Early Clinical Features, Time to Secondary Progression, and Disability Milestones in Polish Multiple Sclerosis Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Data Collection
2.2. Stastistical Analysis
3. Results
3.1. Population Characteristics
3.2. Clinical Features
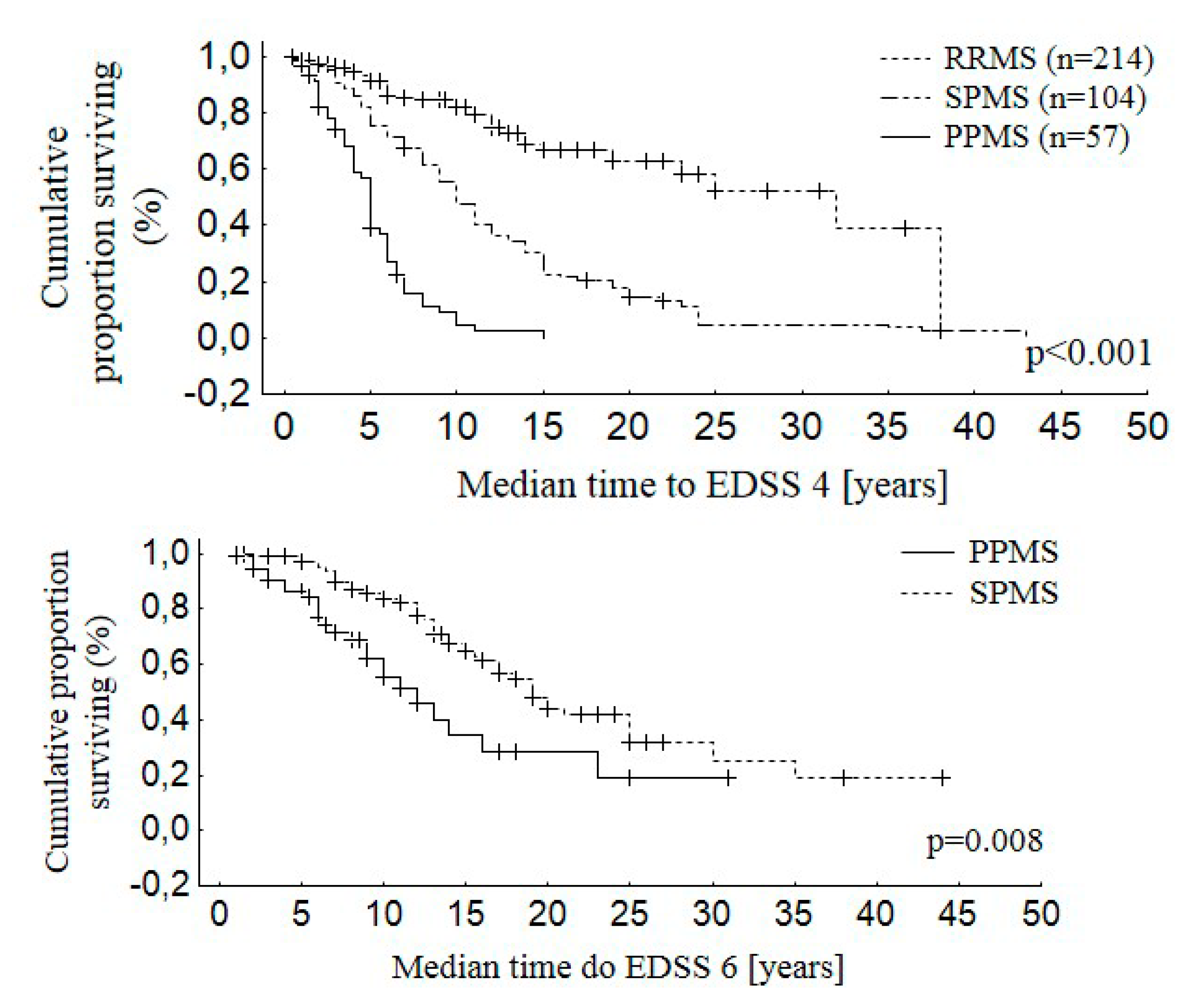
3.3. Disability
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lublin, F.D.; Reingold, S.C. Defining the clinical course of multiple sclerosis: Results of an international survey. National Multiple Sclerosis Society (USA) Advisory Committee on Clinical Trials of New Agents in Multiple Sclerosis. Neurology 1996, 46, 907–911. [Google Scholar] [CrossRef]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis: An overview. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Sadovnick, A.D.; Ebers, G.C. Epidemiology of multiple sclerosis: A critical overview. Can. J. Neurol. Sci. 1993, 20, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Leray, E.; Yaouanq, J.; Le Page, E.; Coustans, M.; Laplaud, D.; Oger, J.; Edan, G. Evidence for a two-stage disability progression in multiple sclerosis. Brain 2010, 133, 1900–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinshenker, B.G.; Bass, B.; Rice, G.P.; Noseworthy, J.; Carriere, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study: I: Clinical course and disability. Brain 1989, 112, 133–146. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S.; Adeleine, P. Early clinical predictors and progression of irreversible disability in multiple sclerosis: An amnesic process. Brain 2003, 126, 770–782. [Google Scholar] [CrossRef]
- Confavreux, C.; Vukusic, S. Natural history of multiple sclerosis: A unifying concept. Brain 2006, 129, 606–616. [Google Scholar] [CrossRef]
- Tremlett, H.; Paty, D.; Devonshire, V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006, 66, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Tremlett, H.; Zhao, Y.; Devonshire, V. Natural history of secondary-progressive multiple sclerosis. Mult. Scler. 2008, 14, 314–324. [Google Scholar] [CrossRef] [PubMed]
- Scalfari, A.; Neuhaus, A.; Degenhardt, A.; Rice, G.P.; Muraro, P.A.; Daumer, M.; Ebers, G.C. The natural history of multiple sclerosis: a geographically based study 10: Relapses and long-term disability. Brain 2010, 133, 1914–1929. [Google Scholar] [CrossRef] [PubMed]
