, median (IQR), U/L | 22 (17–33) | 16 (12–23) | 0.154 |
| AST, median (IQR), U/L | 24 (20–28) | 24 (20–30) | 0.114 |
| Urea, mean ± SD, mg/dL | 10.1 ± 2.3 | 10 ± 2.1 | 0.772 |
| Total protein, mean ± SD, g/dL | 7.3 ± 1.1 | 7.0 ± 0.7 | 0.160 |
| Hemoglobin, mean ± SD, g/dL | 13.6 ± 1.1 | 13.3 ± 1.5 | 0.364 |
| Platelet count, mean ± SD, 103/mm3 | 333 ± 84 | 292 ± 83 | 0.022 |
| RDW, mean ± SD, % | 14.1 ± 3.3 | 13.5 ± 1.1 | 0.288 |
| LV ejection fraction, mean ± SD, % | 70 ± 2 | 70 ± 2 | 0.883 |
| IVS, mean ± SD, mm | 8.0 ± 0.2 | 7.2 ± 0.2 | 0.132 |
| Posterior wall thickness, mean ± SD, mm | 7.9 ± 0.2 | 7.4 ± 0.2 | 0.232 |
| Mitral E velocity, mean ± SD, cm/s | 95 ± 14 | 94 ± 14 | 0.924 |
| Mitral A velocity, mean ± SD, cm/s | 66 ± 17 | 63 ± 17 | 0.440 |
| E/A, mean ± SD | 1.5 ± 0.3 | 1.4 ± 0.3 | 0.187 |
