Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Substances
2.2. Animals
2.3. Experimental Design
2.4. Preparation of Test Substances
2.5. Preparation and Analysis of Blood Serum
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Holick, M.F. The Vitamin D deficiency pandemic: Approaches for diagnosis, treatment and prevention. Rev. Endocr. Metab. Disord. 2017, 18, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Wacker, M.; Holick, M.F. Sunlight and vitamin D. Dermato-Endocrinology 2013, 5, 51–108. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F. Biological effects of sunlight, ultraviolet radiation, visible light, infrared radiation and vitamin D for health. Anticancer Res. 2016, 36, 1345–1356. [Google Scholar] [PubMed]
- Holick, M.F. Vitamin D deficiency. N. Engl. J. Med. 2007, 357, 266–281. [Google Scholar] [CrossRef] [PubMed]
- Hossein-nezhad, A.; Holick, M.F. Vitamin D for health: A global perspective. Mayo Clin. Proc. 2013, 88, 720–755. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the institute of medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Poskitt, E.M.; Cole, T.J.; Lawson, D.E. Diet, sunlight, and 25-hydroxy vitamin D in healthy children and adults. Br. Med. J. 1979, 1, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Heaney, R.P.; Armas, L.A.G.; French, C. All-source basal vitamin D inputs are greater than previously thought and cutaneous inputs are smaller. J. Nutr. 2013, 143, 571–575. [Google Scholar] [CrossRef]
- Crowe, F.L.; Steur, M.; Allen, N.E.; Appleby, P.N.; Travis, R.C.; Key, T.J. Plasma concentrations of 25-hydroxyvitamin D in meat eaters, fish eaters, vegetarians and vegans: Results from the EPIC–Oxford study. Public Health Nutr. 2011, 14, 340–346. [Google Scholar] [CrossRef]
- Schmid, A.; Walther, B. Natural vitamin D content in animal products. Adv. Nutr. 2013, 4, 453–462. [Google Scholar] [CrossRef]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in vitamin D activation and function. J. Am. Osteopath. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M. Evaluation, treatment, and prevention of vitamin D deficiency: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine of the National Academies. Dietary Reference Intakes for Calcium and Vitamin D; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Davies, M.; Mawer, E.B.; Krawitt, E.L. Comparative absorption of vitamin D3 and 25-hydroxyvitamin D3 in intestinal disease. Gut 1980, 21, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.; Sidhom, G.; Whiting, S.J.; Rousseau, D.; Vieth, R. The bioavailability of vitamin D from fortified cheeses and supplements is equivalent in adults. J. Nutr. 2008, 138, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.; Gleize, B.; Roi, S.; Nowicki, M.; Dhaussy, A.; Huertas, A.; Amiot, M.-J.; Reboul, E. Fatty acids affect micellar properties and modulate vitamin D uptake and basolateral efflux in Caco-2 cells. J. Nutr. Biochem. 2013, 24, 1751–1757. [Google Scholar] [CrossRef] [PubMed]