- Kremenchutzky, M.; Rice, G.P.; Baskerville, J.; Wingerchuk, D.M.; Ebers, G.C. The natural history of multiple sclerosis: A geographically bases study. 9: Observations on the progressive phase of the disease. Brain 2006, 129, 584–594. [Google Scholar] [CrossRef]
- Tutuncu, M.; Tang, J.; Zeid, N.A.; Kale, N.; Crusan, D.J.; Atkinson, E.J.; Siva, A.; Pittock, S.J.; Pirko, I.; Keegan, B.M. Onset of progressive phase is an age-dependent clinical milestone in multiple sclerosis. Mult. Scler. 2013, 19, 188–198. [Google Scholar] [CrossRef]
- Confavreux, C.; Aimard, G.; Devic, M. Course and prognosis of multiple sclerosis assessed by the computerized data processing of 349 patients. Brain 1980, 103, 281–300. [Google Scholar] [CrossRef] [PubMed]
- Kantarci, O.; Siva, A.; Eraksoy, M.; Karabudak, R.; Sütlaş, N.; Ağaoğlu, J.; Turan, F.; Ozmenoğlu, M.; Toğrul, E.; Demirkiran, M. Survival and predictors of disability in Turkish MS patients. Turkish Multiple Sclerosis Study Group (TUMSSG). Neurology 1998, 51, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Debouverie, M.; Pittion-Vouyovitch, S.; Louis, S.; Guillemin, F.; LORSEP Group. Natural history of multiple sclerosis in a population-based cohort. Eur. J. Neurol. 2008, 15, 916–921. [Google Scholar] [CrossRef] [PubMed]
- Poser, C.M.; Paty, D.W.; Scheinberg, L.; McDonald, W.I.; Davis, F.A.; Ebers, G.C.; Johnson, K.P.; Sibley, W.A.; Silberberg, D.H.; Tourtellotte, W.W. New diagnostic criteria for multiple sclerosis: Guidelines for research protocols. Ann. Neurol. 1983, 13, 227–231. [Google Scholar] [CrossRef] [PubMed]
- McDonald, W.I.; Compston, A.; Edan, G.; Goodkin, D.; Hartung, H.P.; Lublin, F.D.; McFarland, H.F.; Paty, D.W.; Polman, C.H.; Reingold, S.C.; et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001, 50, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Polman, C.H.; Reingold, S.C.; Edan, G.; Filippi, M.; Hartung, H.P.; Kappos, L.; Lublin, F.D.; Metz, L.M.; McFarland, H.F.; O’Connor, P.W.; et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the "McDonald Criteria". Ann. Neurol. 2005, 58, 840–846. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Multiple Sclerosis International Federation (MSIF). Atlas of MS 2013: Mapping Multiple Sclerosis Around the World. Available online: www.msif.org/wp-content/uploads/2014/09/Atlas-of-MS.pdf (accessed on 15 January 2019).