| Left atrial diameter, mean ± SD, cm | 3.1 ± 0.4 | 3.0 ± 0.3 | 0.516 |
| Obese Subjects (n = 59) | Non-Obese Subjects (n = 38) | p | |
|---|---|---|---|
| PA Septum, mean ± SD, ms | 45 ± 6 | 41 ± 5 | 0.001 |
| PA Lateral, mean ± SD, ms | 60 ± 8 | 52 ± 7 | <0.001 |
| PA Tricuspid, mean ± SD, ms | 37 ± 5 | 36 ± 5 | 0.299 |
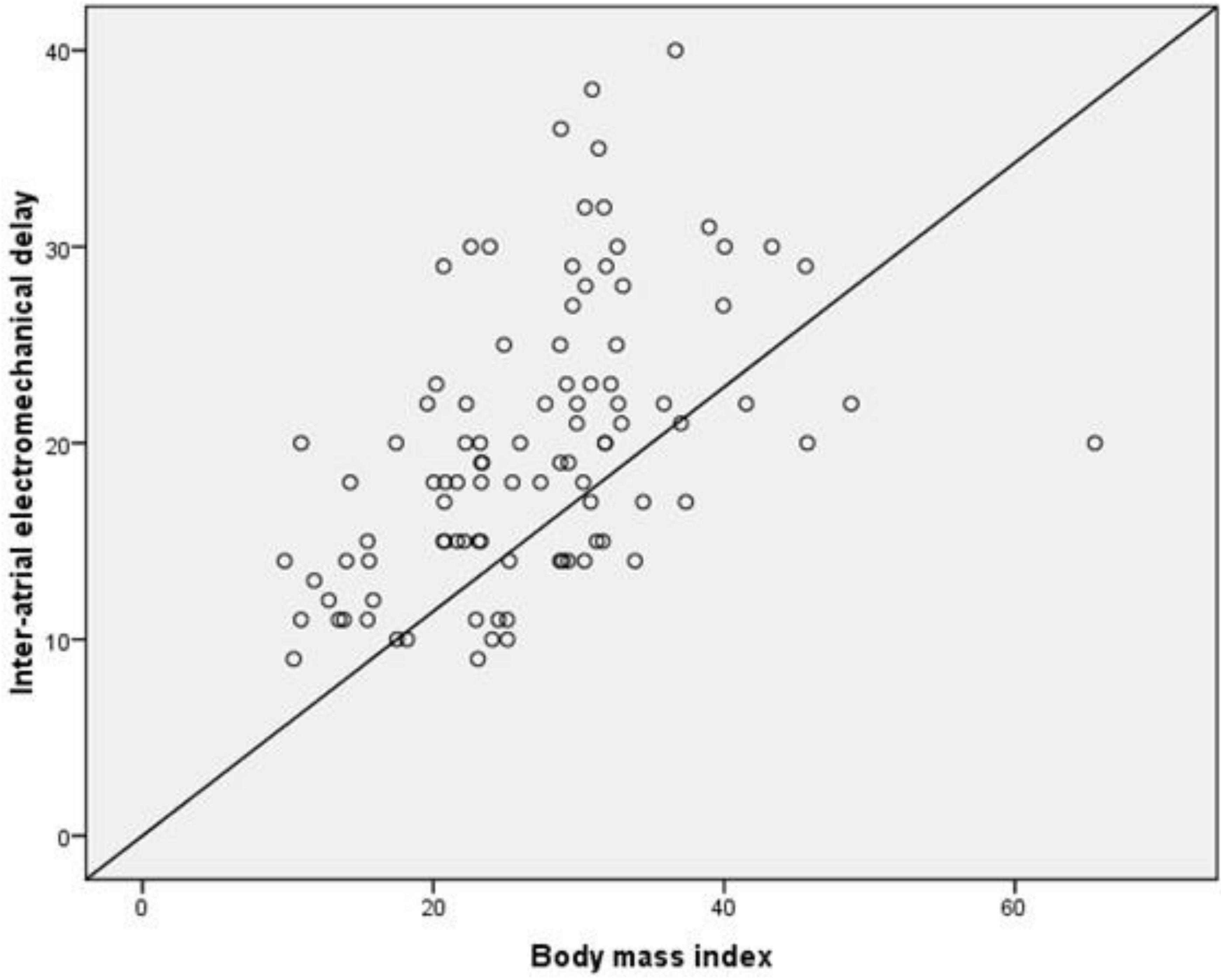
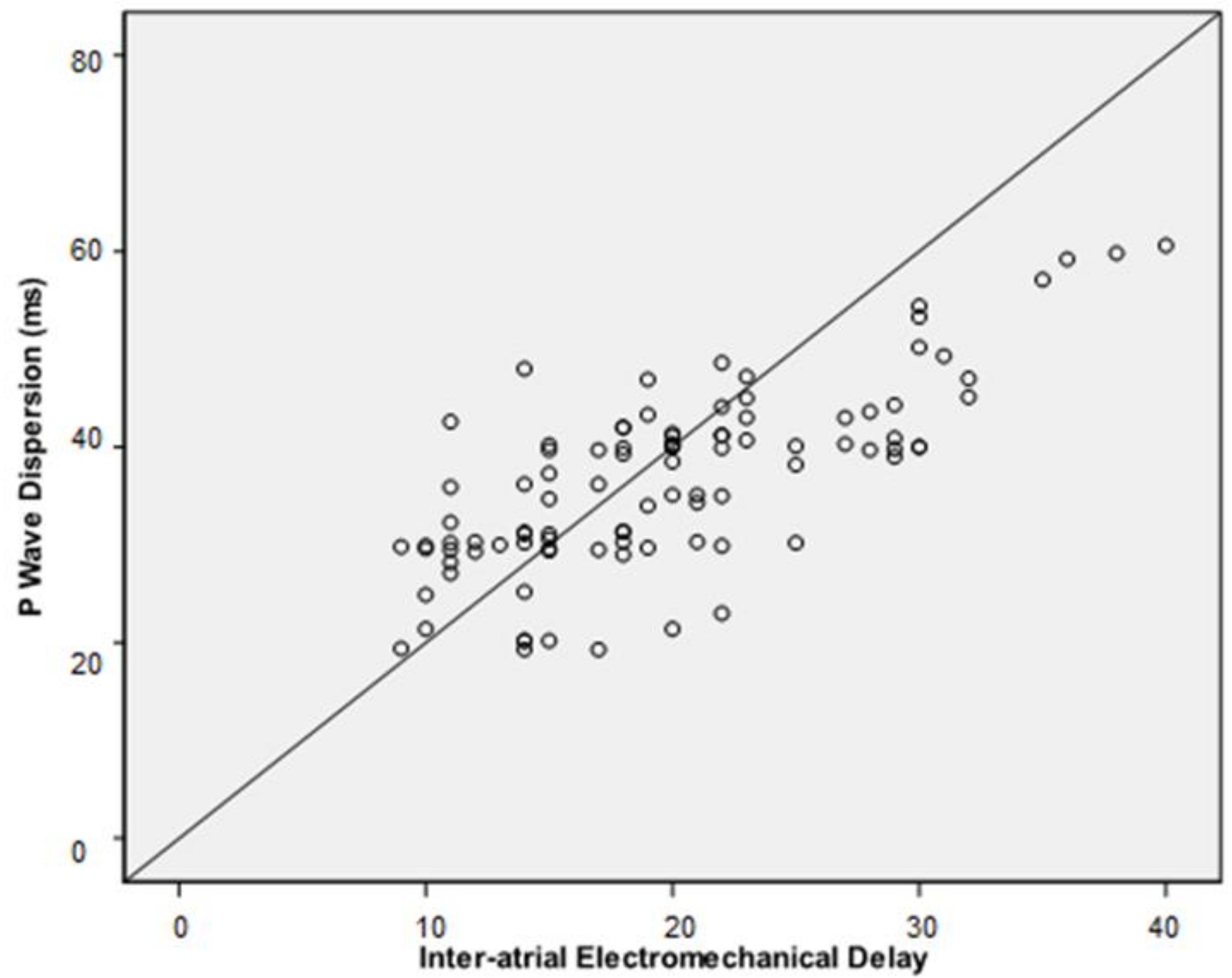
| Inter-atrial EMD, mean ± SD, ms | 22 ± 7 | 16 ± 5 | <0.001 |
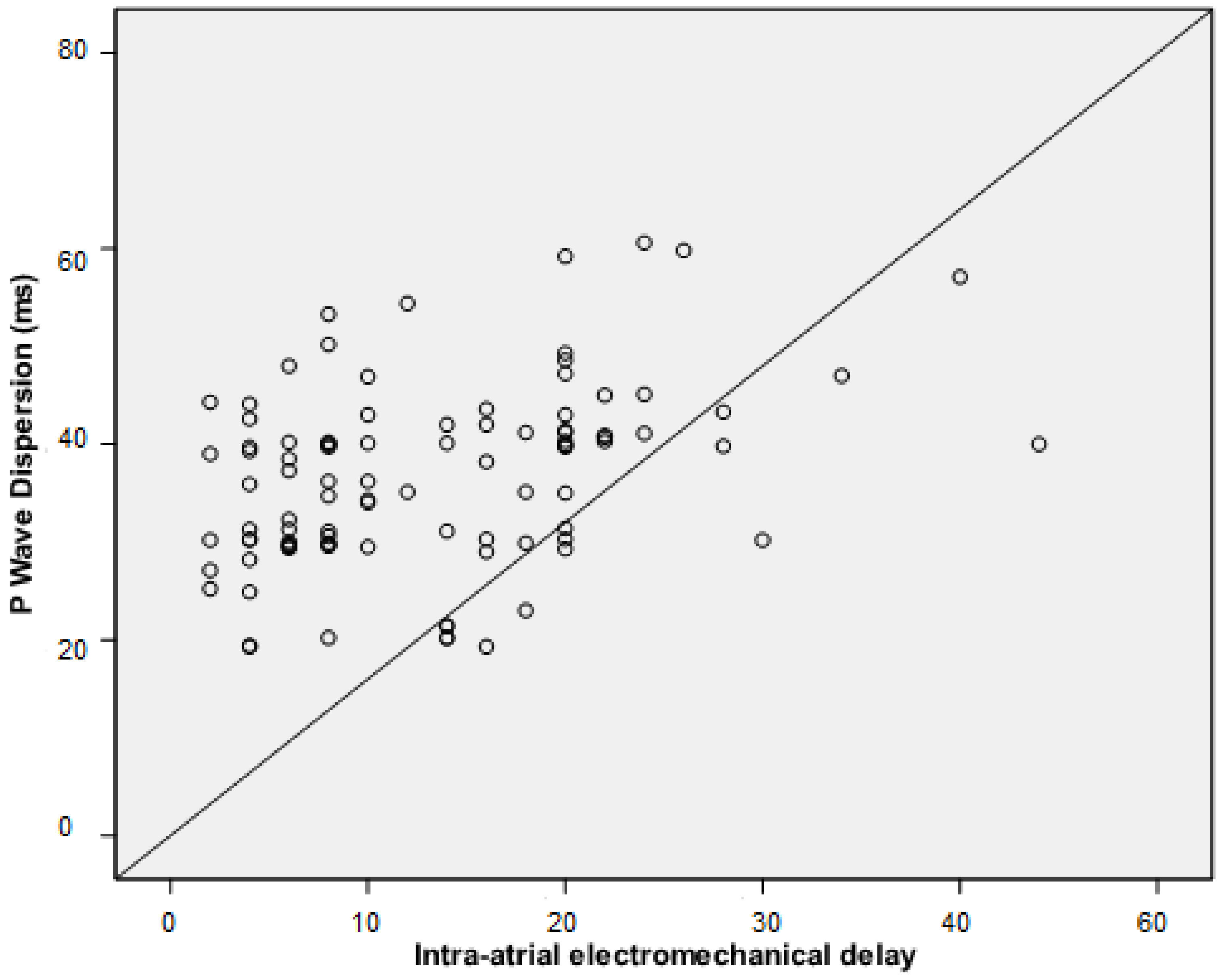
| Intra-atrial EMD, median (IQR), ms | 7 (4–10) | 4 (2–8) | <0.001 |
| Obese Subjects (n = 59) | Non-Obese Subjects (n = 38) | p | |
|---|---|---|---|
| Pmax, median (IQR), ms | 98.1 (89.3–104) | 85.2 (76.4–98.1) | <0.001 |
| Pmin, median (IQR), ms | 57.3 (55–60) | 49 (45.2–64.3) | 0.007 |
| Pd, median (IQR), ms | 40 (34–43.6) | 30.3 (29.3–39.4) | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Temiz, F.; Güneş, H.; Güneş, H. Evaluation of Atrial Electromechanical Delay in Children with Obesity. Medicina 2019, 55, 228. https://doi.org/10.3390/medicina55060228
Temiz F, Güneş H, Güneş H. Evaluation of Atrial Electromechanical Delay in Children with Obesity. Medicina. 2019; 55(6):228. https://doi.org/10.3390/medicina55060228
Chicago/Turabian StyleTemiz, Fatih, Hatice Güneş, and Hakan Güneş. 2019. "Evaluation of Atrial Electromechanical Delay in Children with Obesity" Medicina 55, no. 6: 228. https://doi.org/10.3390/medicina55060228
APA StyleTemiz, F., Güneş, H., & Güneş, H. (2019). Evaluation of Atrial Electromechanical Delay in Children with Obesity. Medicina, 55(6), 228. https://doi.org/10.3390/medicina55060228




