Pulmonary Vascular Underperfusion Score in Premature Infants with Bronchopulmonary Dysplasia and Pulmonary Hypertension
Abstract
:1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Steven, H.A.; Georg, H.; Stephen, L.; Archer, D.; Dunbar, I.; Ian, A.; Wendy, K.C.; Brian, D.H.; Erika, B.; Rosenzweig, J.; et al. Pediatric pulmonary hypertension: Guidelines from the American Heart Association and American Thoracic Society. Circulation 2015, 132, 2037–2099. [Google Scholar]
- Mourani, P.M.; Sontag, M.K.; Younoszai, A.; Miller, J.I.; Kinsella, J.P.; Baker, C.D.; Poindexter, B.B.; Ingram, D.A.; Abman, S.H. Early Pulmonary Vascular Disease in Preterm Infants at Risk for Bronchopulmonary Dysplasia. Am. J. Respir. Crit. Care Med. 2015, 191, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isayama, T.; Lee, S.K.; Yang, J.; Lee, D.; Daspal, S.; Dunn, M.; Shah, P.S. Revisiting the definition of bronchopulmonary dysplasia: Effect of changing panoply of respiratory support for preterm neonates. JAMA Pediatr. 2017, 171, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Ehrenkranz, R.A. Validation of the National Institutes of Health Consensus Definition of Bronchopulmonary Dysplasia. Pediatrics 2005, 116, 1353–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, B.B.; Jadotte, M.M.; Mills, J.; Chan, K.C. Digital subtraction pulmonary angiography in children with pulmonary hypertension due to bronchopulmonary dysplasia. Med. Sci. 2019, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Maislin, G.; Fishman, A.P. Vasodilator Therapy for Primary Pulmonary Hypertension in Children. Circulation 1999, 99, 1197–1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, G.K.; Levy, P.T.; Holland, M.R.; Hamvas, A. Novel Methods for Assessment of Right Heart Structure and Function in Pulmonary Hypertension. Clin. Perinatol. 