- Pugliatti, M.; Rosati, G.; Carton, H.; Riise, T.; Drulovic, J.; Vécsei, L.; Milanov, I. The epidemiology of multiple sclerosis in Europe. Eur. J. Neurol. 2006, 13, 700–722. [Google Scholar] [CrossRef] [Green Version]
- Kułakowska, A.; Bartosik-Psujek, H.; Hożejowski, R.; Mitosek-Szewczyk, K.; Drozdowski, W.; Stelmasiak, Z. Selected aspects of the epidemiology of multiple sclerosis in Poland - A multicentre pilot study. Neurol. Neurochir. Pol. 2010, 44, 443–452. [Google Scholar] [CrossRef]
- Jacobs, L.D.; Wende, K.E.; Brownscheidle, C.M.; Apatoff, B.; Coyle, P.K.; Goodman, A.; Gottesman, M.H.; Granger, C.V.; Greenberg, S.J.; Herbert, J.; et al. A profile of multiple sclerosis: The New York State Multiple Sclerosis Consortium. Mult. Scler. 1999, 5, 369–376. [Google Scholar] [CrossRef]
- Alonso, A.; Jick, S.S.; Olek, M.J.; Hernán, M.A. Incidence of multiple sclerosis in the United Kingdom: Findings from a population-based cohort. J. Neurol. 2007, 254, 1736–1741. [Google Scholar] [CrossRef]
- Brola, W.; Fudala, M.; Flaga, S.; Ryglewicz, D.; Potemkowski, A. Polski rejestr chorych na stwardnienie rozsiane – Stan obecny, perspektywy i problemy. Aktualn. Neurol. 2015, 15, 68–73. [Google Scholar] [CrossRef]
- Chitnis, T.; Glanz, B.; Jaffin, S.; Healy, B. Demographics of pediatric-onset multiple sclerosis in an MS center population from the Northeastern United States. Mult. Scler. 2009, 15, 627–631. [Google Scholar] [CrossRef]
- Scalfari, A.; Knappertz, V.; Cutter, G.; Goodin, D.S.; Ashton, R.; Ebers, G.C. Mortality in patients with multiple sclerosis. Neurology 2013, 81, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Pierzchala, K.; Adamczyk-Sowa, M.; Dobrakowski, P.; Kubicka-Baczyk, K.; Niedziela, N.; Sowa, P. Demographic characteristics of MS patients in Poland’s upper Silesia region. Int. J. Neurosci. 2015, 125, 344–351. [Google Scholar] [CrossRef]
- Rose, A.S.; Kuzma, J.W.; Kurtzke, J.F.; Namerow, N.S.; Sibley, W.A.; Tourtellotte, W.W. Cooperative study in the evaluation of therapy in multiple sclerosis. ACTH vs. placebo – Final report. Neurology 1970, 20, 1–59. [Google Scholar]
- Amato, M.P.; Ponziani, G. A prospective study on the prognosis of multiple sclerosis. Neurol. Sci. 2000, 21, S831–S838. [Google Scholar] [CrossRef]
- Eriksson, M.; Andersen, O.; Runmarker, B. Long-term follow up of patients with clinically isolated syndromes, relapsing-remitting and secondary progressive multiple sclerosis. Mult. Scler. 2003, 9, 260–274. [Google Scholar] [CrossRef] [PubMed]
- Lorscheider, J.; Buzzard, K.; Jokubaitis, V.; Spelman, T.; Havrdova, E.; Horakova, D.; Trojano, M.; Izquierdo, G.; Girard, M.; Duquette, P.; et al. Defining secondary progressive multiple sclerosis. Brain 2016, 139, 2395–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeholm, H.; Lycke, J.; Skoog, B.; Lisovskaja, V.; Hillert, J.; Dahle, C.; Fagius, J.; Fredrikson, S.; Landtblom, A.M.; Malmeström, C.; et al. Time to secondary progression in patients with multiple sclerosis who were treated with first generation immunomodulating drugs. Mult. Scler. 2013, 19, 765–774. [Google Scholar] [CrossRef]