- Kanicky, J.R.; Shah, D.O. Effect of degree, type, and position of unsaturation on the PKa of long-chain fatty acids. J. Colloid Interface Sci. 2002, 256, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; Davidov-Pardo, G.; McClements, D.J. Nanotechnology for increased micronutrient bioavailability. Trends Food Sci. Technol. 2014, 40, 168–182. [Google Scholar] [CrossRef]
- Fox, C.B.; Kim, J.; Le, L.V.; Nemeth, C.L.; Chirra, H.D.; Desai, T.A. Micro/nanofabricated platforms for oral drug delivery. J. Control. Release 2015, 219, 431–444. [Google Scholar] [CrossRef] [Green Version]
- Rautureau, M.; Rambaud, J.C. Aqueous solubilisation of vitamin D3 in normal man. Gut 1981, 22, 393–397. [Google Scholar] [CrossRef]
- Dawson-Hughes, B. Serum 25-hydroxyvitamin D and functional outcomes in the elderly. Am. J. Clin. Nutr. 2008, 88, 537S–540S. [Google Scholar] [CrossRef] [Green Version]
- Lenders, C.M.; Feldman, H.A.; Von Scheven, E.; Merewood, A.; Sweeney, C.; Wilson, D.M.; Lee, P.D.K.; Abrams, S.H.; Gitelman, S.E.; Wertz, M.S.; et al. Relation of body fat indexes to vitamin D status and deficiency among obese adolescents. Am. J. Clin. Nutr. 2009, 90, 459–467. [Google Scholar] [PubMed] [Green Version]
- Strickley, R.G. Solubilizing excipients in oral and injectable formulations. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, R.E.; Tangpricha, V. Evaluation of vehicle substances on vitamin D bioavailability: A systematic review. Mol. Nutr. Food Res. 2010, 54, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Aisha, A.F.; Majid, A.M.S.; Ismail, Z. Preparation and characterization of nano liposomes of orthosiphon stamineus ethanolic extract in Soybean PHOSPHOLIPIDS. BMC Biotechnol. 2014, 14, 23. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Uechi, S.; Takara, K.; Asikin, Y.; Wada, K. Evaluation of an oral carrier system in rats: Bioavailability and antioxidant properties of liposome-encapsulated curcumin. J. Agric. Food Chem. 2009, 57, 9141–9146. [Google Scholar] [PubMed]
- Lopes, C.M.; Martins-Lopes, P.; Souto, E.B. Nanoparticulate carriers (NPC) for oral pharmaceutics and nutraceutics. J. Clin. Endocrinol. Metab. 2010, 65, 75–82. [Google Scholar]
- Rahman, A.; Al-Awadi, A.A.; Khan, K.M. Lead affects vitamin D metabolism in rats. Nutrients 2018, 10, 264. [Google Scholar] [CrossRef]

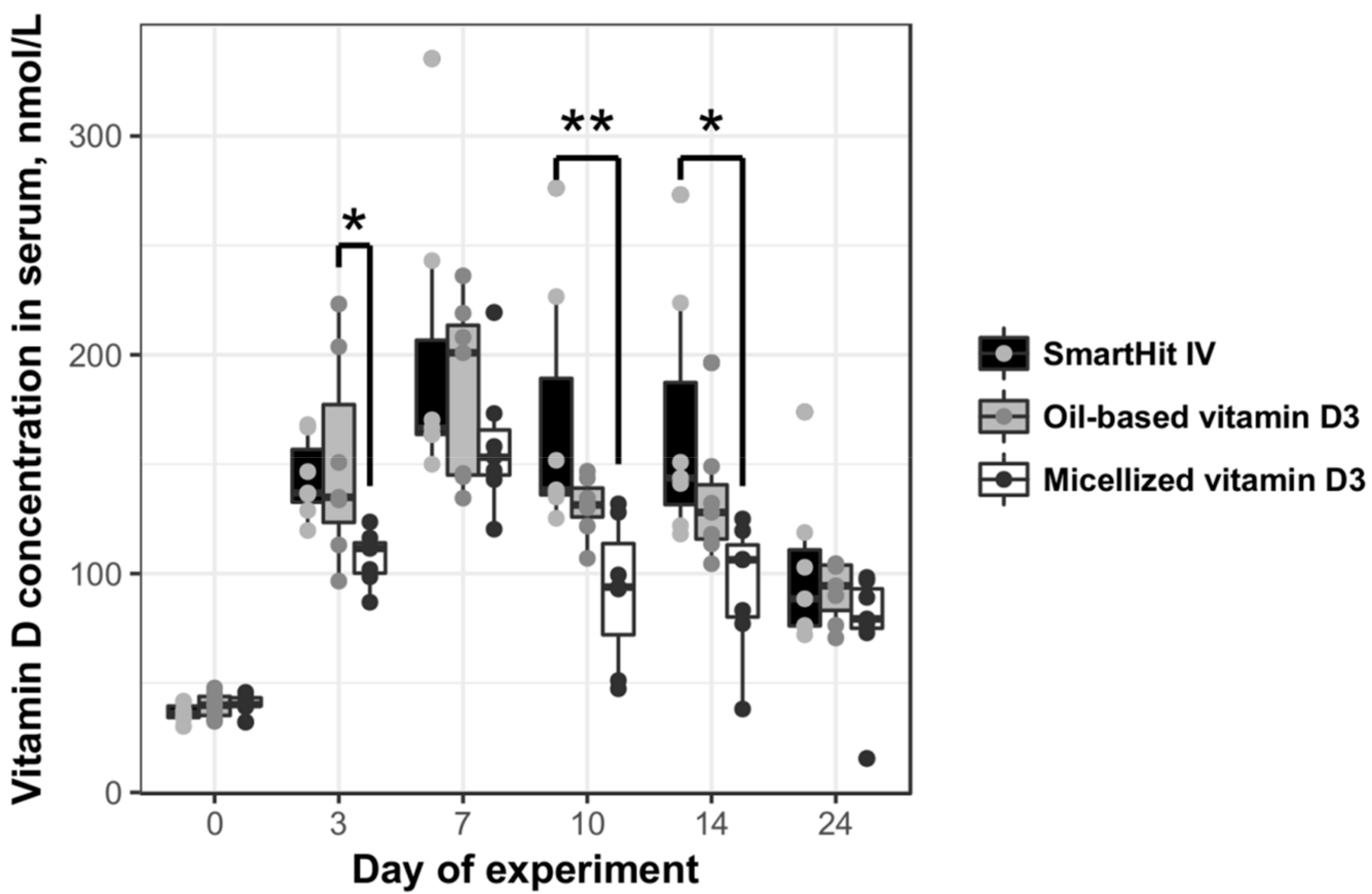
| Group | AUC | AUCx/AUCOil-Based | CMax Vitamin D Concentration in Blood Serum, nmol/L | Day of Maximum Concentration of Vitamin D |
|---|---|---|---|---|
| SmartHit IV™ | 2651.00 | 1.24 | 335.4 | 7 |
| Oil-based vitamin D3 | 2135.60 | 1.00 | 236.1 | 7 |
| Micellized vitamin D3 | 1378.45 | 0.65 | 216.4 | 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimoliūnas, E.; Rinkūnaitė, I.; Bukelskienė, Ž.; Bukelskienė, V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina 2019, 55, 265. https://doi.org/10.3390/medicina55060265
Šimoliūnas E, Rinkūnaitė I, Bukelskienė Ž, Bukelskienė V. Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model. Medicina. 2019; 55(6):265. https://doi.org/10.3390/medicina55060265
Chicago/Turabian StyleŠimoliūnas, Egidijus, Ieva Rinkūnaitė, Živilė Bukelskienė, and Virginija Bukelskienė. 2019. "Bioavailability of Different Vitamin D Oral Supplements in Laboratory Animal Model" Medicina 55, no. 6: 265. https://doi.org/10.3390/medicina55060265