2012, 39, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, G.R.; Wyman, W.L.; Jonathan, A.; Lanqi, H.; Handschumacher, M.D.; Krishnaswamy, C.; Scott, D.S.; Eric, K.L.; Nelson, B.S. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by European Association of Echocardiography; a registered branch of the European Society of Cardiology; and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713. [Google Scholar]
- Herbert, S.; Tulloh, R. Sildenafil Pulmonary hypertension and bronchopulmonary dysplasia. Early Hum. Dev. 2016, 102, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Berkelhamer, S.K.; Mestan, K.K.; Steinhorn, R.H. Pulmonary Hypertension in Bronchopulmonary Dysplasia. Semin. Perinatol. 2013, 37, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Menahem, S.; Allison, B.J.; Miller, S.L.; Polglase, G.R. Preterm growth restriction and bronchopulmonary dysplasia: The vascular hypothesis and related physiology. J. Physiol. 2019, 597, 1209–1220. [Google Scholar]
- Arjaans, S.; Zwart, E.A.H.; Ploegstra, M.-J.; Bos, A.F.; Kooi, E.M.W.; Hillege, H.L.; Berger, R.M.F. Identification of gaps in the current knowledge on pulmonary hypertension in extremely preterm infants: A systematic review and meta-analysis. Paediatr. Périnat. Epidemiol. 2018, 32, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, U.; Feinstein, J.A.; Adatia, I.; Austin, E.D.; Mullen, M.P.; Hopper, R.K.; Hanna, B.; Romer, L.; Keller, R.L.; Fineman, J.; et al. Evaluation and Management of Pulmonary Hypertension in Children with Bronchopulmonary Dysplasia. J. Pediatr. 