- Potemkowski, A. Epidemiologiczne badania czasu trwania choroby i długości życia chorych na stwardnienie rozsiane. Zdrow. Publiczne 1999, 1, 5–11. [Google Scholar]
- Sand, I.K.; Krieger, S.; Farrell, C.; Miller, A.E. Diagnostic uncertainty during the transition to secondary progressive multiple sclerosis. Mult. Scler. 2014, 20, 1654–1657. [Google Scholar] [CrossRef] [PubMed]
- Rovaris, M.; Confavreux, C.; Furlan, R.; Kappos, L.; Comi, G.; Filippi, M. Secondary progressive multiple sclerosis: Current knowledge and future challenges. Lancet Neurol. 2006, 5, 343–354. [Google Scholar] [CrossRef]
- Cottrell, D.A.; Kremenchutzky, M.; Rice, G.P.; Koopman, W.J.; Hader, W.; Baskerville, J.; Ebers, G.C. The natural history of multiple sclerosis: A geographically based study. The clinical features and natural history of primary progressive multiple sclerosis. Brain 1999, 122, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Vukusic, S.; Confavreux, C. Prognostic factors for progression of disability in the secondary progressive phase of multiple sclerosis. J. Neurol. Sci. 2003, 206, 135–137. [Google Scholar] [CrossRef]
- Langer-Gould, A.; Popat, R.A.; Huang, S.M.; Cobb, K.; Fontoura, P.; Gould, M.K.; Nelson, L.M. Clinical and demographic predictors of long-term disability in patients with relapsing-remitting multiple sclerosis: A systematic review. Arch. Neurol. 2006, 63, 1686–1691. [Google Scholar] [CrossRef]


| Characteristics of the Study Population | Total MS Patients | Female | Male |
|---|---|---|---|
| All patients | 375 | 260 (69.3%) | 115 (30.7%) |
| Mean age (years) | 43.1 ± 12.5 | 42.2 ± 12.6 | 44.9 ± 12.0 |
| Median time of disease duration (years) | 9.0 | 8.0 | 9.0 |
| Mean age at the first clinical manifestation of disease (years) | 32.3 | 31.7 ± 10.9 | 33.5 ± 10,9 |
| Mean Expanded Disability Status Scale (EDSS) Score at Censoring (1–8) | 3.7 ± 1.7 | 3.5 ± 1.7 | 4.3 ± 1.7 |
| 0–3.5 | 188 (50.1%) | 143 (55%) | 45 (39.1%) |
| 4.0–5.5 | 120 (32.0%) | 80 (30.8%) | 40 (34.8%) |
| 6.0–10.0 | 67 (17.9%) | 37 (14.2%) | 30 (26.1%) |
| Clinical Course at Censoring | |||
| Relapsing-remitting MS (RRMS) | 214 (57.1%) | 166 (63.8%) | 48 (41.7%) |
| Primary progressive MS (PPMS) | 57 (15.2%) | 28 (10.8%) | 29 (25.2%) |
| Secondary progressive MS (SPMS) | 104 (27.7%) | 66 (25.4%) | 38 (33.1%) |
| Localization of the First Demyelinating Lesions | |||
| Supratentorial structures and optic nerves | 138 (64.8%) | ||
| Spinal cord | 132 (35.2%) | ||
| Cerebellum | 60 (16.2%) | ||
| Brain stem | 45 (12.0%) | ||
| Nature of Initial Symptoms | |||
| Motor deficit | 133 (35.5%) | ||