2017, 188, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Higgins, R.D.; Jobe, A.H.; Koso-Thomas, M.; Bancalari, E.; Viscardi, R.M.; Hartert, T.V.; Ryan, R.M.; Kallapur, S.G.; Steinhorn, R.H.; Konduri, G.G.; et al. Bronchopulmonary Dysplasia: Executive Summary of a Workshop. J. Pediatr. 2018, 197, 300–308. [Google Scholar] [CrossRef] [PubMed]



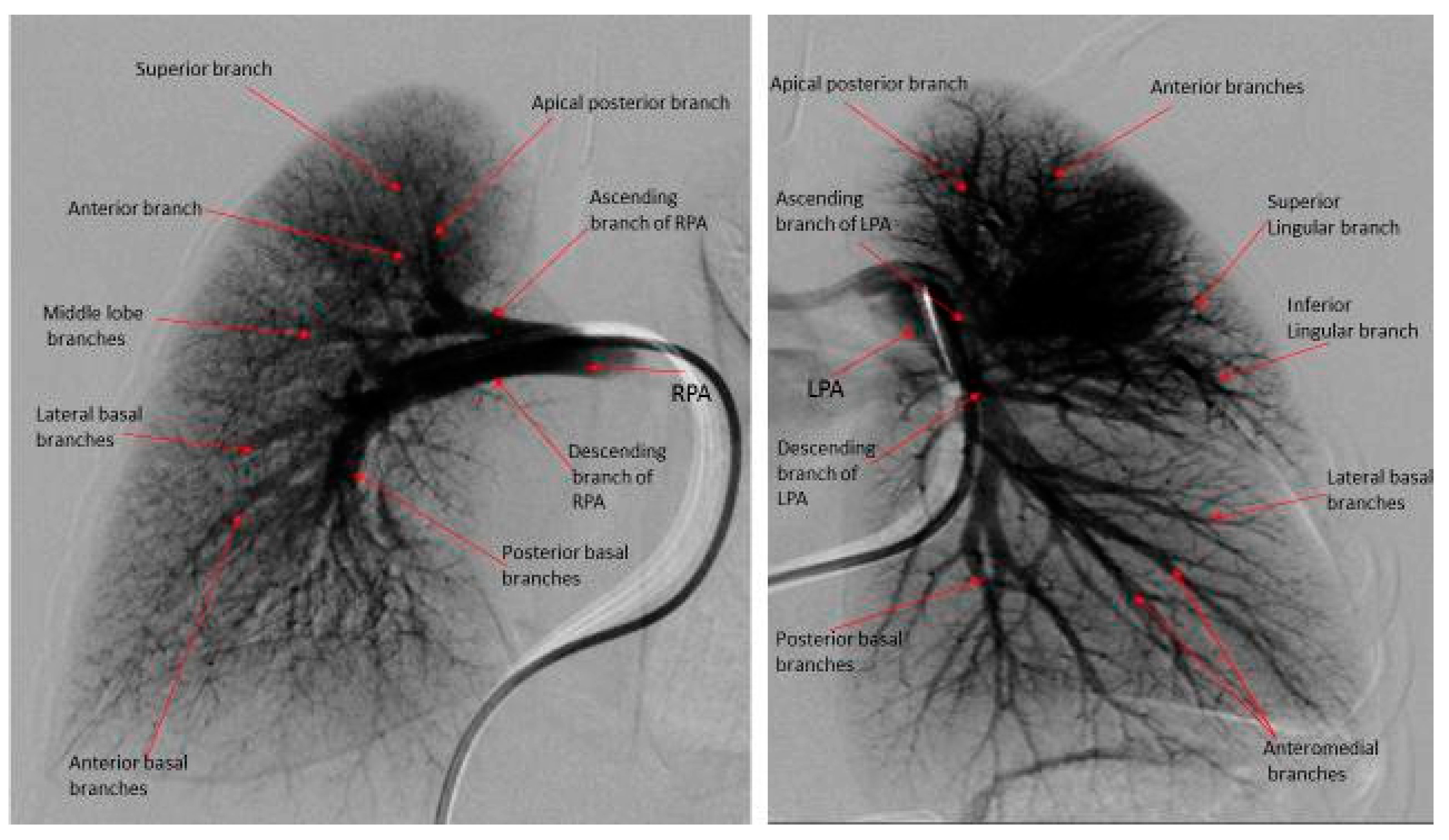

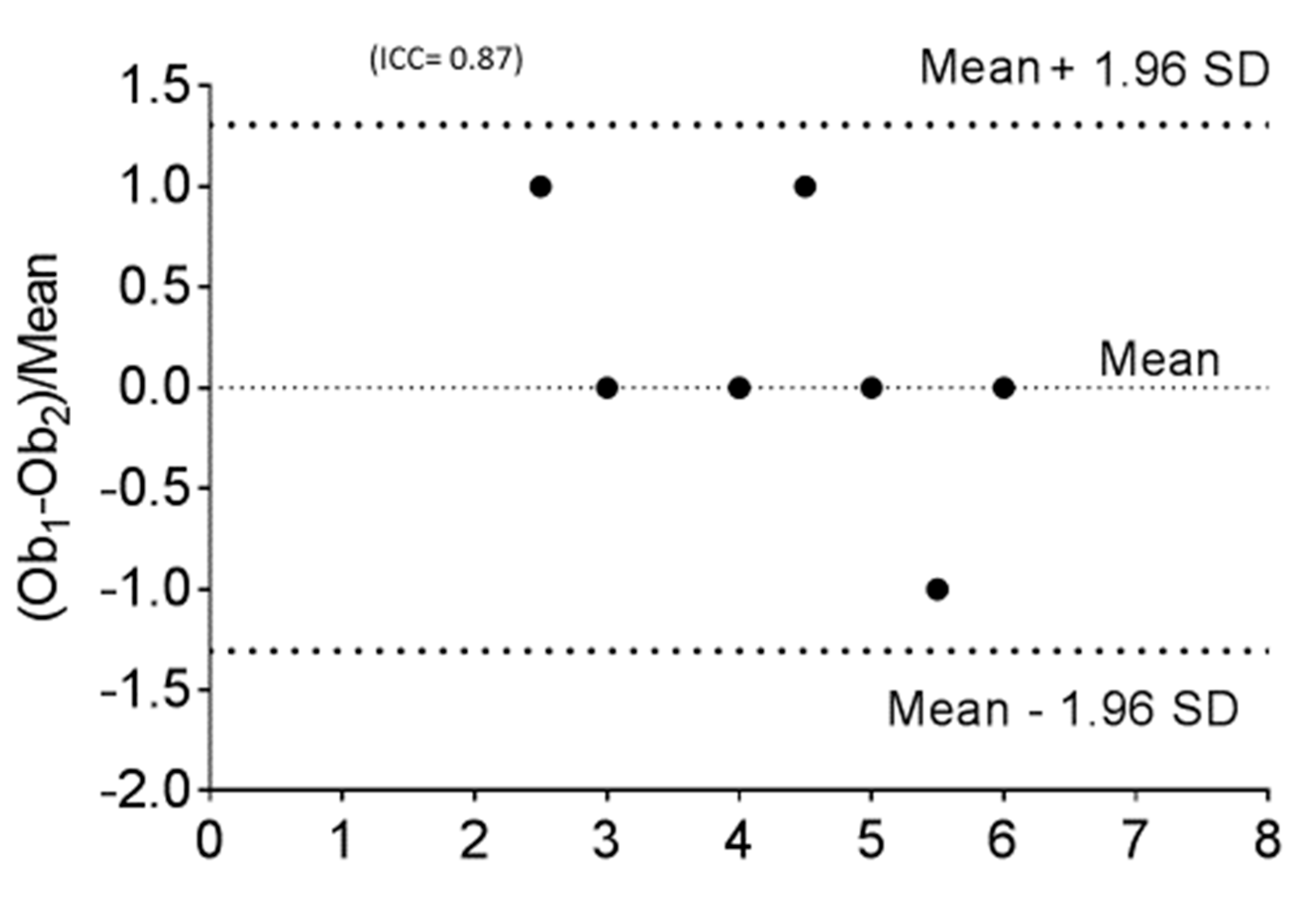
| Cases (N = 10) | |
|---|---|
| Gestational age (weeks ± SD) | 25.1 ± 2.02 |
| Birth weight (grams ± SD) | 706 ± 240 |
| SGA (n) (%) | 3 (30) |
| Gender: Male, n (%) | 4 (40) |
| C-section delivery, n (%) | 7 (70) |
| Antenatal steroid, n (%) | 9 (90) |
| Preeclampsia (Yes), n (%) | 4 (40) |
| HELLP (Yes), n (%) | 1 (10) |
| Pregnancy-induced hypertension (Yes), n (%) | 1 (10) |
| Chronic hypertension (Yes), n (%) | 3 (30) |
| Oligohydramnios (Yes), n (%) | 1 (10) |
| Polyhydramnios (Yes), n (%) | 1 (10) |
| Clinical chorioamnionitis (Yes), n (%) | 0 (0) |
| GBS (+), n (%) | 1 (10) |
| APGAR at 5 min, (minutes ± SD) | 5.6 ± 1.2 |
| Received surfactant after birth (Yes), n (%) | 5 (50) |
| PDA (Yes), n (%) | 5 (50) |
| PFO/ASD (Yes), n (%) | 6 (60) |
| Intubation after birth), n (%) | 7 (70) |
| Duration of intubation (days ± SD) | 45 ± 38 |
| Tracheostomy, n (%) | 2 (20) |
| Necrotizing enterocolitis (Bell’s stage 2 +), n (%) | 4 (40) |
| Severe intraventricular hemorrhage, n (%) | 2 (20) |
| Retinopathy of prematurity, n (%) | 4 (40) |
| Variables | AVT Responder (N = 6) | AVT Non-Responder (N = 4) | P |
|---|---|---|---|
| Right heart catheterization: | |||
| Age at cardiac catheterization (months ± SD) | 9.6 ± 2.8 | 9.7 ± 2.8 | 0.96 |
| Weight at cardiac catheterization (kg ± SD) | 3.9 ± 0.64 | 4.4 ± 0.6 | 0.28 |