| Sensory symptoms | 126 (33.6%) | ||
| Optic neuritis | 93 (24.8%) | ||
| Balance disturbances | 74 (19.7%) | ||
| Vertigo | 41 (10.9%) | ||
| Double vision | 35 (9.3%) | ||
| Cranial nerves dysfunction | 22 (5.9%) | ||
| Sphincter dysfunctions | 13 (3.5%) | ||
| Gait dysfunctions | 8 (2.1%) | ||
| Coordination impairment | 7 (1.9%) | ||
| Neuralgias | 6 (1.6%) | ||
| Monosymptomatic onset | 240 (64%) | ||
| Polysymptomatic onset | 135 (36%) | ||
| Treatment | |||
| Immunomodulatory drugs IMDs | 125 (33.3%) |
| Symptoms | PPMS n = 57 | RRMS n = 318 | p | ||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Motor deficits | 44 | 77.2% | 89 | 28.0% | <0.001 |
| Sensory loss | 11 | 19.3% | 115 | 36.2% | 0.013 |
| Optic neuritis | 0 | 0.0% | 93 | 29.3% | <0.001 |
| Balance disturbances | 21 | 36.8% | 53 | 16.7% | <0.001 |
| Vertigo | 9 | 15.8% | 32 | 10.1% | 0.202 |
| Double vision | 2 | 3.5% | 33 | 10.4% | 0.100 |
| Cranial nerves dysfunctions | 7 | 12.3% | 15 | 4.7% | 0.025 |
| Sphincter dysfunctions | 5 | 8.8% | 8 | 2.5% | 0.017 |
| Gait dysfunctions | 4 | 7.0% | 4 | 1.3% | 0.006 |
| Coordination impairment | 1 | 1.8% | 6 | 1.9% | 0.940 |
| Neuralgias | 0 | 0.0% | 6 | 1.9% | 0.296 |
| Symptoms | Conversion from RRMS to SPMS (years), n = 103 | p | |||
|---|---|---|---|---|---|
| Present Symptoms | Absent Symptoms | ||||
| n | Mean ± SD | n | Mean ± SD | ||
| Motor deficits | 38 | 8.3 ± 4.4 | 65 | 15.3 ± 7.6 | <0.001 |
| Sensory loss | 34 | 13.5 ± 8.0 | 69 | 11.5 ± 5.9 | 0.233 |
| Optic neuritis | 24 | 16.8 ± 5.2 | 79 | 11.5 ± 7.6 | 0.002 |
| Balance disturbances | 25 | 11.2 ± 6,8 | 78 | 13.2 ± 7.6 | 0.230 |
| Vertigo | 14 | 14.6 ± 11.7 | 89 | 12,4 ± 6.6 | 0.299 |
| Double vision | 8 | 13.3 ± 11.5 | 95 | 12.7 ± 7.1 | 0.834 |
| Cranial nerves dysfunctions | 5 | 13.2 ± 2.8 | 98 | 12.7 ± 7.6 | 0.883 |
| Sphincter dysfunctions | 2 | 8.5 ± 2.1 | 101 | 12.8 ± 7.5 | 0.420 |
| Coordination impairment | 2 | 5.5 ± 2.1 | 101 | 12.9 ± 7.4 | 0.167 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rzepiński, Ł.; Zawadka-Kunikowska, M.; Maciejek, Z.; Newton, J.L.; Zalewski, P. Early Clinical Features, Time to Secondary Progression, and Disability Milestones in Polish Multiple Sclerosis Patients. Medicina 2019, 55, 232. https://doi.org/10.3390/medicina55060232
Rzepiński Ł, Zawadka-Kunikowska M, Maciejek Z, Newton JL, Zalewski P. Early Clinical Features, Time to Secondary Progression, and Disability Milestones in Polish Multiple Sclerosis Patients. Medicina. 2019; 55(6):232. https://doi.org/10.3390/medicina55060232
Chicago/Turabian StyleRzepiński, Łukasz, Monika Zawadka-Kunikowska, Zdzisław Maciejek, Julia L. Newton, and Paweł Zalewski. 2019. "Early Clinical Features, Time to Secondary Progression, and Disability Milestones in Polish Multiple Sclerosis Patients" Medicina 55, no. 6: 232. https://doi.org/10.3390/medicina55060232