| Mean RAP mmHg (mean ± SD) | 11 ± 4.7 | 9 ± 1.3 | 0.31 |
| Mean PAP mmHg (mean ± SD) | 40 ± 7 | 45 ± 7 | 0.32 |
| Mean PVRi (WU·m2) (mean ± SD) | 3.3 ± 2.5 | 3.5 ± 2.3 | 0.13 |
| Mean SVRi (WU·m2) (mean ± SD) | 9.8 ± 9.5 | 10.2 ± 6.8 | 0.53 |
| PVRi/SVRi (mean ± SD) | 0.32 ± 0.1 | 0.34 ± 0.04 | 0.71 |
| Mean Cardiac Index (L/min/m2) (mean ± SD) | 3.6 ± 1.3 | 3.9 ± 1.6 | 0.45 |
| Digital subtraction pulmonary angiogram: | |||
| Pulmonary Vascular Underperfusion Score (PVUS) a (mean ± SD) | 2.66 ± 0.47 | 5 ± 0.81 | 0.0048 |
| Echocardiographic parameters (not done with simultaneous cardiac catheterization) | |||
| b RVSP based on TR jet mmHg (mmHg ± SD) | 40 ± 4 | 53 ± 10.6 | 0.06 |
| c RV Fractional shortening area change % (mean ± SD) | 0.39 ± 0.09 | 0.31 ± 0.09 | 0.07 |
| d TAPSE (cm ± SD) | 12.4 ± 1.6 | 10.5 ± 1.9 | 0.15 |
| e AT/RVET (mean ± SD) | 0.33 ± 0.02 | 0.29 ± 0.13 | 0.13 |
| f RV/LV ratio (mean ± SD) | 0.94 ± 0.05 | 0.96 ± 0.04 | 0.89 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, B.B.; Jadotte, M.-M.; Chan, K.-C. Pulmonary Vascular Underperfusion Score in Premature Infants with Bronchopulmonary Dysplasia and Pulmonary Hypertension. Medicina 2019, 55, 359. https://doi.org/10.3390/medicina55070359
Das BB, Jadotte M-M, Chan K-C. Pulmonary Vascular Underperfusion Score in Premature Infants with Bronchopulmonary Dysplasia and Pulmonary Hypertension. Medicina. 2019; 55(7):359. https://doi.org/10.3390/medicina55070359
Chicago/Turabian StyleDas, Bibhuti B., Michelle-Marie Jadotte, and Kak-Chen Chan. 2019. "Pulmonary Vascular Underperfusion Score in Premature Infants with Bronchopulmonary Dysplasia and Pulmonary Hypertension" Medicina 55, no. 7: 359. https://doi.org/10.3390/medicina55070359






